Food Chain Length Associated with Environmental Factors Affected by Large Dam along the Yangtze River
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Site Descriptions
2.2. Sample Collection and Stable Isotope Analysis
2.3. Estimates of Food Chain Length
2.4. Characterizing Ecosystem Size, Resource Availability, and Disturbance
2.5. Statistical Analysis
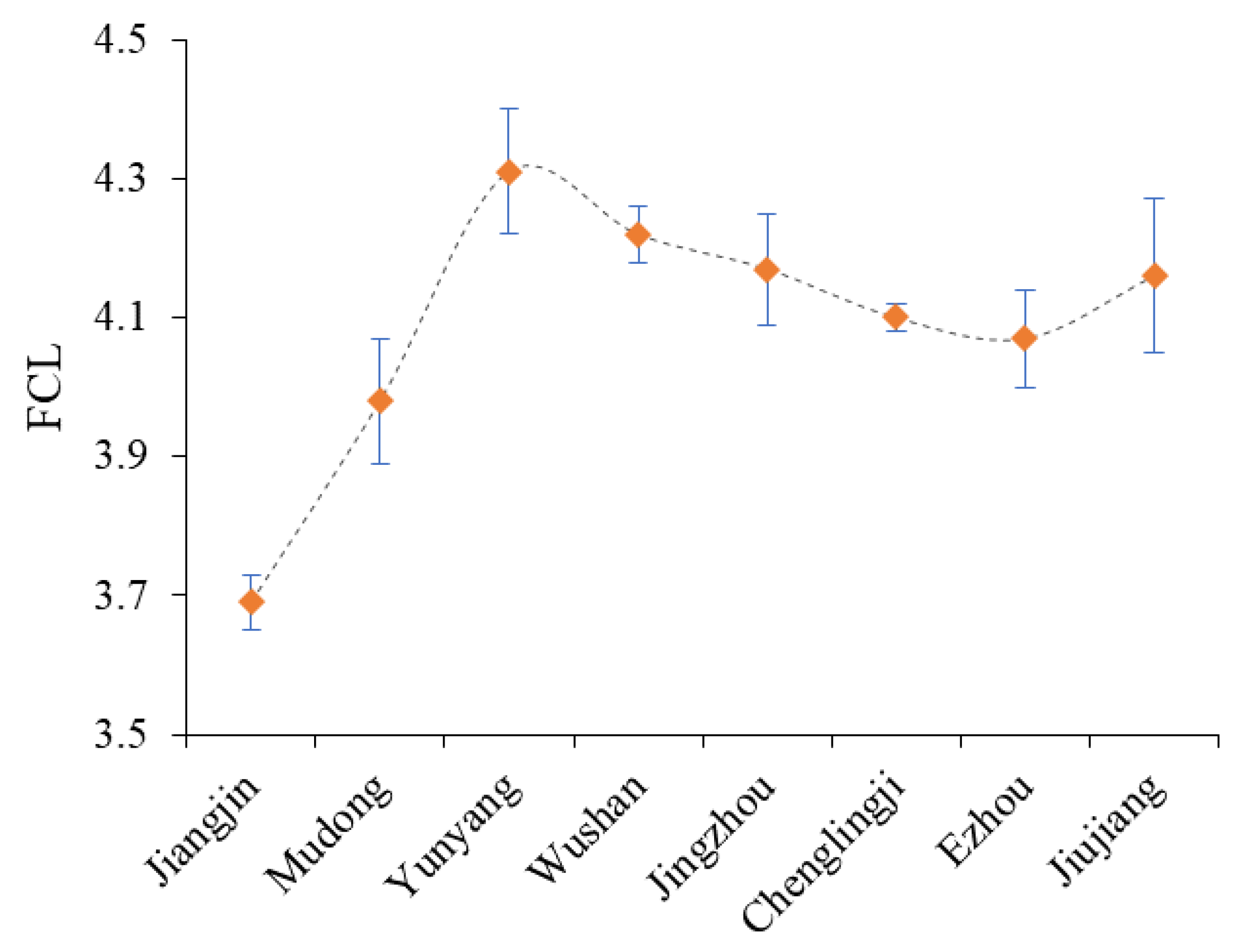
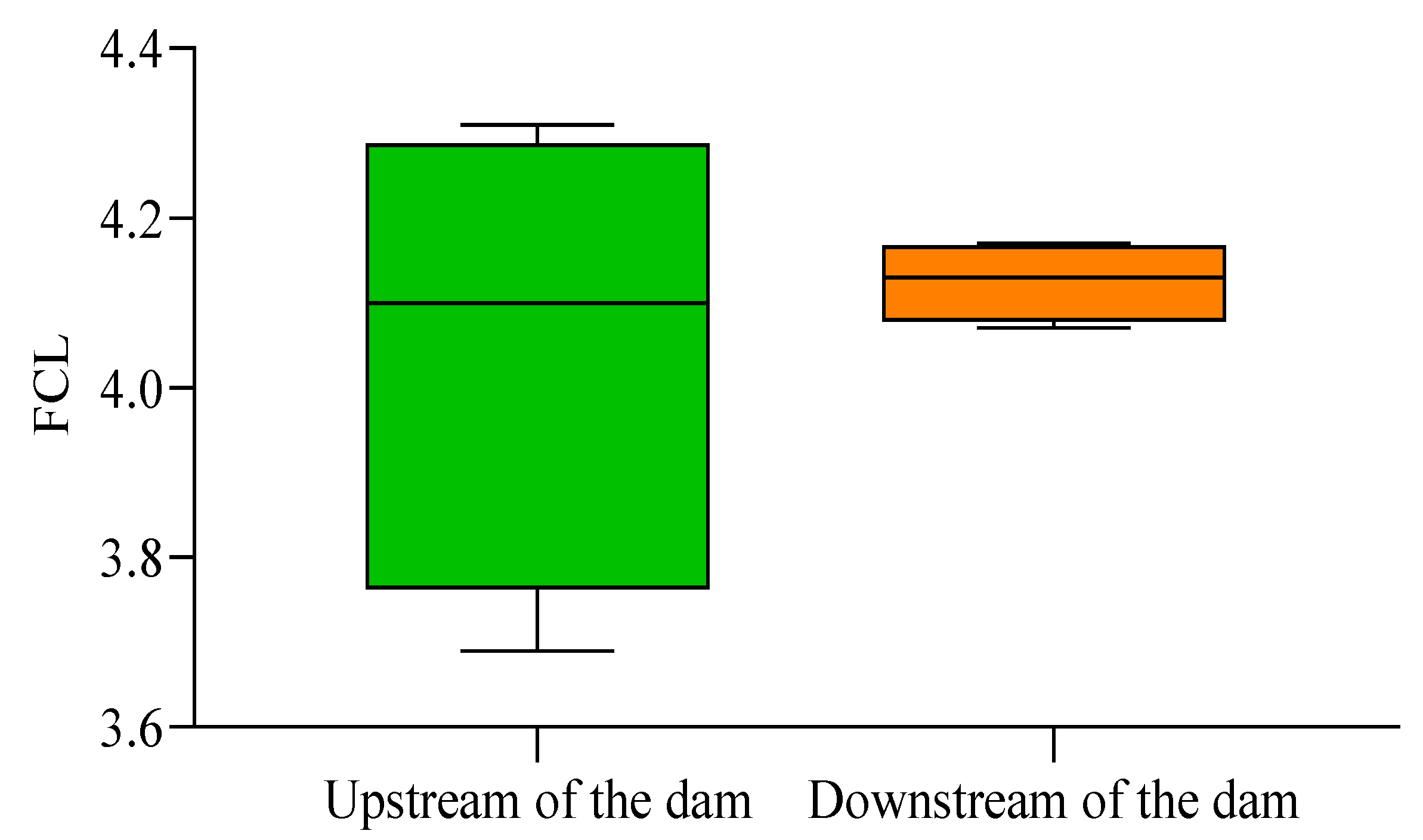
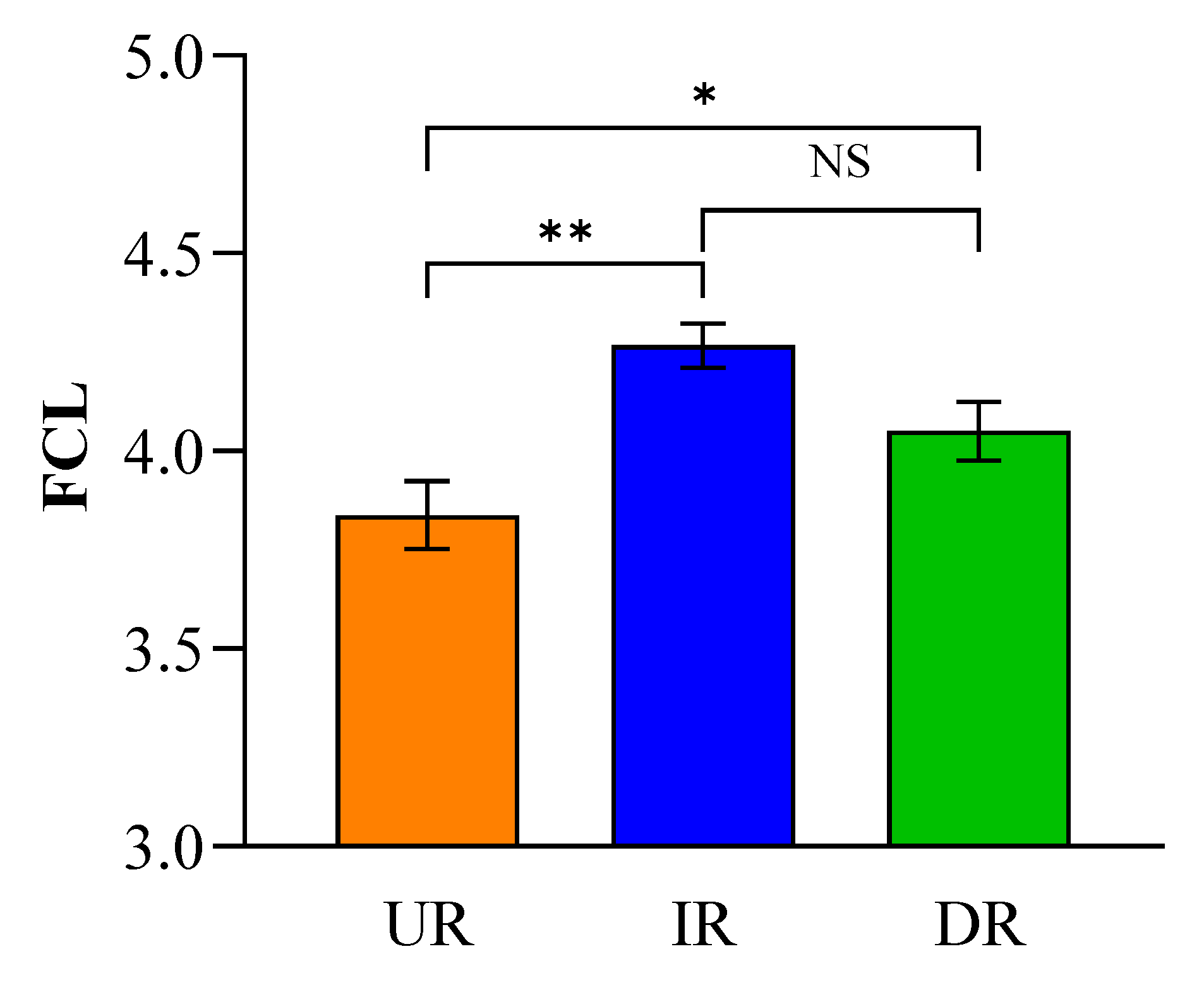
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pimm, S.L. Food Webs; Chapman & Hall: London, UK, 1982. [Google Scholar]
- Schoener, T.W. Food webs from the small to the large. Ecology 1989, 79, 2013–2018. [Google Scholar]
- Wang, Y.Y.; Xu, J.; Lei, G.C. Proximate and ultimate determinants of food chain length. Acta Ecol. Sin. 2013, 33, 5990–5996. [Google Scholar] [CrossRef][Green Version]
- Post, D.M. The long and short of food-chain length. Trends Ecol. Evol. 2002, 17, 269–277. [Google Scholar] [CrossRef]
- Zhang, H.; He, L.; Zheng, P.Y. Food chain length theory: A review. Acta Ecol. Sin. 2012, 33, 7630–7643. [Google Scholar]
- Cabana, G.; Rasmussen, J.B. Modelling food chain structure and contaminant bioaccumulation using stable nitrogen isotopes. Nature 1994, 372, 255–257. [Google Scholar] [CrossRef]
- Sabo, J.L.; Finlay, J.C.; Post, D.M. Food Chains in Freshwaters. Ann. N. Y. Acad. Sci. 2010, 1162, 187–220. [Google Scholar] [CrossRef]
- Saigo, M.; Ruffener, L.; Scarabotti, P.; Marchese, M. Food chain length in a large floodplain river: Planktonic or benthic reliance as a limiting factor. Mar. Freshw. Res. 2016, 65, 249–255. [Google Scholar] [CrossRef]
- Post, D.M. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 2002, 83, 703–718. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, M.; Xie, P. Variability of stable nitrogen isotopic baselines and its consequence for trophic modeling. J. Lake Sci. 2010, 22, 8–20. [Google Scholar]
- Wang, Y.Y.; Xu, J.; Zhang, L.; Lei, G.C. Seasonal variability in baseline δ15N and usage as a nutrient indicator in Lake Poyang, China. J. Freshw. Ecol. 2013, 28, 365–373. [Google Scholar] [CrossRef]
- Perkins, M.J.; Mcdonald, R.A.; Frank, V.V.F.J.; Kelly, S.D.; Rees, G.; Bearhop, S. Application of Nitrogen and Carbon Stable Isotopes (δ15N and δ13C) to Quantify Food Chain Length and Trophic Structure. PLoS ONE 2014, 9, e93281. [Google Scholar] [CrossRef] [PubMed]
- Post, D.M.; Pace, M.L.; Hairston, N.G. Ecosystem size determines food-chain length in lakes. Nature 2000, 405, 1047–1049. [Google Scholar] [CrossRef]
- Takimoto, G.K.; Post, D.M. Proximate Structural Mechanisms for Variation in Food-Chain Length. Oikos 2007, 116, 775–782. [Google Scholar]
- Hette-Tronquart, N.; Roussel, J.M.; Dumont, B.; Archaimbault, V.; Pont, D.; Oberdorff, T.; Belliard, J. Variability of water temperature may influence food-chain length in temperate streams. Hydrobiologia 2013, 718, 159–172. [Google Scholar] [CrossRef]
- Xiao, X.W.; Wang, Y.Y.; Zhang, H.; Yu, X.B. Effects of primary productivity and ecosystem size on food-chain length in Raohe River, China. Acta Ecol. Sin. 2015, 35, 29–34. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Xiao, X.W.; Yu, X.B.; Xu, J.; Cai, Y.J.; Lei, G.C. Resource availability determines food chain length in Chinese subtropical rivers. Aquat. Ecol. 2016, 50, 187–195. [Google Scholar] [CrossRef]
- Fetzer, W.W.; Vander Zanden, M.J. Global Patterns of Aquatic Food Chain Length. Oikos 2007, 116, 1378–1388. [Google Scholar]
- Kautza, A.; Sullivan, S.M. Anthropogenic and natural determinants of fish food-chain length in a midsize river system. Freshw. Sci. 2016, 35, 895–908. [Google Scholar] [CrossRef]
- Ruhi, A.; Muñoz, I.; Tornés, E.; Batalla, R.; Vericat, D.; Ponsatí, L.; Acuña, V.; Von Schiller, D.; Marcé, R.; Bussi, G.; et al. Flow regulation increases food-chain length through omnivory mechanisms in a Mediterranean river network. Freshw. Biol. 2016, 61, 1536–1549. [Google Scholar] [CrossRef]
- Warfe, D.M.; Jardine, T.D.; Pettit, N.E.; Hamilton, S.K.; Pusey, B.J.; Bunn, S.E.; Davies, M.P.; Douglas, M.M. Productivity, disturbance and ecosystem size have no influence on food chain length in seasonally connected rivers. PLoS ONE 2013, 8, e66240. [Google Scholar] [CrossRef]
- Post, D.M. Testing the productive-space hypothesis: Rational and power. Oecologia 2007, 153, 973–984. [Google Scholar] [CrossRef]
- Mchugh, P.A.; McIntosh, A.R.; Jellyman, P.G. Dual influences of ecosystem size and disturbance on food chain length in streams. Ecol. Lett. 2010, 13, 881–890. [Google Scholar] [CrossRef]
- Takimoto, G.; Post, D.M. Environmental determinants of food-chain length: A meta-analysis. Ecol. Res. 2013, 28, 675–681. [Google Scholar] [CrossRef]
- Walters, A.W.; Post, D.M. An experimental disturbance alters fish size structure but not food chain length in streams. Ecology 2008, 89, 3261–3267. [Google Scholar] [CrossRef]
- Sabo, J.L.; Finlay, J.C.; Kennedy, T.; Post, D.M. The role of discharge variation in scaling of drainage area and food chain length in rivers. Science 2010, 330, 965–967. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.M.; Townsend, C.R. Energy availability, spatial heterogeneity and ecosystem size predict food-web structure in streams. Oikos 2005, 108, 137–148. [Google Scholar] [CrossRef]
- Zanden, M.J.V.; Shuter, B.J.; Lester, N.; Rasmussen, A.J.B. Patterns of food chain length in lakes: A stable isotope study. Am. Nat. 1999, 154, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, B.; Kitching, R.L.; Pimm, S.L. Productivity, disturbance and food web structure at a local spatial scale in experimental container habitats. Oikos 2016, 65, 249–255. [Google Scholar] [CrossRef]
- Sullivan, S.M.P.; Hossler, K.; Cianfrani, C.M. Ecosystem structure emerges as a strong determinant of food-chain length in linked stream-riparian ecosystems. Ecosystems 2015, 18, 1356–1372. [Google Scholar] [CrossRef]
- González-Bergonzoni, I.; Kristensen, P.B.; Baattrup-Pedersen, A.; Kristensen, E.A.; Alnoee, A.B.; Riis, T. Riparian forest modifies fuelling sources for stream food webs but not food-chain length in lowland streams of Denmark. Hydrobiologia 2017, 805, 291–310. [Google Scholar] [CrossRef]
- Zhang, H.; Kang, M.; Shen, L.; Wu, J.M.; Li, J.Y.; Du, H.; Wang, C.Y.; Yang, H.L.; Zhou, Q.; Liu, Z.G.; et al. Rapid change in Yangtze fisheries and its implications for global freshwater ecosystem management. Fish. Fish 2020, 21, 60–623. [Google Scholar] [CrossRef]
- Liu, X.J.; Qin, J.J.; Xu, Y.; Ouyang, S.; Wu, X.P. Biodiversity decline of fish assemblages after the impoundment of the Three Gorges Dam in the Yangtze River Basin, China. Rev.: Methods Technol. Fish Biol. Fish. 2019, 29, 177–195. [Google Scholar] [CrossRef]
- Cheng, F.; Li, W.; Castello, L.; Murphy, B.R.; Xie, S.G. Potential effects of dam cascade on fish: Lessons from the Yangtze River. Rev. Fish Biol. Fish. 2015, 25, 569–585. [Google Scholar] [CrossRef]
- Wu, J.G.; Huang, J.H.; Han, X.G.; Gao, X.M.; He, F.L.; Jiang, M.X.; Jiang, Z.G.; Primack, R.B.; Shen, Z.H. The Three Gorges Dam: An Ecological Perspective. Front. Ecol. Environ. 2004, 2, 241–248. [Google Scholar] [CrossRef]
- Jordi-René, M.; Albert, R.; Elisabet, T.; Héctor, V.; Isabel, M.; Sergi, S. Dam regulation and riverine food-web structure in a Mediterranean river. Sci. Total Environ. 2017, 625, 301–310. [Google Scholar]
- Zhang, S. Distribution of Nutrient and Plankton Mass in Three Gorges Reservoir Water Body. Ph.D. Thesis, Southwest Agricultural University, Chongqi, China, May 2005. [Google Scholar]
- Fearnside, P.M. Dams in the Amazon: Belo Monte and Brazil’s hydroelectric development of the Xingu river basin. Environ. Manag. 2006, 38, 16–27. [Google Scholar] [CrossRef]
- Li, B.; Wang, Z.J.; Yang, J.P.; Yue, X.J.; Qi, Z.M.; Zhang, Y.G. The dynamic and seasonal variation of the fish food webs in the mainstream of Three Gorges Reservoir. J. Fish. China 2013, 37, 1015–1022. [Google Scholar] [CrossRef]
- Ba, J.W.; Deng, H.T.; Duan, X.B.; Liu, S.P.; Li, Y.; Cheng, D.Q. Trophic level analysis on main fish species in the middle reaches of Yangtze River by δ13C and δ15N analysis. Chin. J. Zool. 2015, 50, 537–546. [Google Scholar]
- Ban, X.; Du, H.; Wei, Q.W. Fish preference for hydraulic habitat in typical middle reaches of Yangtze River, China. J. Appl. Ichthyol. 2013, 29, 1408–1415. [Google Scholar] [CrossRef]
- Ministry of Environmental Protection of the People′s Republic of China. Water Quality-Determination of Chlorophyll a-Spectrophotometric Method: HJ 897-2017; Ministry of Environmental Protection of the People’s Republic of China: Beijing, China, 2017.
- Burnhan, K.P.; Anderson, D.R. Model Selection and Multi-Model Inference: A Practical Information-Theoretic Approach; Springer: New York, NY, USA, 2004. [Google Scholar]
- Hoeinghaus, D.J.; Winemiller, K.O.; Agostinho, A.A. Hydrogeomorphology and river impoundment affect food-chain length of diverse Neotropical food webs. Oikos 2008, 117, 984–995. [Google Scholar] [CrossRef]
- Tang, C.J. Evolution, driving mechanism and control strategy for eutrophication in Changjiang River Basin. Yangtze River 2020, 51, 80–87. [Google Scholar]
- Akin, S.; Winemiller, K.O. Seasonal variation in food web composition and structure in a temperate Tidal Estuary. Estuaries Coasts 2006, 29, 552–567. [Google Scholar] [CrossRef]
- Takimoto, G.; Spiller, D.A.; Post, D.M. Ecosystem size, but not disturbance, determines food-chain length on islands of the bahamas. Ecology 2008, 89, 3001–3007. [Google Scholar] [CrossRef]
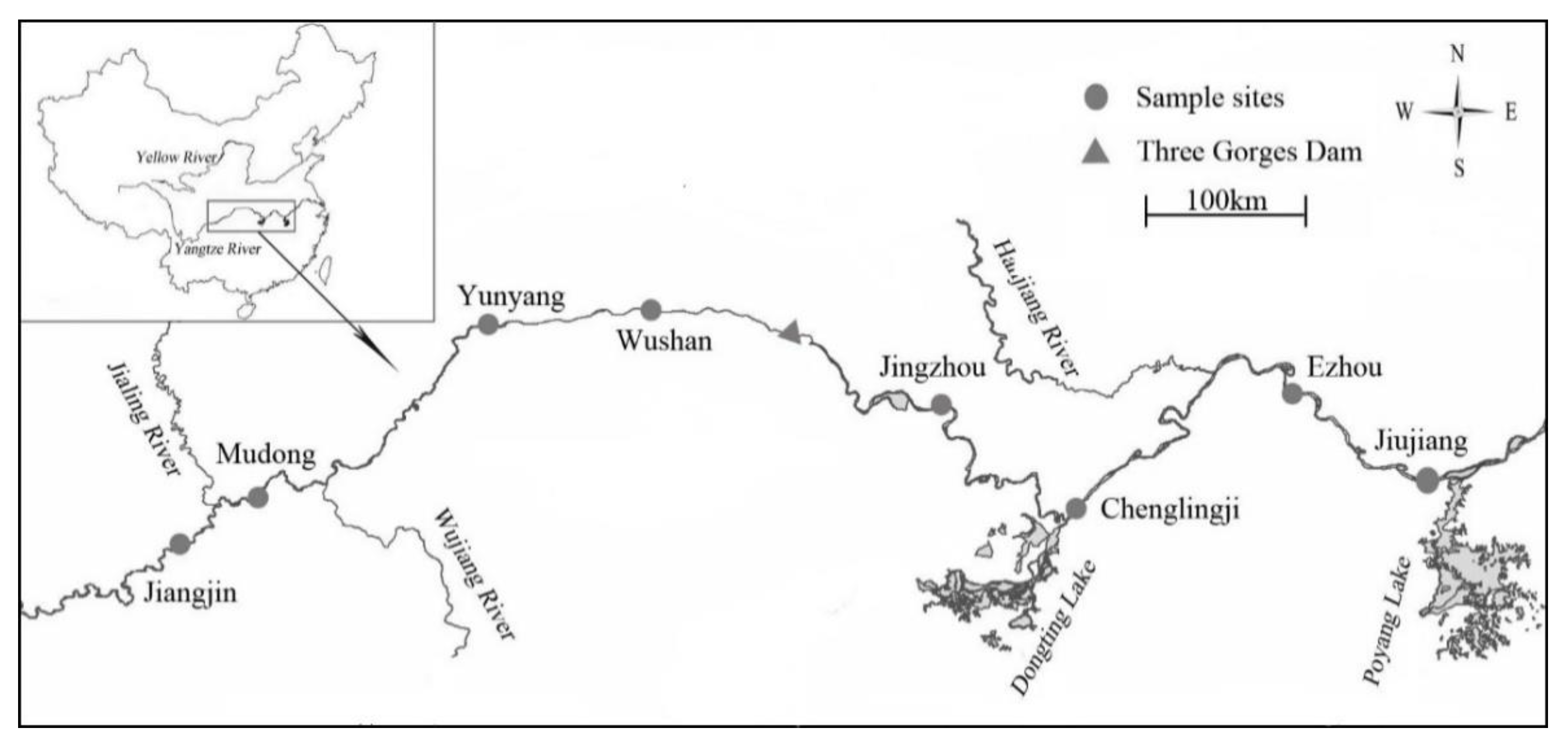


| Sample Reach | Ecosystem Type | Altitude (m) | Distance from the Three Gorges Dam |
|---|---|---|---|
| Jiangjin | River | 209 | 644 km (upstream of the dam) |
| Mudong | River | 181 | 543 km (upstream of the dam) |
| Yunyang | Reservoir | 175 | 248 km (upstream of the dam) |
| Wushan | Reservoir | 174 | 121 km (upstream of the dam) |
| Jingzhou | River | 43 | 185 km (downstream of the dam) |
| Chenglingji | River | 37 | 392 km (downstream of the dam) |
| Ezhou | River | 26 | 710 km (downstream of the dam) |
| Jiujiang | River | 23 | 853 km (downstream of the dam) |
| Component Models | R2 | p | AICc | △AICc | wi | Evidence Ratio |
|---|---|---|---|---|---|---|
| Ecosystem size | 0.63 | 0.01 | −1.71 | 0.00 | 0.72 | 1.0 |
| Resource availability | 0.58 | 0.03 | 0.24 | 1.95 | 0.27 | 2.7 |
| Disturbance | 0.04 | 0.63 | 6.62 | 8.33 | 0.01 | 72.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, C.; Deng, H.; Ba, J.; Li, S.; Chen, Z.; Tao, Y.; Duan, X.; Liu, S.; Li, Y.; Chen, D. Food Chain Length Associated with Environmental Factors Affected by Large Dam along the Yangtze River. Water 2020, 12, 3157. https://doi.org/10.3390/w12113157
He C, Deng H, Ba J, Li S, Chen Z, Tao Y, Duan X, Liu S, Li Y, Chen D. Food Chain Length Associated with Environmental Factors Affected by Large Dam along the Yangtze River. Water. 2020; 12(11):3157. https://doi.org/10.3390/w12113157
Chicago/Turabian StyleHe, Chun, Huatang Deng, Jiawen Ba, Sheng Li, Zheyu Chen, Yixi Tao, Xinbin Duan, Shaoping Liu, Yun Li, and Daqing Chen. 2020. "Food Chain Length Associated with Environmental Factors Affected by Large Dam along the Yangtze River" Water 12, no. 11: 3157. https://doi.org/10.3390/w12113157
APA StyleHe, C., Deng, H., Ba, J., Li, S., Chen, Z., Tao, Y., Duan, X., Liu, S., Li, Y., & Chen, D. (2020). Food Chain Length Associated with Environmental Factors Affected by Large Dam along the Yangtze River. Water, 12(11), 3157. https://doi.org/10.3390/w12113157






