Mass Production of Plasma Activated Water: Case Studies of Its Biocidal Effect on Algae and Cyanobacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hydrodynamic Cavitation Plasma Jet Device (HCPJ)
2.2. Treatment Procedures of Contaminated Water
2.3. Diagnostical Methods of the Plasma Jet
2.4. Model of Hydrodynamic Cavitation at Reduced Backpressure
2.5. Algae and Cyanobacteria Culturing Conditions, Growth Inhibition Test
3. Results and Discussion
3.1. Phenomenological Description of HCPJ Generated at Sub-Atmospheric Pressure
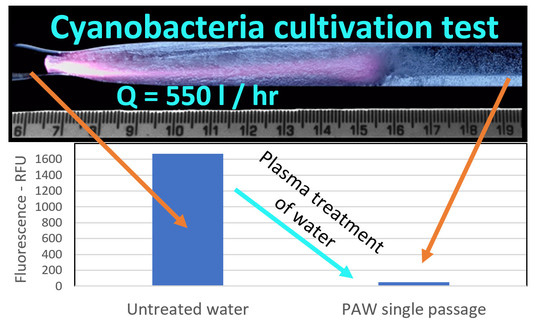
3.2. Treatment of Water Contaminated with Algae and Cyanobacteria
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
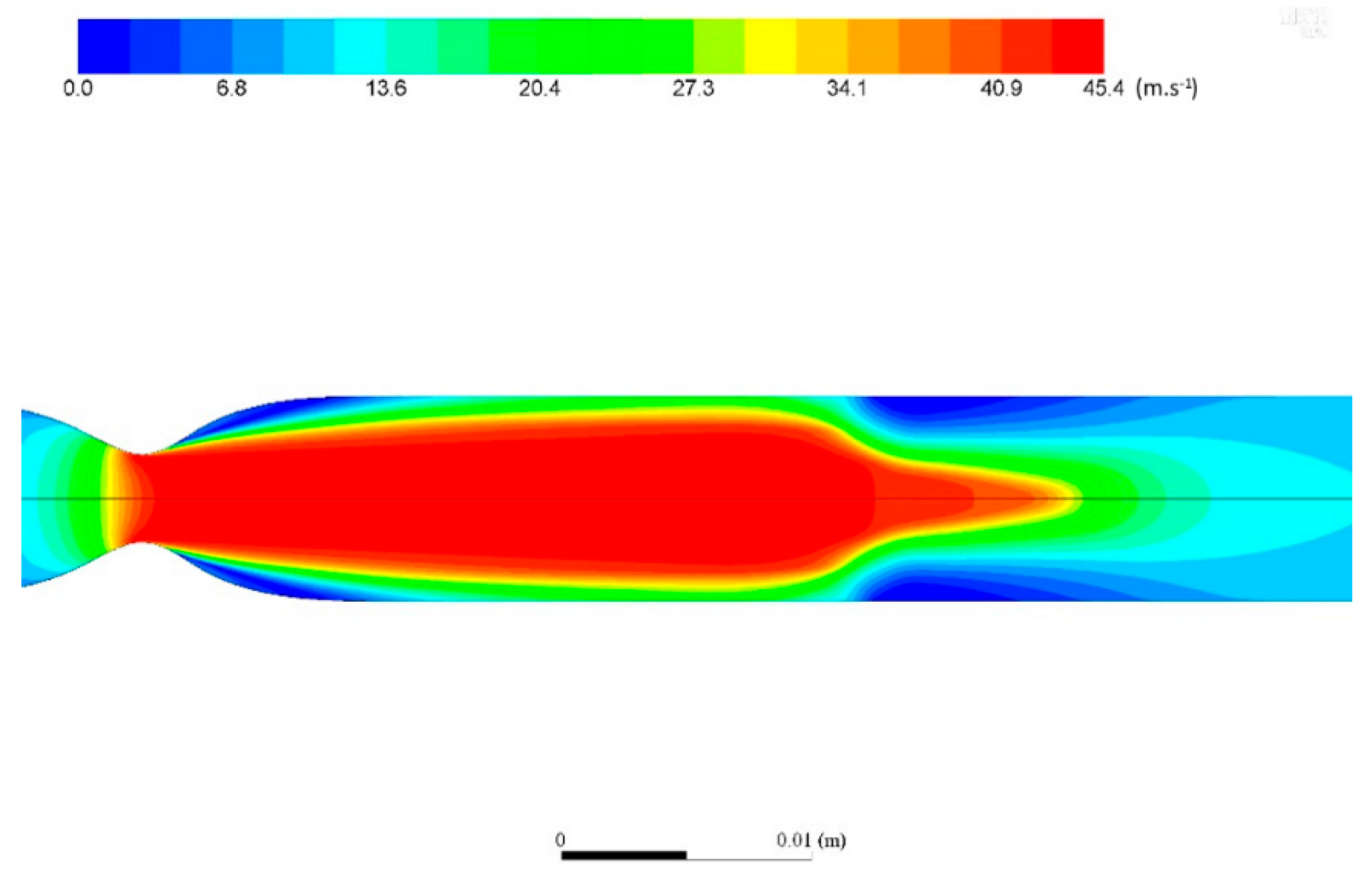
Appendix A. Numerical Model
Appendix A.1. Governing Equations
Appendix A.2. Geometry and Computational Mesh






References
- Yasuda, J.; Yoshizawa, S.; Umemura, S. Efficient generation of cavitation bubbles and reactive oxygen species using triggered high-intensity focused ultrasound sequence for sonodynamic treatment. Jpn. J. Appl. Phys. 2016, 55, 07KF24. [Google Scholar] [CrossRef]
- Bruggeman, P.J.; Kushner, M.J.; Locke, B.R.; Gardeniers, J.G.; Graham, W.G.; Graves, D.B.; Hofman-Caris, R.C.H.M.; Maric, D.; Reid, J.P.; Ceriani, E.; et al. Plasma-liquid interactions: A review and roadmap. Plasma Sources Sci. Technol. 2016, 25, 053002. [Google Scholar] [CrossRef]
- Machala, Z.; Tarabová, B.; Sersenová, D.; Janda, M.; Hensel, K. Chemical and antibacterial effects of plasma activated water: Correlation with gaseous and aqueous reactive oxygen and nitrogen species, plasma sources and air flow conditions. J. Phys. D Appl. Phys. 2019, 52, 034002. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Ghimire, B.; Li, Y.; Adhikari, M.; Veerana, M.; Kaushik, N.; Jha, N.; Adhikari, B.; Lee, S.J.; Masur, K.; et al. Biological and medical applications of plasma-activated media, water and solutions. Biol. Chem. 2018, 400, 39–62. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, R.; Bogaerts, A.; Bongers, W.; Fridman, A.; Fridman, G.; Locke, B.R.; Miller, V.; Reuter, S.; Schiorlin, M.; Verreycken, T.; et al. White paper on the future of plasma science in environment, for gas conversion and agriculture. Plasma Process. Polym. 2019, 16, 1700238. [Google Scholar] [CrossRef] [Green Version]
- Thirumdas, R.; Kothakota, A.; Annapure, U.; Siliveru, K.; Blundell, R.; Gatt, R.; Valdramidis, V.P. Plasma activated water (PAW): Chemistry, physico-chemical properties, applications in food and agriculture. Trends Food Sci. Technol. 2018, 77, 21–31. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, R.; Tian, Y.; Su, B.; Wang, K.; Yu, S.; Zhang, J.; Fang, J. Sterilization Efficiency of a Novel Electrochemical Disinfectant against Staphylococcus aureus. Environ. Sci. Technol. 2016, 50, 3184–3192. [Google Scholar] [CrossRef]
- Lu, P.; Boehm, D.; Cullen, P.; Bourke, P. Controlled cytotoxicity of plasma treated water formulated by open-air hybrid mode discharge. Appl. Phys. Lett. 2017, 110, 264102. [Google Scholar] [CrossRef] [Green Version]
- Ito, M.; Oh, J.-S.; Ohta, T.; Shiratani, M.; Hori, M. Current status and future prospects of agricultural applications using atmospheric-pressure plasma technologies. Plasma Process. Polym. 2018, 15, 1700073. [Google Scholar] [CrossRef]
- Gamaleev, V.; Iwata, N.; Ito, G.; Hori, M.; Hiramatsu, M.; Ito, M. Scalable treatment of flowing organic liquids using ambient-air glow discharge for agricultural applications. Appl. Sci. 2020, 10, 801. [Google Scholar] [CrossRef] [Green Version]
- Rezaei, F.; Nikiforov, A.; Morent, R.; De Geyter, N. Plasma Modification of Poly Lactic Acid Solutions to Generate High Quality Electrospun PLA Nanofibers. Sci. Rep. 2018, 8, 2241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.; Yoo, S.; Kim, K. Synthesis of Carbon-Coated TiO2 by Underwater Discharge with Capillary Carbon Electrode. IEEE Trans. Plasma Sci. 2019, 47, 1482–1486. [Google Scholar] [CrossRef]
- Yayci, A.; Baraibar, Á.G.; Krewing, M.; Fueyo, E.F.; Hollmann, F.; Alcalde, M.; Kourist, R.; Bandow, J.E. Plasma-Driven in Situ Production of Hydrogen Peroxide for Biocatalysis. ChemSusChem 2020, 13, 2072–2079. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, D.; Patel, J.; Švrček, V.; Maguire, P. Plasma-liquid interactions at atmospheric pressure for nanomaterials synthesis and surface engineering. Plasma Process. Polym. 2012, 9, 1074–1085. [Google Scholar] [CrossRef]
- Liguori, A.; Gallingani, T.; Padmanaban, D.B.; Laurita, R.; Velusamy, T.; Jain, G.; Macias-Montero, M.; Mariotti, D.; Gherardi, M. Synthesis of Copper-Based Nanostructures in Liquid Environments by Means of a Non-equilibrium Atmospheric Pressure Nanopulsed Plasma Jet. Plasma Chem. Plasma Process. 2018, 38, 1209–1222. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Kaushik, N.; Linh, N.; Ghimire, B.; Pengkit, A.; Sornsakdanuphap, J.; Lee, S.-J.; Choi, E. Plasma and Nanomaterials: Fabrication and Biomedical Applications. Nanomaterials 2019, 9, 98. [Google Scholar] [CrossRef] [Green Version]
- Lukeš, P.; Locke, B.R. Degradation of substituted phenols in a hybrid gas-liquid electrical discharge reactor. Ind. Eng. Chem. Res. 2005, 44, 2921–2930. [Google Scholar] [CrossRef]
- Kozáková, Z.; Nejezchleb, M.; Krčma, F.; Halamová, I.; Čáslavský, J.; Dolinová, J. Removal of organic dye Direct Red 79 from water solutions by DC diaphragm discharge: Analysis of decomposition products. Desalination 2010, 258, 93–99. [Google Scholar] [CrossRef]
- Magureanu, M.; Dobrin, D.; Mandache, N.B.; Bradu, C.; Medvedovici, A.; Parvulescu, V.I. The mechanism of plasma destruction of enalapril and related metabolites in water. Plasma Process. Polym. 2013, 10, 459–468. [Google Scholar] [CrossRef]
- Jiang, B.; Zheng, J.; Qiu, S.; Wu, M.; Zhang, Q.; Yan, Z.; Xue, Q. Review on electrical discharge plasma technology for wastewater remediation. Chem. Eng. J. 2014, 236, 348–368. [Google Scholar] [CrossRef]
- Tampieri, F.; Giardina, A.; Bosi, F.J.; Pavanello, A.; Marotta, E.; Zaniol, B.; Neretti, G.; Paradisi, C. Removal of persistent organic pollutants from water using a newly developed atmospheric plasma reactor. Plasma Process. Polym. 2018, 15, e170027. [Google Scholar] [CrossRef]
- Meirovich, A.; Parkansky, N.; Boxman, R.L.; Berkh, O.; Barkay, Z.; Rosenberg, Y. Treatment of Methylene Blue water solution by submerged pulse arc in multi-electrode reactor. J. Water Process Eng. 2016, 13, 53–60. [Google Scholar] [CrossRef]
- Šimor, M.; Krump, H.; Hudec, I.; Ráheľ, J.; Brablec, A.; Černák, M. Atmospheric pressure H2O plasma treatment of polyester cord threads. Acta Phys. Slovaca 2004, 54, 43–48. [Google Scholar]
- Nikiforov, A.Y.; Leys, C. Surface treatment of cotton yarn by underwater capillary electrical discharge. Plasma Chem. Plasma Process. 2006, 26, 415–423. [Google Scholar] [CrossRef]
- Galmiz, O.; Zemánek, M.; Pavliňák, D.; Černák, M. Plasma treatment of polyethylene tubes in continuous regime using surface dielectric barrier discharge with water electrodes. J. Phys. D Appl. Phys. 2018, 51, 195201. [Google Scholar] [CrossRef] [Green Version]
- Locke, B.R.; Sato, M.; Sunka, P.; Hoffmann, M.R.; Chang, J.-S. Electrohydraulic discharge and nonthermal plasma for water treatment. Ind. Eng. Chem. Res. 2006, 45, 882–905. [Google Scholar] [CrossRef]
- Shan, M.; Chen, B.; Yao, C.; Han, Q.; Zhu, C.; Yang, Y. Electric characteristic and cavitation bubble dynamics using underwater pulsed discharge. Plasma Sci. Technol. 2019, 21, 074002. [Google Scholar] [CrossRef]
- Maršálek, B.; Maršálková, E.; Odehnalová, K.; Pochylý, F.; Rudolf, P.; Stahel, P.; Rahel, J.; Čech, J.; Fialová, S.; Zezulka, Š. Removal of Microcystis aeruginosa through the combined effect of plasma discharge and hydrodynamic cavitation. Water 2020, 12, 8. [Google Scholar] [CrossRef] [Green Version]
- Ihara, S.; Hirohata, T.; Kominato, Y.; Yamabe, C.; Ike, H.; Hakiai, K.; Hirabayashi, K.; Tamagawa, M. Water treatment using discharge generated in cavitation field with micro bubble cloud. Electr. Eng. Jpn. (Engl. Transl. Denki Gakkai Ronbunshi) 2014, 186, 1–10. [Google Scholar] [CrossRef]
- Abramov, V.O.; Abramova, A.V.; Cravotto, G.; Nikonov, R.V.; Fedulov, I.S.; Ivanov, V.K. Flow-mode water treatment under simultaneous hydrodynamic cavitation and plasma. Ultrason. Sonochem. 2020, 70, 105323. [Google Scholar] [CrossRef]
- Rudolf, P.; Pochylý, F.; Sťahel, P.; Ráheľ, J.; Čech, J.; Maršálek, B. Apparatus for purifying liquids and a method for purifying liquids using this apparatus. Czech Patent No. 308532, 13 December 2019. [Google Scholar]
- Ihara, S.; Sakai, T.; Yoshida, Y.; Nishiyama, H. Fundamental characteristics of discharge plasma generated in a water cavitation field. J. Electrost. 2018, 93, 110–117. [Google Scholar] [CrossRef]
- Oka, Y.; Ohnishi, K.; Asami, K.; Suyama, M.; Nishimura, Y.; Hashimoto, T.; Yonezawa, K.; Nakamura, T.; Yatsuzuka, M. Dispersion of carbon nanotubes into water without dispersant using cavitation bubble plasma. Vacuum 2017, 136, 209–213. [Google Scholar] [CrossRef]
- Navratil, Z.; Trunec, D.; Smid, R.; Lazar, L. A software for optical emission spectroscopy-problem formulation and application to plasma diagnostics. Czechoslov. J. Phys. 2006, 56, B944. [Google Scholar] [CrossRef]
- Voráč, J.; Synek, P.; Procházka, V.; Hoder, T. State-by-state emission spectra fitting for non-equilibrium plasmas: OH spectra of surface barrier discharge at argon/water interface. J. Phys. D Appl. Phys. 2017, 50, 294002. [Google Scholar] [CrossRef]
- Voráč, J.; Synek, P.; Potočńáková, L.; Hnilica, J.; Kudrle, V. Batch processing of overlapping molecular spectra as a tool for spatio-temporal diagnostics of power modulated microwave plasma jet. Plasma Sources Sci. Technol. 2017, 26, 025010. [Google Scholar] [CrossRef]
- ANSYS Fluent Users Guide. Available online: http://www.ansys.com (accessed on 3 August 2020).
- Sauer, J.; Schnerr, G.H. Development of a new cavitation model based on bubble dynamics. J. Appl. Math. Mech. 2001, 81, 561–562. [Google Scholar] [CrossRef]
- Briels, T.M.P.; Kos, J.; Winands, G.J.J.; van Veldhuizen, E.M.; Ebert, U. Positive and negative streamers in ambient air: Measuring diameter, velocity and dissipated energy. J. Phys. D Appl. Phys. 2008, 41, 234004. [Google Scholar] [CrossRef] [Green Version]
- Shamsborhan, H.; Coutier-Delgosha, O.; Caignaert, G.; Nour, F.A. Experimental determination of the speed of sound in cavitating flows. Exp. Fluids 2010, 49, 1359–1373. [Google Scholar] [CrossRef]
- Bruggeman, P.J.; Sadeghi, N.; Schram, D.C.; Linss, V. Gas temperature determination from rotational lines in non-equilibrium plasmas: A review. Plasma Sources Sci. Technol. 2014, 23, 023001. [Google Scholar] [CrossRef] [Green Version]
- Comninellis, C.; Kapalka, A.; Malato, S.; Parsons, S.A.; Poulios, I.; Mantzavinos, D. Advanced oxidation processes for water treatment: Advances and trends for R&D. J. Chem. Technol. Biotechnol. 2008, 83, 769–776. [Google Scholar] [CrossRef]









| Application | Reactor/Electrode Configuration | Volume Efficiency | Refs. |
|---|---|---|---|
| PAW generation | plasma jet to the water surface | 10 mL/min | [10] |
| spark to the water surface | 0.7 mL/min | [8] | |
| plasma electrospray | 1.7 mL/min | [3] | |
| dielectric barrier discharge (DBD) on or above the water surface; immersed Ar + O2 or air plasma jets | 0.5–25 mL/min | [6] and references therein | |
| Pollution removal (phenols, dyes, etc.) | hybrid (corona pulse + water surface DBD) | 7 mL/min | [17] |
| direct current (DC) diaphragm discharge | 38 mL/min | [18] | |
| pulsed DBD | 5 mL/min | [19] | |
| wetted wall DBD; hybrid; DC corona in water; DC diaphragm | 0.4–300 mL/min | [20] and references therein | |
| pulsed submerged arc | 600 mL/min | [22] | |
| Cyanobacteria removal | HPCJ | 24,000 mL/min | [28] 1 |
| Organic dye removal | alternating current arc in a cavitation field | 400 mL/min | [29] |
| Organic dye and E. coli removal | discharge in cavitation field | 16,700 mL/min | [30] |
| Water Flow Rate | PAW Production | Ozonisation Treatment | Backpressure (kPa) |
|---|---|---|---|
| 9 230 mL/min (0.55 m3/h) | Single pass of media through the reactor, i.e., 13 s of total treatment time of 2 L PAW | - | 40 |
| Triple pass of media through the reactor, i.e., 39 s of total treatment time of 2 L PAW | - | 40 | |
| Quintuple pass of media through the reactor, i.e., 65 s of total treatment time of 2 L PAW | - | 40 | |
| - | 3 min in total | 100 | |
| - | 6 min in total | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čech, J.; Sťahel, P.; Ráheľ, J.; Prokeš, L.; Rudolf, P.; Maršálková, E.; Maršálek, B. Mass Production of Plasma Activated Water: Case Studies of Its Biocidal Effect on Algae and Cyanobacteria. Water 2020, 12, 3167. https://doi.org/10.3390/w12113167
Čech J, Sťahel P, Ráheľ J, Prokeš L, Rudolf P, Maršálková E, Maršálek B. Mass Production of Plasma Activated Water: Case Studies of Its Biocidal Effect on Algae and Cyanobacteria. Water. 2020; 12(11):3167. https://doi.org/10.3390/w12113167
Chicago/Turabian StyleČech, Jan, Pavel Sťahel, Jozef Ráheľ, Lubomír Prokeš, Pavel Rudolf, Eliška Maršálková, and Blahoslav Maršálek. 2020. "Mass Production of Plasma Activated Water: Case Studies of Its Biocidal Effect on Algae and Cyanobacteria" Water 12, no. 11: 3167. https://doi.org/10.3390/w12113167
APA StyleČech, J., Sťahel, P., Ráheľ, J., Prokeš, L., Rudolf, P., Maršálková, E., & Maršálek, B. (2020). Mass Production of Plasma Activated Water: Case Studies of Its Biocidal Effect on Algae and Cyanobacteria. Water, 12(11), 3167. https://doi.org/10.3390/w12113167