Net Primary Production Predicted by the Proportion of C:N:P Stoichiometric Ratio in the Leaf-Stem and Root of Cynodon Dactylon (Linn.) in the Riparian Zone of the Three Gorges Reservoir
Abstract
:1. Introduction
2. Materials and Methods
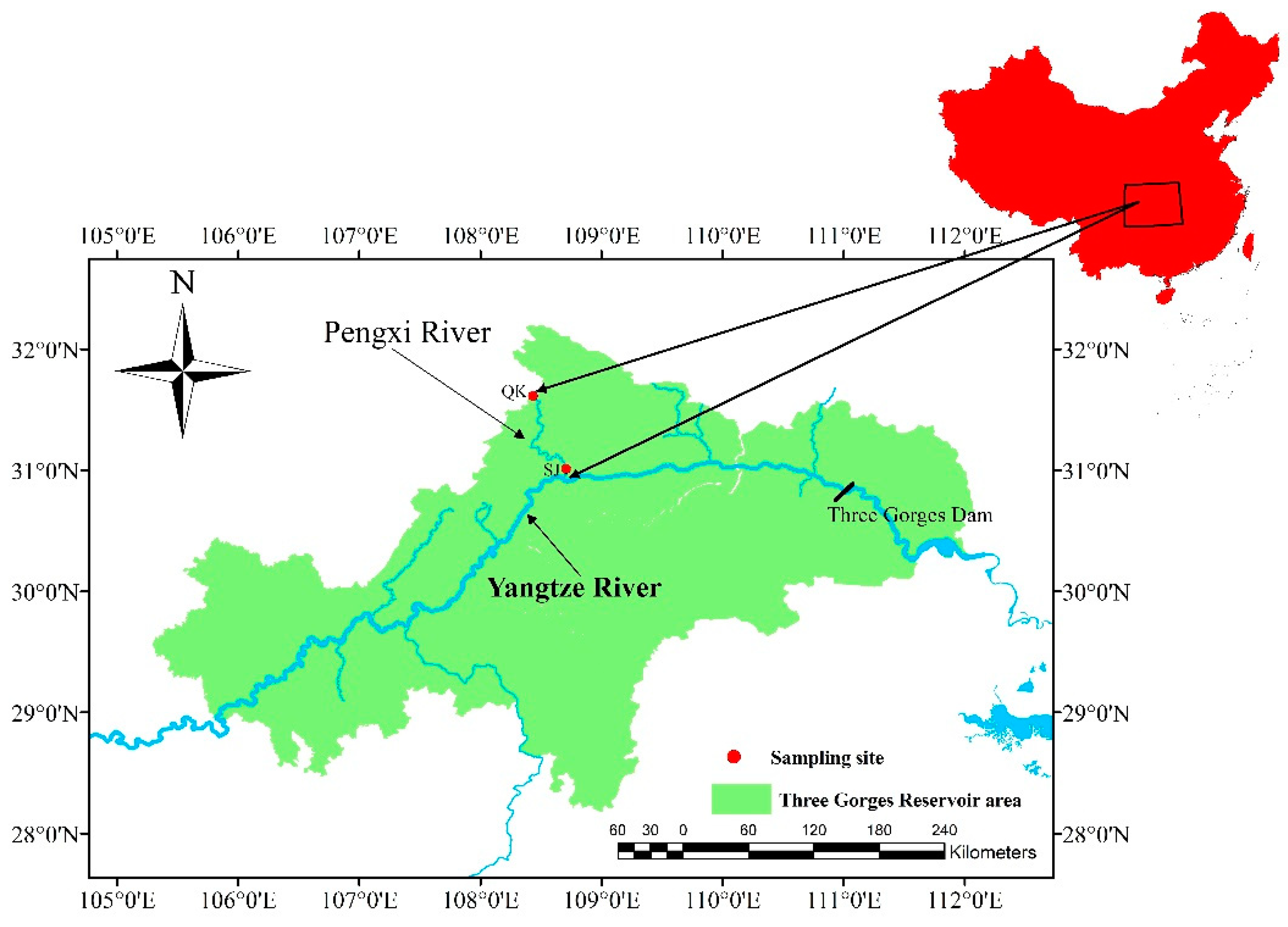
2.1. Study Areas
2.2. Sampling
2.3. Laboratory Analyses
2.4. Data Processing
2.5. Statistical Analysis
3. Results
3.1. Flooding Time and Net Primary Production (NPP)
3.2. C, N, and P in Leaf-Stem and Root
3.3. Leaf-Stem and Root C:N:P Stoichiometry with NPP
3.4. Exploring the Indicators of NPP
4. Discussion
4.1. Flooding Stress and NPP
4.2. Nutrient Allocation and NPP
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Lin, J.; Fu, C.; Zhang, X.; Xie, K.; Yu, Z. Heavy Metal Contamination in the Water-Level Fluctuating Zone of the Yangtze River within Wanzhou Section, China. Biol. Trace Elem. Res. 2012, 145, 268–272. [Google Scholar] [CrossRef]
- Wu, J.; Huang, J.; Han, X.; Gao, X.; He, F.; Jiang, M.; Jiang, Z.; Primack, R.B.; Shen, Z. The three gorges dam: An ecological perspective. Front. Ecol. Environ. 2004, 2, 241–248. [Google Scholar] [CrossRef]
- Yang, F.; Liu, W.-W.; Wang, J.; Liao, L.; Wang, Y. Riparian vegetation’s responses to the new hydrological regimes from the Three Gorges Project: Clues to revegetation in reservoir water-level-fluctuation zone. Acta Ecol. Sin. 2012, 32, 89–98. [Google Scholar] [CrossRef]
- Lu, Z.-J.; Li, L.-F.; Jiang, M.-X.; Huang, H.-D.; Bao, D.-C. Can the soil seed bank contribute to revegetation of the drawdown zone in the Three Gorges Reservoir Region? Plant Ecol. 2010, 209, 153–165. [Google Scholar] [CrossRef]
- Ye, C.; Butler, O.M.; Chen, C.; Liu, W.; Du, M.; Zhang, Q. Shifts in characteristics of the plant-soil system associated with flooding and revegetation in the riparian zone of Three Gorges Reservoir, China. Geoderma 2020, 361, 114015. [Google Scholar] [CrossRef]
- Li, W.; Cao, T.; Ni, L.; Zhang, X.; Zhu, G.; Xie, P. Effects of water depth on carbon, nitrogen and phosphorus stoichiometry of five submersed macrophytes in an in situ experiment. Ecol. Eng. 2013, 61, 358–365. [Google Scholar] [CrossRef]
- Güsewell, S. N: P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef]
- Hessen, D.O.; Ågren, G.I.; Anderson, T.R.; Elser, J.J.; De Ruiter, P.C. Carbon sequestration in ecosystems: The role of stoichiometry. Ecology 2004, 85, 1179–1192. [Google Scholar] [CrossRef]
- Huang, D.; Wang, D.; Ren, Y. Using leaf nutrient stoichiometry as an indicator of flood tolerance and eutrophication in the riparian zone of the Lijang River. Ecol. Indic. 2019, 98, 821–829. [Google Scholar] [CrossRef]
- Wang, W.; Sardans, J.; Wang, C.; Zeng, C.; Tong, C.; Bartrons, M.; Asensio, D.; Peñuelas, J. Shifts in plant and soil C, N and P accumulation and C:N:P stoichiometry associated with flooding intensity in subtropical estuarine wetlands in China. Estuar. Coast. Shelf Sci. 2018, 215, 172–184. [Google Scholar] [CrossRef]
- Larmola, T.; Alm, J.; Juutinen, S.; Saarnio, S.; Martikainen, P.J.N.; Silvola, J. Floods can cause large interannual differences in littoral net ecosystem productivity. Limnol. Oceanogr. 2004, 49, 1896–1906. [Google Scholar] [CrossRef]
- Liao, J.; Jiang, M.; Li, L. Effects of simulated submergence on survival and recovery growth of three species in water fluctuation zone of the Three Gorges reservoir. Acta Ecol. Sin. 2010, 30, 216–220. [Google Scholar] [CrossRef]
- Peng, C.; Zhang, L.; Qin, H.; Li, D. Revegetation in the water level fluctuation zone of a reservoir: An ideal measure to reduce the input of nutrients and sediment. Ecol. Eng. 2014, 71, 574–577. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zhang, S.; Liu, D.; Yu, Z.; Zhou, S.; Li, R.; Liu, Z.; Lin, J. Nutrient inputs from the leaf decay of Cynodon dactylon (L.) Pers in the water level fluctuation zone of a Three Gorges tributary. Sci. Total Environ. 2019, 688, 718–723. [Google Scholar] [CrossRef]
- Singh, K.; Pandey, V.C.; Singh, R.P. Cynodon dactylon: An efficient perennial grass to revegetate sodic lands. Ecol. Eng. 2013, 54, 32–38. [Google Scholar] [CrossRef]
- Zhang, Z.; Wan, C.; Zheng, Z.; Hu, L.; Feng, K.; Chang, J.; Xie, P. Plant community characteristics and their responses to environmental factors in the water level fluctuation zone of the three gorges reservoir in China. Environ. Sci. Pollut. Res. 2013, 20, 7080–7091. [Google Scholar] [CrossRef]
- Li, H.; Liu, L.; Lou, Y.; Hu, T.; Fu, J. Genetic diversity of Chinese natural bermudagrass (Cynodon dactylon) germplasm using ISSR markers. Sci. Hortic. 2011, 127, 555–561. [Google Scholar] [CrossRef]
- Lin, J.; Tang, Y.; Liu, D.; Zhang, S.; Lan, B.; He, L.; Yu, Z.; Zhou, S.; Chen, X.; Qu, Y. Characteristics of organic nitrogen fractions in sediments of the water level fluctuation zone in the tributary of the Yangtze River. Sci. Total Environ. 2019, 653, 327–333. [Google Scholar] [CrossRef]
- Shi, Y.; Xu, G.; Wang, Y.; Engel, B.A.; Peng, H.; Zhang, W.; Cheng, M.; Dai, M. Modelling hydrology and water quality processes in the Pengxi River basin of the Three Gorges Reservoir using the soil and water assessment tool. Agric. Water Manag. 2017, 182, 24–38. [Google Scholar] [CrossRef]
- Huang, Y.; Yasarer, L.M.W.; Li, Z.; Sturm, B.S.M.; Zhang, Z.; Guo, J.; Shen, Y. Air–water CO2 and CH4 fluxes along a river–reservoir continuum: Case study in the Pengxi River, a tributary of the Yangtze River in the Three Gorges Reservoir, China. Environ. Monit. Assess. 2017, 189, 223. [Google Scholar] [CrossRef]
- Schermelleh-Engel, K.; Moosbrugger, H.; Müller, H. Evaluating the fit of structural equation models: Tests of significance and descriptive goodness-of-fit measures. Methods Psychol. Res. Online 2003, 8, 23–74. [Google Scholar]
- Lin, J.; Zhou, S.; Liu, D.; Zhang, S.; Yu, Z.; Yang, X. Relative contribution of environmental and nutritional variables to net primary production of Cynodon dactylon (Linn.) Pers in the riparian zone of a Three Gorges tributary. Ecol. Evol. 2020, 10, 7073–7081. [Google Scholar] [CrossRef]
- Zhang, J.; He, N.; Liu, C.; Xu, L.; Chen, Z.; Li, Y.; Wang, R.; Yu, G.; Sun, W.; Xiao, C.; et al. Variation and evolution of C:N ratio among different organs enable plants to adapt to N-limited environments. Glob. Chang. Biol. 2020, 26, 2534–2543. [Google Scholar] [CrossRef]
- Colman, B.P.; Schimel, J.P. Drivers of microbial respiration and net N mineralization at the continental scale. Soil Biol. Biochem. 2013, 60, 65–76. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, J.; Zhang, M.; Wang, J. Effect of Cynodon dactylon community on the conservation and reinforcement of riparian shallow soil in the Three Gorges Reservoir area. Ecol. Process. 2015, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Stagg, C.L.; Cai, Y.; Lü, X.; Wang, X.; Shen, R.; Lan, Z. Scaling responses of leaf nutrient stoichiometry to the lakeshore flooding duration gradient across different organizational levels. Sci. Total Environ. 2020, 740, 139740. [Google Scholar] [CrossRef]
- Lambert, A.M.; Dudley, T.L.; Robbins, J. Nutrient enrichment and soil conditions drive productivity in the large-statured invasive grass Arundo donax. Aquat. Bot. 2014, 112, 16–22. [Google Scholar] [CrossRef]
- Zhao, N.; Yu, G.; He, N.; Xia, F.; Wang, Q.; Wang, R.; Xu, Z.; Jia, Y. Invariant allometric scaling of nitrogen and phosphorus in leaves, stems, and fine roots of woody plants along an altitudinal gradient. J. Plant Res. 2016, 129, 647–657. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Su, Y.; Yang, R. Biomass and nutrient allocation strategies in a desert ecosystem in the Hexi Corridor, northwest China. J. Plant Res. 2017, 130, 699–708. [Google Scholar] [CrossRef]
- Koerselman, W.; Meuleman, A.F. The vegetation N: P ratio: A new tool to detect the nature of nutrient limitation. J. Appl. Ecol. 1996, 33, 1441–1450. [Google Scholar] [CrossRef]
- Gong, H.; Li, Y.; Yu, T.; Zhang, S.; Gao, J.; Zhang, S.; Sun, D. Soil and climate effects on leaf nitrogen and phosphorus stoichiometry along elevational gradients. Glob. Ecol. Conserv. 2020, 23, e01138. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, X.; Yuan, Z.; Lock, T.R.; Kallenbach, R.L. Plant nutritional adaptations under differing resource supply for a dryland grass Leymus chinensis. J. Arid Environ. 2020, 172, 104037. [Google Scholar] [CrossRef]
- Dai, L.; Ke, X.; Du, Y.; Zhang, F.; Li, Y.; Li, Q.; Lin, L.; Peng, C.; Shu, K.; Cao, G.; et al. Nitrogen controls the net primary production of an alpine Kobresia meadow in the northern Qinghai-Tibet Plateau. Ecol. Evol. 2019, 9, 8865–8875. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Wu, H.; He, N.; Lü, X.; Wang, Z.; Elser, J.J.; Wu, J.; Han, X. Testing the Growth Rate Hypothesis in Vascular Plants with Above- and Below-Ground Biomass. PLoS ONE 2012, 7, e32162. [Google Scholar] [CrossRef] [Green Version]
- Rana, K.; Kumar, M.; Kumar, A. Assessment of Annual Shoot Biomass and Carbon Storage Potential of Grewia optiva: An Approach to Combat Climate Change in Garhwal Himalaya. Water Air Soil Pollut. 2020, 231, 450. [Google Scholar] [CrossRef]





| Riparian Zone Altitude (masl) | Tissue | Replicate | C (%) | N (%) | P (%) | C:N | C:P | N:P |
|---|---|---|---|---|---|---|---|---|
| 145–155 | leaf-stem | 6 | 39.46 | 1.77 | 0.28 | 22.37 | 143.93 | 6.41 |
| root | 6 | 39.71 | 0.97 | 0.18 | 40.67 | 220.55 | 5.41 | |
| 155–165 | leaf-stem | 6 | 41.20 | 1.38 | 0.23 | 30.06 | 187.69 | 6.30 |
| root | 6 | 43.45 | 0.82 | 0.16 | 53.18 | 278.87 | 5.35 | |
| 165–175 | leaf-stem | 6 | 41.35 | 2.16 | 0.27 | 35.16 | 220.61 | 7.49 |
| root | 6 | 40.43 | 0.86 | 0.17 | 52.62 | 311.79 | 5.33 | |
| Results from linear mixed models | ANOVA P-values | |||||||
| Main effect | Altitude | 0.076 | 0.424 | 0.259 | 0.064 | 0.008 | 0.819 | |
| Tissue | 0.579 | 0.002 | 0.000 | 0.000 | 0.000 | 0.100 | ||
| Interaction effect | Altitude × Tissue | 0.392 | 0.495 | 0.720 | 0.851 | 0.945 | 0.792 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; He, L.; Yu, Z.; Liu, Z.; Lin, J. Net Primary Production Predicted by the Proportion of C:N:P Stoichiometric Ratio in the Leaf-Stem and Root of Cynodon Dactylon (Linn.) in the Riparian Zone of the Three Gorges Reservoir. Water 2020, 12, 3279. https://doi.org/10.3390/w12113279
Liu D, He L, Yu Z, Liu Z, Lin J. Net Primary Production Predicted by the Proportion of C:N:P Stoichiometric Ratio in the Leaf-Stem and Root of Cynodon Dactylon (Linn.) in the Riparian Zone of the Three Gorges Reservoir. Water. 2020; 12(11):3279. https://doi.org/10.3390/w12113279
Chicago/Turabian StyleLiu, Dan, Liping He, Zhiguo Yu, Zhengxue Liu, and Junjie Lin. 2020. "Net Primary Production Predicted by the Proportion of C:N:P Stoichiometric Ratio in the Leaf-Stem and Root of Cynodon Dactylon (Linn.) in the Riparian Zone of the Three Gorges Reservoir" Water 12, no. 11: 3279. https://doi.org/10.3390/w12113279
APA StyleLiu, D., He, L., Yu, Z., Liu, Z., & Lin, J. (2020). Net Primary Production Predicted by the Proportion of C:N:P Stoichiometric Ratio in the Leaf-Stem and Root of Cynodon Dactylon (Linn.) in the Riparian Zone of the Three Gorges Reservoir. Water, 12(11), 3279. https://doi.org/10.3390/w12113279







