Abstract
Notwithstanding the widespread use of natural and pre-exchanged zeolites for zootechnical effluent treatment, little attention has been dedicated to the variation in the chemical composition of the treated slurries, besides the effects on their NH4+ content. This paper aimed at elucidating the compositional variations in terms of major and trace elements of a raw pig-slurry (PS) after three sequential treatment cycles (TC) with three different grain sizes of natural and Na-exchanged zeolite-rich volcanic tuffs (natural ZTs and NaZTs). A series of laboratory batch experiments revealed that all ZTs had profoundly influenced the final PS chemical composition. As expected, the NaZTs were more efficient in terms of NH4+ removal than the natural ZTs, reaching almost 60% reduction of the initial content after three TCs. A parallel effect of this efficient removal was the remarkable increase in Na+. The Na-forms of ZTs led also to stronger competition with K+ ions, resulting in adsorption of this macronutrient and hence in a reduction of the fertilization value of the PS. In terms of heavy metals and other trace elements, all the treatments with ZTs had significantly increased the Li, Ba, Rb, Sr, Ga, and U content in the PS.
1. Introduction
The impact of livestock activities on the environmental compartment is huge, and it is rapidly increasing [1]. This sector is worldwide currently undergoing a quick and complex shift towards intensification and industrialization; by consequence, also the pig industry has been significantly restructured in recent years, resulting in the trend “fewer farms are producing more pigs” with an augmented volume of pig-slurry (PS) to be managed at each farm. As stated by the European Commission, “in 2016, the production of pork in the EU amounted to 23.4 million tons. This translated to 45.9 kg per each EU inhabitant and was one and a half kg per person more than in 2006” (data from ec.europa.eu/eurostat).
PS, as most of the animal slurries, can be used as an organic fertilizer because of its high nutrient level (mainly N, P, and K) and carbon (C) content [2,3]. When correctly distributed, the use of PS as organic fertilizer to agricultural lands generally improves crop yield and hence decreases the need for chemical fertilizers, allowing farmers to reduce fertilizer costs while increasing soil fertility. PS generally contains low levels of heavy metals (HMs); however, salts containing Cu and Zn are commonly used in feed additives to prevent or treat animal disease and increase feed efficiency [4].
Besides their fertilizer value, many ecological concerns derive from PS storage and application to farmlands. Some examples are represented by the runoff or leaching of N and P that contribute significantly to eutrophication of water bodies [5,6]. Because of their high N and C content, various gaseous emissions (i.e., CH4, CO2, and N2O) evolves during PS storage and after their application to farmlands, which can significantly impair atmosphere quality [7,8]. Moreover, other harmful gasses are emitted from PS during these phases, such as ammonia (NH3), which can affect both air, soil, and water quality [9]. Additionally, when PS application to farmlands is based only on its N load, an over-supply of other nutrients, such as K, may induce soil nutrient imbalances [10]. PS (and all farm effluents in general) also contains many pathogens that can potentially contaminate the water supplies of animals and humans [11,12].
In this framework, PS poor management may directly influence the soil nutrient loads and their subsequent transfer to surface and groundwater resources [13]. By consequence, modern intensive livestock is recognized as an activity with an extremely high environmental impact and a subject of increasing concerns for both developed and developing countries. In 1991, the European Community firstly introduced restrictions in N application to address this issue. Later, the EU-27 aimed at improving water quality by introducing the water framework regulation [13]. Following EU directives, a wide spectrum of technologies has been developed over the years by large farms to reduce the environmental impact of PS and farm effluents, in general.
Some of the methods available for reducing the N fraction of PS are represented by aerated lagoons, fixed-bed reactors, activated sludge with an anoxic tank, activated sludge with intermittent aeration, and sequencing batch reactors [14]. Although some of these treatments give important results, their impact on gaseous emissions, particularly N2O and NH3, should be addressed with more attention and precisely evaluated. To the best of the authors’ knowledge, the main challenge is now to find “how to implement such technologies at a wide scale and in an economically feasible way, making the processes more environmentally sustainable”. Moreover, considering the actual policies in terms of environmental protection, there is a strong need to invest in green methodologies with low environmental impact, and that may represent opportunities for a close “circular” loop in an economically sustainable way. A valid alternative to the above-mentioned processes may be represented by the use of natural geo-materials with a high adsorption capacity for the removal of (mainly) NH4+ from PS [10,15]. Many materials have been tested among the years to this purpose, but rocks containing significant amounts of natural zeolites remain one of the most studied adsorption media.
Zeolites are aluminosilicate minerals with an open 3D-structure formed by linked tetrahedra of [SiO4]4− and [AlO4]5−, which constitute the primary building units–PBUs. The replacements of Si4+ by Al3+ induce a negative charge of the zeolite framework, which is compensated by the presence of extra-framework cations (counterions) [16]. Natural zeolites are generally found in many areas of the globe as a component of volcanic tuffs. Recently, it has been proposed the name of “zeolitites” (ZTs) for those rocks having a zeolite content >50 wt% [17]. ZTs, in general, can be easily modified from their natural state by enrichment processes; the adsorption of a specific cation (e.g., Na+) originates a “homoionic” zeolite, where all or most of the cation exchange sites are occupied by the selected type of ion, increasing their effectiveness in NH4+ removal from solutions [18]. The Na-homoionic zeolite form is preferred, with respect to those where other alkali or alkaline-earth ions are used, for the easier production process and because Na+ is generally weakly bonded in zeolites extra-framework sites, facilitating the exchange with other ions in solution [18,19,20]. During the modification, Na+ also replace divalent cations, such as Mg2+ and Ca2+, resulting in a production of larger pores and cavities in the modified zeolite, improving the adsorption performances [21]. Other forms of pre-exchanged zeolites have been found to increase adsorption performances, such as H-exchanged forms [22]. The use of different kinds of natural and pre-exchanged ZTs as a soil amendment has been studied extensively in terms of modification of the soil physic-chemical characteristics, reduced N leaching, increased N use efficiency, water use efficiency, and crop yield [23,24,25,26,27,28,29,30,31]. Zeolites mitigation potential concerning some greenhouse gasses (like CO2 and N2O) and other harmful gasses like NOx and, especially NH3, has also been addressed in several studies [32,33,34,35,36]. Concerning the use of ZTs in the treatment of PS, [15] used Italian chabazite-rich ZT for removing NH4+ from PS and creating an NH4-enriched ZT to be used as a soil amendment. Through a series of batch experiments, they obtained a reduction from 15% to 30% of the total amount of NH4+ of the treated PS; the process was also scaled to a farm level with a small prototype tank (10 m3 volume) that confirmed the obtained results. The NH4-enriched ZT produced through the prototype was then used as a soil amendment in the ZeoLIFE project (LIFE10 ENV/IT/000321) with interesting results in terms of crop yield increments, chemical fertilizers reduction, and environmental protection (reduced NO3− leaching) [25,36,37].
Beyond the discussion about the effectiveness of natural or homoionic ZTs in the treatment of PS for NH4+ removal, few attentions have been dedicated to the PS compositional variation after the treatments. The use of ZTs induce a reversible ion exchange process, where zeolite-affine ions are sorbed from the liquid, but contemporaneously other ions, contained in the extra-framework sites of the mineral, are released. This is reflected in a complex shift of the PS composition that may result as beneficial or harmful. The use of homoionic ZTs in Na-form (NaZTs) is, in fact, widely recognized as an efficient procedure to improve the removal efficiency of NH4+ but it may lead to the release of consistent amounts of Na+ into the slurry, increasing the Na-risk for soils and waters following their application to an agricultural context.
This paper was, therefore, aiming at elucidating the changes in PS chemical composition, following sequential treatments using natural ZTs and NaZTs in terms of NH4+, major and trace elements, through a series of laboratory batch experiments.
2. Materials and Methods
2.1. PS and ZT Employed in the Experimentation
The PS samples employed in the batch experiment derived from livestock located at Isernia (Molise Region, Italy). The PS was a product of mechanical separation from the solid fraction with a low amount of suspended solid (<3%). It was delivered to the laboratory in a 25 L tank, and it was equilibrated at a temperature of 20 °C before the beginning of the experiment.
Concerning the ZT used as adsorption media, the chosen material was delivered by an Italian company and comes from a quarry located in central Italy (42°41’20.65” N, 11°44’26.29” E, Sorano, Grosseto), the same exploited by [24,26,30,37]. The quarried rock is a thick zeolitized pyroclastic deposits belonging to the Sorano formation, a unit, which is part of the lithic yellow tuff erupted during quaternary by the latera volcanic complex [38]. These rocks (in particular their glassy fraction) underwent extensive zeolitization due to the activity of pore fluids heated by the thermal energy of the pyroclastic deposit itself, resulting in a sort of “geoautoclave” [39]. The main zeolite species present in the rocks are K-rich, Na-poor chabazite, phillipsite, and analcime; a detailed mineralogical and chemical characterization of this material has been carried out by [40]. The quarry is mainly dedicated to the production of construction bricks, but, from the cutting process, high amounts of ZT remains unused, constituting an interesting and precious granular by-product, which can be used for many purposes, including the use as adsorption media for PS treatments and as soil amendment [30].
The quantitative phase analysis of the minerals constituting the employed ZT is displayed in Table 1. The ZT was supplied in three different grain sizes, 0.1–0.7 mm (ZF, “Zeolite Fine-size”), 0.7–2.0 mm (ZM, “Zeolite Medium-size”), and 2.0–5.0 mm (ZG, “Zeolite Gravel-size”). The effective grain sizes were checked by particle-size analysis before the experiment, and results showed that >96% of ZF and ZM were within the range declared by the company, while only 88% of ZG was within the declared grain size range. The material was delivered with a gravimetric water content <3 wt%. The cation exchange capacity (CEC) of the employed ZT was already determined by [40], and, as reported by the authors, the total CEC was 2.17 meq g−1 with Ca2+ as prevalent exchangeable cation (1.46 meq g−1), followed by K+ (0.60 meq g−1), Na+ (0.07 meq g−1), and Mg2+ (0.04 meq g−1).

Table 1.
Quantitative phase analysis of the employed chabazite-rich zeolite at natural state (Natural-ZT) quarried in the Sorano area. Data from [40]. Standard deviation within brackets. TZC refers to total zeolitic content.
Zeolite Enrichment with Na+
Part of the ZT was subjected to enrichment with Na in order to increase its adsorption capacity with respect to NH4+ [18], originating a “Na-homoionic” zeolite. A hundred grams of ZT of each grain size were stirred in 2 L of 2 M >99% pure NaCl solution for 8 h (modified from [18]. At the end of the process, the material was washed several times with Milli-Q water to remove residual Na and Cl and let air-dry before the experiment. The NaZTs were hereafter labeled as Na-ZF, Na-ZM, and Na-ZG, respectively.
2.2. Experimental Set-Up
These experiments were constructed to mimic the operational conditions of field scale treatment systems, which are already being constructed in Italy. For this reason, the effects of some important parameters, such as pH, contact time, and temperature, were not the primary aim of this paper and were kept fixed among the experiments.
The experimental set up was designed to test the following effects on variation in PS chemical composition:
- Zeolite grain size
- Zeolite Na-enrichment
- Number of the treatment cycle
To these aims, a series of batch experiments were performed as described in the following paragraphs.
An amount of 6 g of each ZT type (both natural and NaZTs) was placed in a 100 mL high-density polyethylene (HDPE) plastic bottle in three replicates. After careful homogenization of the PS, a subvolume of ~2.5 L was transferred from the main tank into a smaller recipient, and after further homogenization, 100 mL was added to each HDPE bottle for a total of 18 samples. Three additional samples were prepared for the determination of initial PS characteristics at time 0 (PS T0), and three more samples served as blanks (B) and were subjected to the treatment but without any ZT addition. Each sample (except PS T0) was closed hermetically and placed in a horizontal shaker for 2 h and then let rest for the other 4 h to assure equilibration: this procedure consisted of one treatment cycle (TC). After the end of the 1st TC, the maximum amount of recoverable PS from each sample was transferred in new HDPE bottles and stored overnight at 4 °C. The following day, new “fresh” ZT was added to the recycled PS, maintaining the same solid/liquid ratio. This procedure was repeated for a total of three consecutive TC (total of 63 samples treated and analyzed). The contact time (2 h + 4 h) was chosen on the basis of previous studies on adsorption kinetic, which demonstrated that the majority of the exchange operated by zeolites occurred within the first 3 h [41]. PS’s pH was not buffered in order to limit the use of chemicals and to reproduce the operational conditions that usually occur in field-scale systems, where no buffers are expected to be added to reduce the operational costs [15]; additionally, the pH of the PS (~7.1) was the pH at which [41] found almost the maximum removal efficiency of NH4+ in solution using Chinese zeolites (heulandite).
The dosage of 6 g per 100 mL (6%) was chosen on the basis of previous experiments carried out on the same matrix having the aim of determining the best and most convenient solid/liquid ratio for the NH4+ removal. Additionally, also [41] found the highest removal efficiency at zeolite dosage of ~6%. For these reasons, we did not further investigate the dosage, pH, and contact time effects.
2.3. Analytical Techniques
PS’s pH and electrical conductivity (EC) were measured in each sample by using an Orion 9102BNWP pH-meter connected to an Orion 4star pH–ISE benchtop (Thermo Fisher Scientific, Waltham, MA USA) and a RS 180-7127 (Hanna Instrument, Ronchi di Villafranca Padovana, Italy) probe, respectively. At the end of each TC, 5 mL of PS was transferred in a graduated cylinder, diluted to 1:20 v/v ratio with Milli-Q water, and transferred to HDPE bottles. The diluted samples were analyzed for NH4+ content with an ion-selective electrode (ISE) Orion 95–12 (Thermo Fisher Scientific, Waltham, MA USA) connected to the Orion 4star pH–ISE benchtop.
At the end of each TC, additionally to the aliquot designed for ISE measurement, 1 mL of PS was sampled from each replicate and stored in a 1.5 mL Eppendorf tube at −20 °C until sample preparation and analysis (a few days later). A 0.5 mL aliquot was then added to a 25 mL volumetric flask and diluted with Milli-Q water (Direct-Q UV, Millipore, Burlington, MA, USA) to a ratio of 1:50 v/v and then filtered with a Wathman#40 N-free filter. The filtrate was then further diluted upon reaching a ratio of 1:250 v/v before the analysis. Major (Na, Mg, P, K, Ca) and trace elements (Li, Ti, V, Cr, Mn, Fe, Ni, Cu, Zn, Ga, Rb, Sr, Ba, and U) were determined by an inductively coupled plasma mass spectrometer (ICP-MS) X series Thermo-Scientific, equipped with Collision/reaction Cell Technology (CCT) (Thermo Scientific, Bremen, Germany).
A set of major and trace elements was also analyzed on zeolite tuff by X-ray fluorescence (XRF) on powder pellets, using a wavelength-dispersive automated ARL Advant’X spectrometer (Thermo Electron SA, Ecublens, Switzerland) (data displayed in Supplementary Material Table S1).
2.4. Calculations and Statistical Analysis
The amount of NH4+ removed at each TC was determined, as well as the removal efficiency (RE %), according to the Equation (1):
where C0 and Cf are the initial and final NH4+ concentrations in mg L−1, respectively.
RE (%) = [(C0 − Cf)/C0] × 100
To evaluate significant differences between the treatments at each TC, a series of one-way ANOVA was performed at p = 0.05, and Tukey honestly significant difference (HSD) post hoc multiple comparison test was then executed. Data were tested for normality and homogeneity of variance before the test execution. In case one or both prerequisites were not met, data were log-transformed prior to ANOVA analysis. A series of one-way ANOVA was also been performed to evaluate differences within each treatment among the three TCs.
Correlation matrixes were built using the Pearson product-moment correlation coefficient (PPMCC) to individuate positive or negative correlations between the many variables (p = 0.05). All the statistical tests were performed with Sigmaplot 12.0 (Systat Software, San Jose, CA, USA).
3. Results and Discussion
All the results obtained in the experiments (including the initial PS chemical composition) are reported in Appendix A (Table A1, Table A2 and Table A3) listed at the end of the manuscript. In the following paragraphs, we have focused on the main trends observed.
3.1. Effect of ZT Treatments on PS’s pH and EC
The initial PS’s pH at T0 was characterized by nearly neutral values (7.14 ± 0.03). PS’s pH varied significantly at each TC (p < 0.05) with no particular trends in relation to grain size or typology (natural or NaZTs) (Table A1, Table A2 and Table A3). After the 1st TC, the pH was very close to the starting pH except for the B sample that showed slightly higher values (p < 0.05) (Table A1). Starting from the 2nd TC, pH started increasing toward mean values varying between 7.51 and 7.75 (ZG and B, respectively) (Table A2). During the 3rd TC, pH further increased to more alkaline values from 7.76 to 7.88 (observed in ZG and NaZM) and up to 8.12 in the B sample (p < 0.05) (Table A3). The general tendency was thus a gradual increase of the pH towards sub-alkaline to alkaline values by increasing the TCs (p < 0.05), usually with few differences between the various kinds of ZTs but with a stronger alkalization in the B sample.
This evidence might be the result of different processes, which could involve the possible precipitation of mineral phases or the oxidation of volatile fatty acids (VFA) during the shaking process. Precipitation of many different mineral phases, such as struvite (MgNH4PO4·6H2O), calcite (CaCO3), hydroxyapatite (Ca5(PO4)3OH), and a wide range of calcium, magnesium, and iron phosphates, are, in fact, well known to possibly occur in wastewaters [42,43]. It is also plausible that the shaking of PS resulted in gradual oxidation of VFA, which therefore led to a gradual pH increase over the three performed TC in all the analyzed samples. [44] indicated that aeration of slurry (surely occurred in this experiment during the shaking process) favored the microbial oxidation of VFA and hence the uptake of undissociated or dissociated acids with the consequent removal of H+ in the former case or introduction of OH− in the latter.
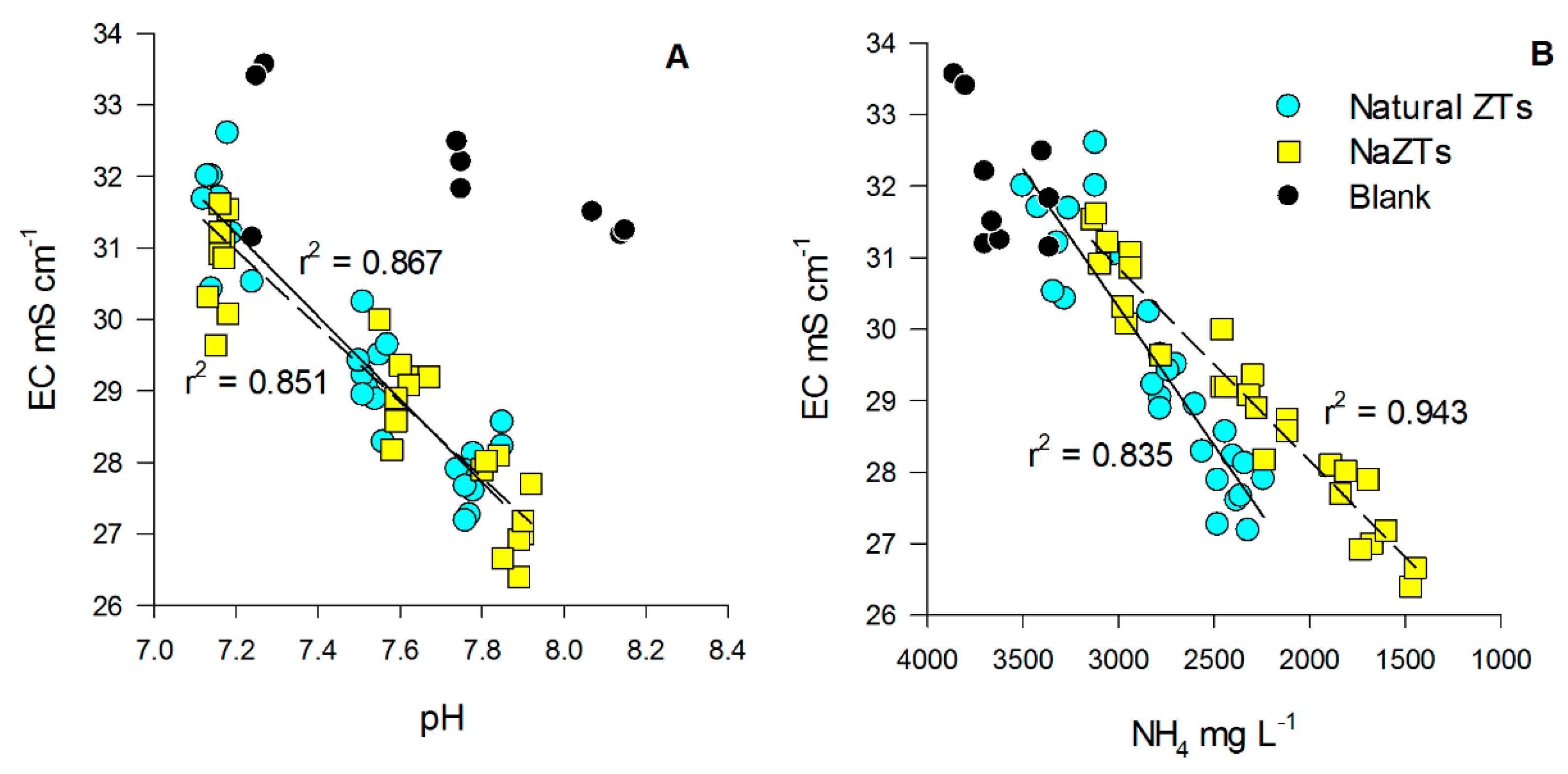
The pH of the treated PS was also well correlated with the EC in all the samples where any kind of ZTs was added (Figure 1A). Additionally, pH in these latter samples was always lower in comparison to the B sample, suggesting that the employed ZTs had a sort of slight buffering effect during the treatment [30].
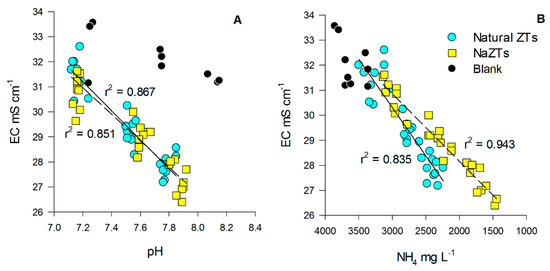
Figure 1.
Relationship between electrical conductivity (EC) vs. pH (A) and EC vs. NH4 (B) in the treated pig-slurry. Black dots represent blank samples (no ZT added), cyan dots represent natural ZTs (all the grain sizes), while yellow squares represent NaZTs (all the grain sizes). Continuous regression lines refer to natural ZTs, while the dotted regression line refers to NaZTs. ZT: zeolite; NaZT: Na-exchanged zeolite.
The EC of the treated PS was initially very high (34.1 ± 0.5 mS cm−1), but it decreased significantly after each TC, especially in the samples treated with ZTs (p < 0.05). EC decreased down to values ranging from 26.7 to 28.0 mS cm−1 after the 3rd TC (Table A3). The reduction of EC could be likely explained by three main factors, which are (i) adsorption of ions from solution, (ii) gaseous losses during the shaking process, and (iii) precipitation of mineral phases. The reason for the stronger decrease in EC observed in the samples treated with any kind of ZTs could be mainly explained by the high sorption capacity of zeolite minerals, which are known to be able to decrease solution EC [45]. EC was, in fact, well positively correlated with NH4+, which was strongly adsorbed in all the samples where any kind of ZT was added, with a higher correlation (r2 = 0.943, p < 0.05) when NaZTs were used (Figure 1B). A minor fraction of the EC reduction could be explained by the volatilization of some compounds and/or precipitation of mineral phases during the experiments (as testified by the lower decrease of EC in the B sample).
3.2. Effects of ZT Treatments on PS’s NH4+ Content
The high affinity of chabazite, as well as most of the natural zeolites for NH4+, is well known and documented in the scientific literature [18,46,47]. Since the volcanic tuff used in these experiments was composed of approximately 70 wt% of zeolite minerals (Table 1), the amount of NH4+ potentially subjected to sorption was relevant.
NH4+ concentration in the initial PS was 3690 ± 270 mg L−1 (Table A1). These concentrations are extremely high but typical of raw pig slurries that are known to have the highest fraction of mineral N among the various animal slurries [48]. These extremely high amounts of mineral N must be absolutely controlled before the utilization for fertilization purposes since NH4+ could be rapidly converted into NH3 and/or be rapidly nitrified when mixed with aerated soils, leading to leaching and gaseous losses with well-known fallouts in terms of environmental problems.
The concentration of NH4+ remained unchanged in the B samples after the three TCs (p > 0.05), confirming that mineral phases, including NH4+, did not precipitate from the PS (e.g., struvite) and that also N losses through NH3 volatilization were negligible (Table A1, Table A2 and Table A3). The starting pH was, in fact, nearly neutral, and it increased toward values favorable to NH3 volatilization only after the 3rd TC; moreover, having conducted the experiments in closed bottles with low free headspace might have probably further reduced the volatilization of NH4+ into NH3.
We observed significantly higher sorption of NH4+ in all the samples treated with NaZTs (p < 0.05) (Table A1, Table A2 and Table A3). This was in agreement with the scientific literature, where it has been widely pointed out that Na-exchanged zeolites are more efficient in NH4+ removal from solutions than the natural forms, which contains many different cations in the extra-framework sites [18].
The main reason for this behavior could be found in the stronger bond of divalent cations (e.g., Ca2+) with the framework of zeolites and a consequent more difficult exchange when they are present at high amounts as exchangeable cations. Na+ could, in fact, easily access to more exchange sites, especially in chabazite zeolite, allowing a faster and a more efficient replacement with NH4+ [18,21,49].
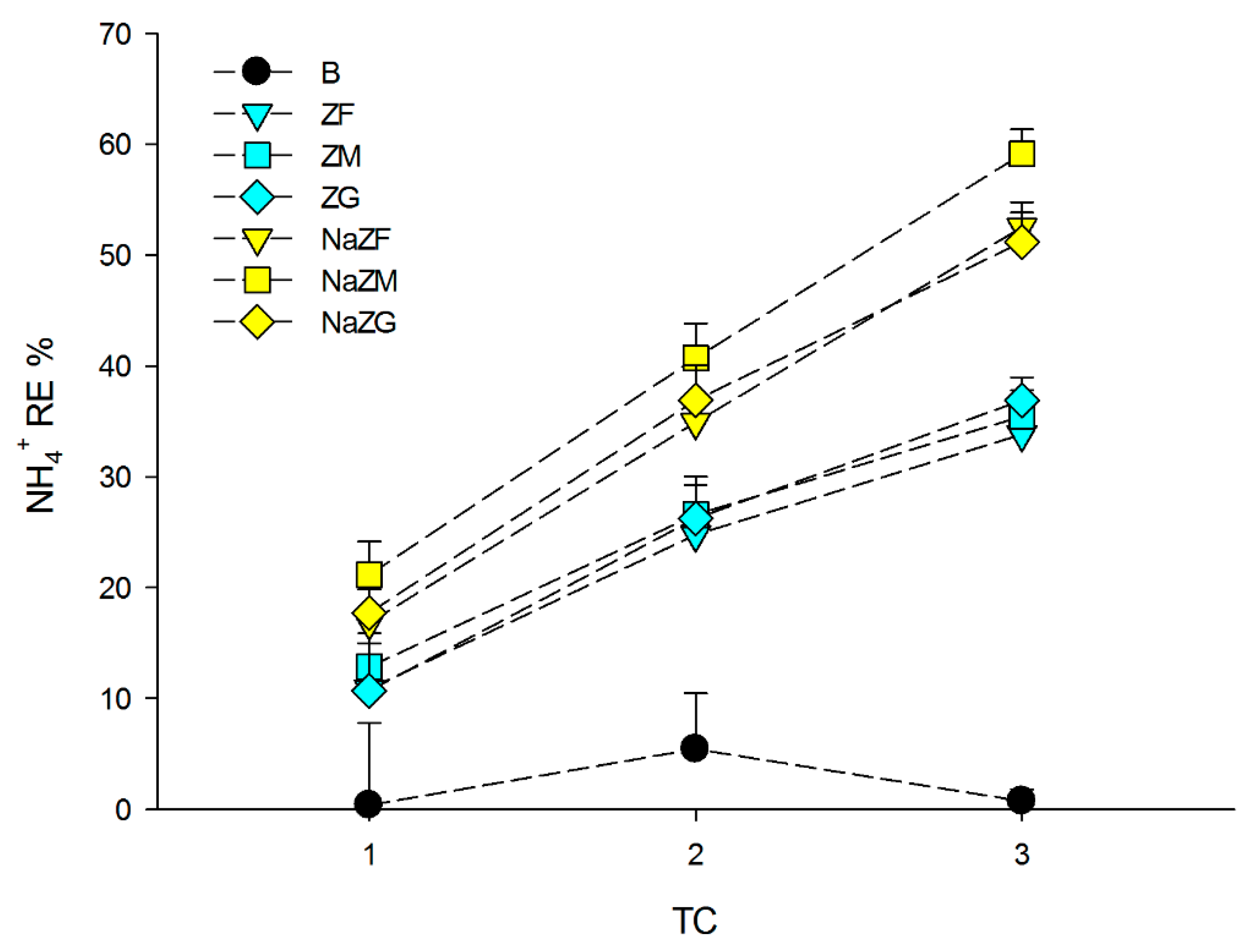
As visible from Figure 2, there was no significant effect of grain size in terms of NH4+ removal by ZF, ZM, and ZG (p > 0.05). In these samples, the RE % after the 1st TC was between 10.67 and 12.84%, while at the 2nd TC, the RE% was between 24.77 and 26.58%, and at the 3rd TC, between 33.82 and 36.89%. As pointed out above, the NaZTs (NaZF, NaZM, and NaZG) showed remarkably higher RE% with a slight effect of grain size only at the 3rd TC where NaZM showed higher RE % compared to the other two grain sizes (p < 0.05). The RE % obtained after the 1st TC with the NaZTs ranged between mean values of 16.82 and 21.16%, while after the 2nd TC, between 34.90 and 40.69%, and at the 3rd TC, between 51.18 and 59.11% (Figure 2). The results obtained within this study exhibited that, in most cases, grain sizes ranging from 0.1 to 5.0 mm had not strongly affected NH4+ adsorption by ZTs with the only exception of NaZM at the 3rd TC, which was slightly higher. Other authors reported that grain size in this range had no particularly evident effects on NH4+ adsorption [50,51]. Contrasting evidence could be found in the scientific literature concerning the grain size effect, since [41,46] found a decrease in the adsorption capacity, increasing zeolite particle size, while [50,52] stated that decreasing zeolite particle size would not affect NH4+ sorption. According to [50], by reducing particle size, the internal surface of zeolites (and hence their cation exchange sites) is not increasing, the only variation being the external specific surface area. Since the main process that leads to NH4+ sorption by zeolites is cation exchange, probably the variation in the external specific surface area within the tested grain sizes is not large enough to allow a significant and systematic variation in NH4+ sorption. By testing a significantly lower grain size (e.g., <0.1 mm as done by [41]), probably the increase in external specific surface area would have been large enough to induce an increase also in the NH4+ sorption. Since this experiment was performed on raw PS, which is intrinsically a complex and heterogeneous matrix and since also the zeolite-rich tuff used is intrinsically not perfectly homogeneous (% of zeolite minerals may be different within grains of the rocks), it could not be excluded that the slightly higher RE% obtained with NaZM on the 3rd TC was related to matrix heterogeneity. Other authors reported similar experiments on wastewaters or biogas sludge, where remarkably higher RE % of NH4+ were obtained using natural ZTs (i.e., [53]). The lower RE % obtained in our experiment at each TC was, in this case, easily explainable by the initial NH4+ load of the treated PS in this study, which was remarkably higher (>3500 mg L−1) in comparison to the other studies found in the literature (generally <1000 mg L−1).
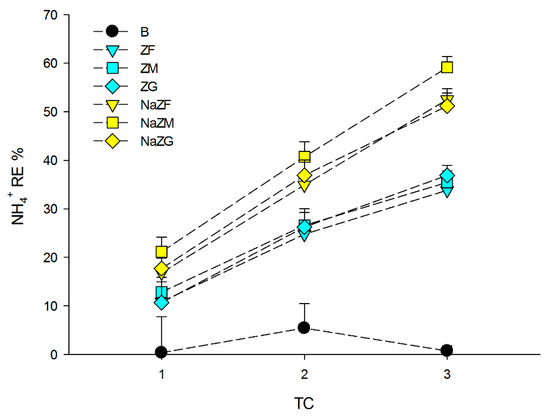
Figure 2.
Removal Efficiency (RE %) of NH4+ among the three performed treatment cycles (TC) obtained during the experiments by treating the pig-slurry with natural ZTs in three grain sizes (ZF = grain size 0.1–0.7 mm, ZM = grain size 0.7–2.0 mm and ZG = grain size 2.0–5.0 mm) and Na-homoionic NaZTs in three grain sizes (NaZF = grain size 0.1–0.7 mm, NaZM = grain size 0.7–2.0 mm, and NaZG = grain size 2.0–5.0 mm). Error bars represent standard deviation.
3.3. Dynamics of Other Major Elements (K+, Na+, Ca2+, Mg2+, and P) in the PS
The effect of competitor cations in the PS surely played a crucial role in the entity of NH4+ removal. The studied PS was, in fact, extremely rich in K+ (1691 ± 11 mg L−1). Beside K+, the other elements present at relevant concentrations in the initial PS were Na+ (305 ± 7.8 mg L−1), Ca2+ (170 ± 26 mg L−1), and Mg2+ (65.0 ± 2.5 mg L−1) (Table A1). Given the starting concentration of each element, it is very likely that the main competitor with NH4+ was K+.
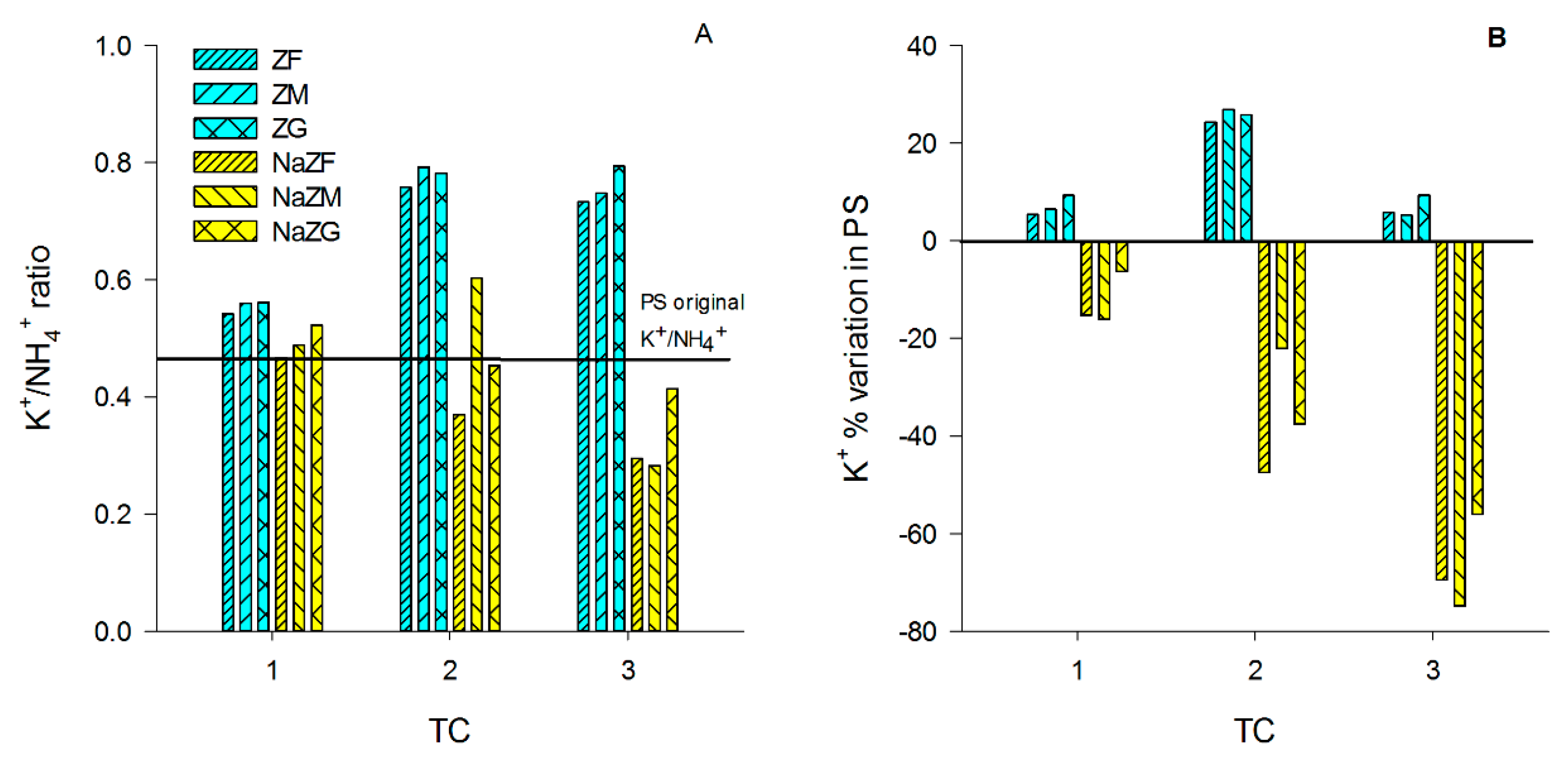
After the 1st TC with ZF, ZM, and ZG, a tendency in increasing K+ of PS (and the B sample) was observed only in the ZG sample (Table A1). This suggested that no significant amounts of K+ were introduced in the PS by ZTs at natural state after one TC. K+ tended to increase in these treatments also after the 2nd TC with no strong differences between the three grain sizes (p > 0.05) to value up to around 2100 mg L−1 (Table A2). However, after the 3rd TC, the K+ concentration in the PS decreased to values similar to that obtained after the 1st TC, again with no significant differences between the three grain sizes (p < 0.05) (Table A3). This behavior might be explained by changes in the K+/NH4+ ratio of the PS during the experiment, which might have induced different levels of competition for NH4+ removal at each TC. At the beginning of the experiment, the K+/NH4+ ratio of the PS was equal to 0.46, while it increased in ZF, ZM, and ZG samples after the 1st TC (~0.55) but especially at 2nd TC (>0.7), and then it remained almost constant until the end of the 3rd TC (Figure 3A). It is plausible that the release of K+ occurred after the 2nd TC caused an increased competition between K+ and NH4+ during the 3rd TC, leading to a decrease in the trend of NH4+ sorption by ZTs at natural state (Figure 2) and to sorption of some K+ released during the previous TCs.
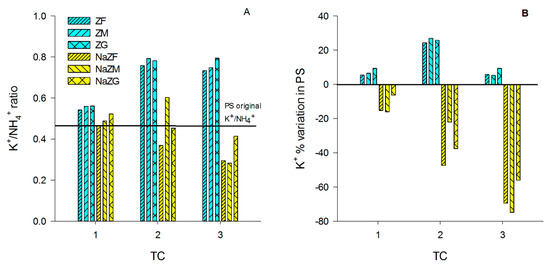
Figure 3.
(A) K+/NH4+ ratio variation among treatment cycles (TC) in all the tested samples, treated with natural ZTs in three grain sizes (ZF = grain size 0.1–0.7 mm, ZM = grain size 0.7–2.0 mm and ZG = grain size 2.0–5.0 mm) and Na-homoionic NaZTs in three grain sizes (NaZF = grain size 0.1–0.7 mm, NaZM = grain size 0.7–2.0 mm, and NaZG = grain size 2.0–5.0 mm; (B) % of K+ variation with respect to the initial values in the pig-slurry among the TCs in all the samples treated with natural and NaZTs.
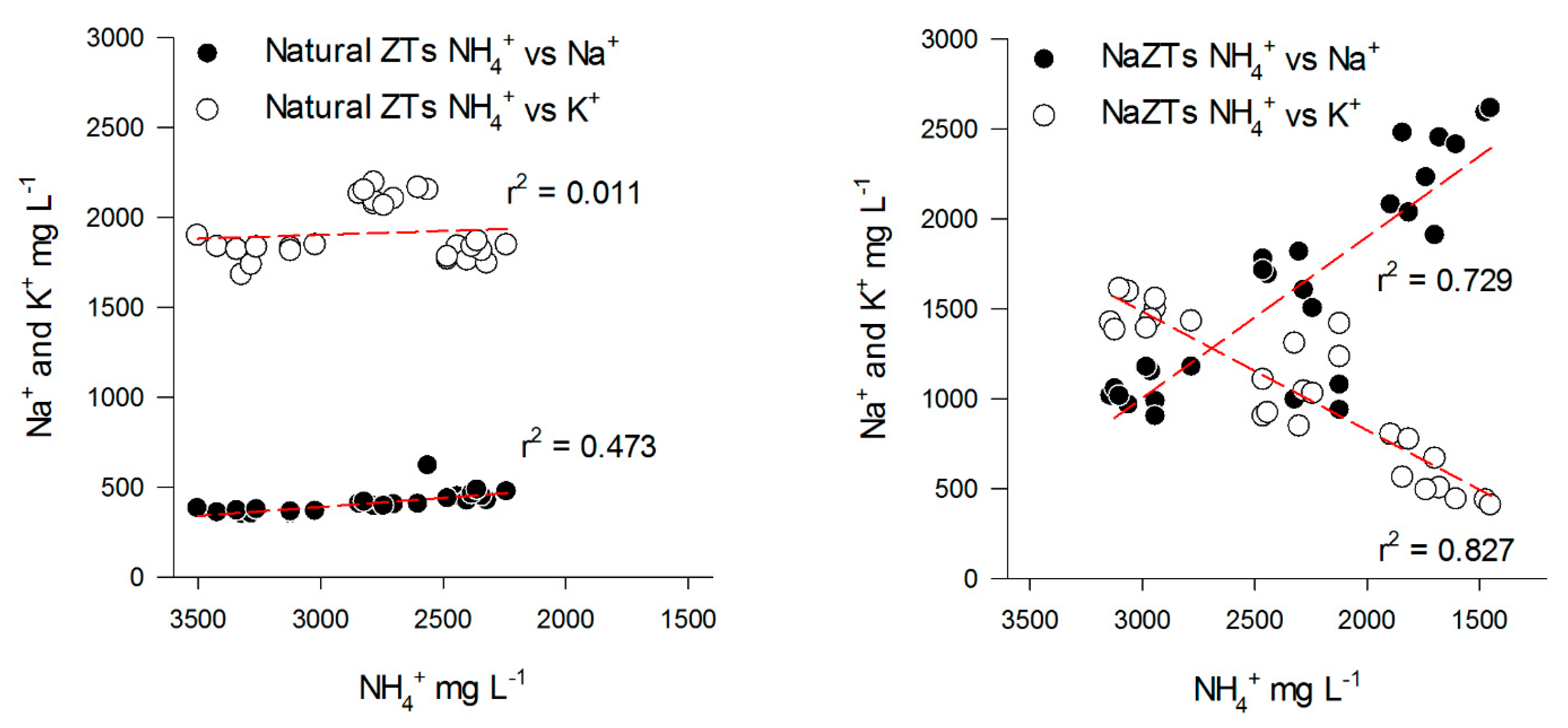
On the contrary, the NaZTs (NaZF, NaZM, and NaZG) showed a clear trend in the adsorption of K+ from the PS with a consequent decrease of the K+/NH4+ ratio over the TCs (Figure 3A,B). This was likely induced by the treatment with 1M NaCl, which extracted most of the exchangeable cations from the accessible exchange sites, replacing them with Na+. By using NaZTs, it was evident that not only the capability of NH4+ sorption was improved, but also the sorption of other important nutrients, such as K+ (Figure 4). The K+ reduction reached 75% of the initial content after the 3rd TC in NaZM, which was indeed also the most performant in reducing NH4+ (Figure 2).
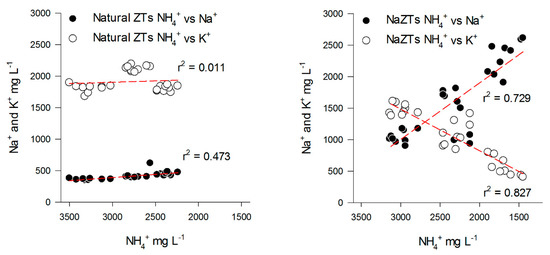
Figure 4.
Relationships between Na+ vs. NH4+ (black dots) and K+ vs. NH4+ (white dots) in the samples treated with NaZTs (all the grain sizes together). Red dotted lines represent linear regressions.
These trends in K+ are of relevant importance for the future utilization of this treated PS as fertilizer since K+ is one of the major nutrients for crop growth, and increasing/decreasing its concentration may have an important impact in terms of fertilization value and soil nutrient balance.
The Na+ content of the initial PS was 304 (±8) mg L−1 (Table A1). The treatments with ZF, ZM, and ZG increased significantly its concentration at each TC, up to values >470 mg L−1 at the end of the 3rd TC (p < 0.05). However, the increase in Na+ observed after the treatments with NaZTs was far more severe (p < 0.05). The total Na+ content in the PS after the 1st TC reached values up to around 1165 mg L−1 and increased almost linearly until the 3rd TC, reaching values up to >2500 mg L−1, meaning a total average increase of more than 500% of the Na+ content (Table A1, Table A2 and Table A3). These trends related to Na+ contents were explainable by the fact that the exchangeable Na+ of the chabazite contained in the ZT at natural state is low (see Section 2.1), but the little fraction present was probably easily released in the PS at each TC. On the contrary, in samples treated with the NaZTs, a considerably higher amount of Na+ was released into the PS. Na-exchanged zeolites have been already tested in synthetic wastewaters or real slurries [18,53], although the question of the significant release of Na+ into the slurry after the treatment has been poorly addressed. This remarkable amount of Na+ might, in fact, cause severe environmental problems, especially if these effluents are later used as fertilizers for agricultural purposes; hence, the enrichment of zeolites with other ions instead of Na+ should be eventually considered.
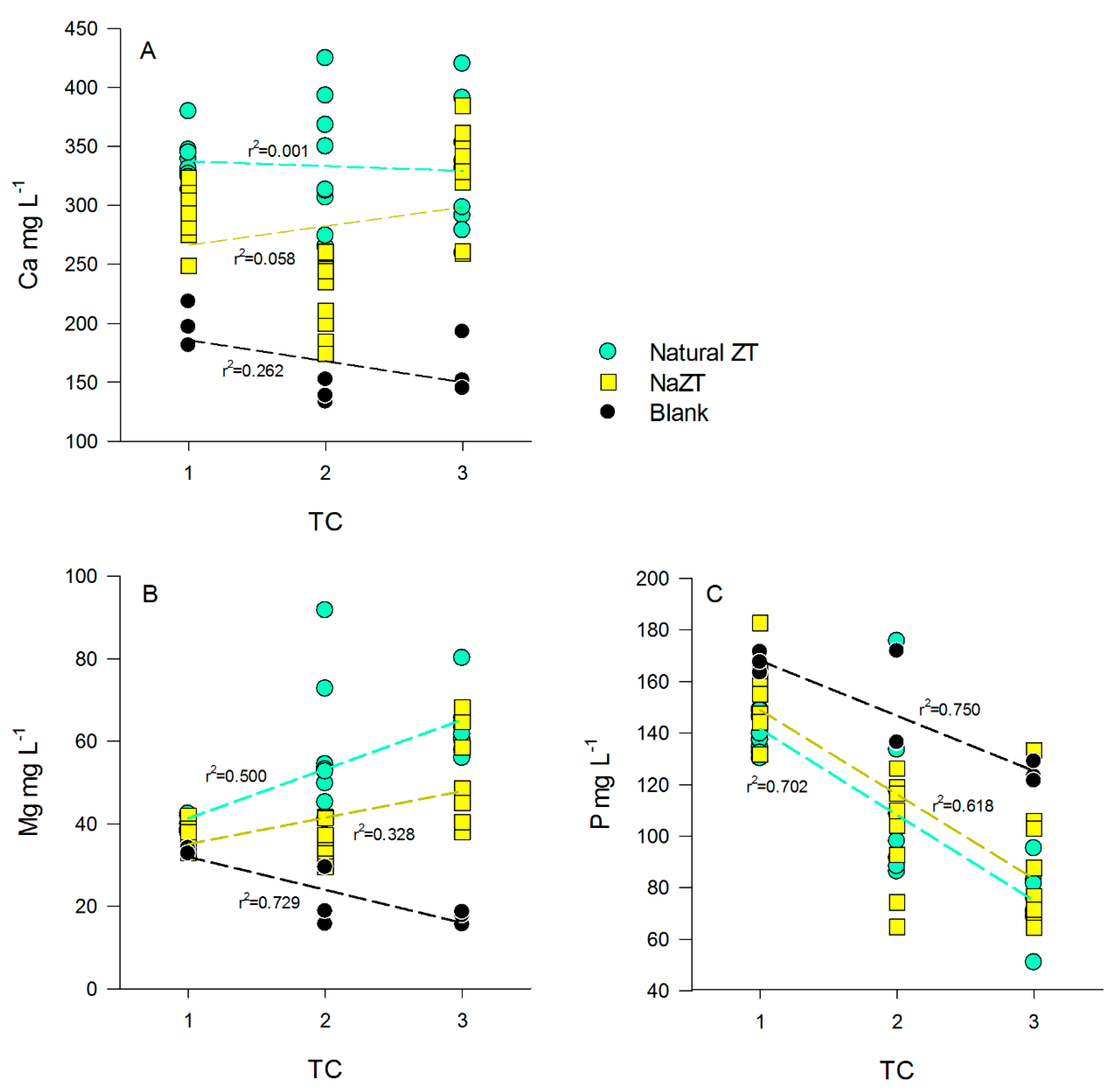
Ca2+ concentration in the initial PS was 170 (±26) mg L−1, and it remained unchanged in the B sample among the TCs (p > 0.05) (Table A1, Table A2 and Table A3). In treatments with ZF, ZM, and ZG, the Ca2+ concentration increased significantly after the 1st TC (p < 0.05) up to values of ~350 mg L−1 and remained almost stable up to the 3rd TC, without any particular effects of grain size (p > 0.05) and thus without correlations with the number of TC performed (Figure 5A). Similar behavior was encountered also for the NaZTs with very similar concentrations and no differences between the various grain sizes (p > 0.05) (Figure 5A). This evidence might indicate that part of the exchangeable Ca2+ in the ZTs was released into the PS after the 1st TC, but this was not confirmed by similar evidence after the 2nd and 3rd TCs. A possible explanation could be found in the precipitation of Ca-phosphates or Ca-Mg-phosphates, which might have played a role in Ca2+ balance at the end of the experimentation, representing an additional output product (not further investigated in this experiment). As pointed out before, precipitation of Ca and Mg-phosphates are indeed quite common in these kinds of effluents. In support of this hypothesis, it could be noticed that Mg2+ decreased significantly in the B at each TC, down to average values of <20 mg L−1 and concomitantly also p content decreased in the B samples (Figure 5B,C and Table A1, Table A2 and Table A3). In the treatments carried out with ZF, ZM, and ZG, Mg2+ decreased significantly after the 1st TC but with no significant differences with respect to grain sizes (p > 0.05) (Table A1). Starting from the 2nd TC, we observed an increase in Mg2+ content with respect to the previous TC, indicating that the ZTs were, however, releasing a small fraction of Mg2+ that, after the 3rd TC, almost restored the initial values of PS, counterbalancing the amount of Mg2+ lost (possibly by precipitation of mineral phases) (Table A2 and Table A3). In these samples, the amount of removed P, in fact, further increased in comparison to B samples, indicating that probably more Mg2+ was available for precipitation of phosphates. It is known that P in wastewaters is generally present as orthophosphate (PO43−), which has a great eutrophication potential [54]. P concentration decreased down to 60/70% with respect to initial PS values after these treatments, with a generally linear decrease (Figure 5C). Grain size had also, in this case, no significant effects on P variations in the PS (p > 0.05).
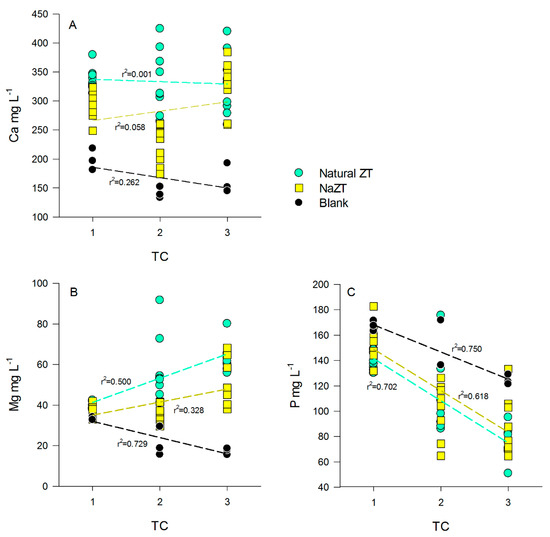
Figure 5.
Relationship between Ca2+ (A), Mg2+ (B), and P (C) vs. the number of treatment cycles (TC) in the treated pig-slurry (PS). Black dots and black regression lines represent blank samples (no ZT added), cyan dots and regression lines to natural ZTs (all the grain sizes), while yellow squares and regression lines to NaZTs (all the grain sizes).
Concerning the NaZTs (NaZF, NaZM, NaZG), the trends observed for Mg2+ were slightly different. As for natural ZTs, the concentrations with respect to initial PS decreased significantly after the 1st TC, but they remained almost constant up to the end of the 2nd TC, increasing only at the end of the 3rd TC (p > 0.05). The final concentrations of Mg2+ after the 3rd TC tended to be lower if compared to samples treated with natural ZTs, indicating that the treatment with Na+ reduced the amount of exchangeable Mg2+ (Figure 5B). This had also fallouts on the entity of P reduction that was indeed lower in comparison of the samples treated with natural ZT (Figure 5C) likely because of a lower amount of available Mg2+ for Mg-phosphates precipitation.
3.4. Dynamics of Heavy Metals (HMs) and Trace Elements in PS
Throughout the years, the execution of thermodynamic studies in mixed-metal contaminated effluents allowed the determination of the affinity series for natural chabazite zeolites, which is known to be the following: Pb > Cd > Zn > Co > Cu > Ni > Cr [55]. These studies, however, were conducted at a relatively higher concentration of such metals in comparison to our PS, and hence the experimental conditions were reasonably hardly comparable, also in terms of competition with other ions (e.g., NH4+, K+, Ca2+, Mg2+, etc.). Raw and undiluted PS is clearly a very complex matrix with high ionic strength, which makes difficult the comparison with laboratory studies performed with synthetic solutions where the competition for ion adsorption is more controlled. Besides these considerations, in this paragraph, we aimed at unveiling the changes in PS heavy-metals (HMs) (Ni, Mn, Cu, Zn, U, Ti, V, Fe, Ga, Cr) and other trace elements (Li, Ba, Rb, Sr) load after each treatment, since the conditions reproduced in this experiment were likely very similar to that of an operational system and might be useful to determine potential introduction or removal of hazardous elements during the sequential treatment with natural or NaZTs.
Several studies have been performed concerning Ni removal from aqueous solution using natural zeolite-rich rocks (mainly clinoptilolite) [55,56,57], but in this particular case, the initial concentration of Ni is much lower if compared to those investigated in these studies. The original PS showed an average Ni concentration of 170 (±12) µg L−1, and it remained constant in the B sample (p > 0.05). At these low concentrations, apparently, all the samples treated with any form of ZT slightly increased the Ni content of the PS in the 1st TC, then it generally decreased in the 2nd and 3rd TC to values not significantly different with respect to the initial PS values (p > 0.05) (Table A1, Table A2 and Table A3).
Italian chabazite-phillipsite rich tuffs from the Neapolitan yellow tuff formation have been investigated in the literature as possible media for Cr removal from waters by ion exchange in the past [58]. In our experiment, a significant trend in Cr removal from the PS (p > 0.05) was not observed (Table A1, Table A2 and Table A3). Similarly, no clear and consistent significant trends for Ti, Mn, Cu, Zn, and V between the various treatments were observed (Table A1, Table A2 and Table A3).
Fe in solution remained stable in the B sample (p > 0.05) and showed a general tendency in increasing concentrations in solution in the samples treated with natural and NaZTs. However, the trend was not always significant (high standard deviation within replicates), as testified by the fact that NaZG was not significantly different from the initial Fe content of PS (p > 0.05).
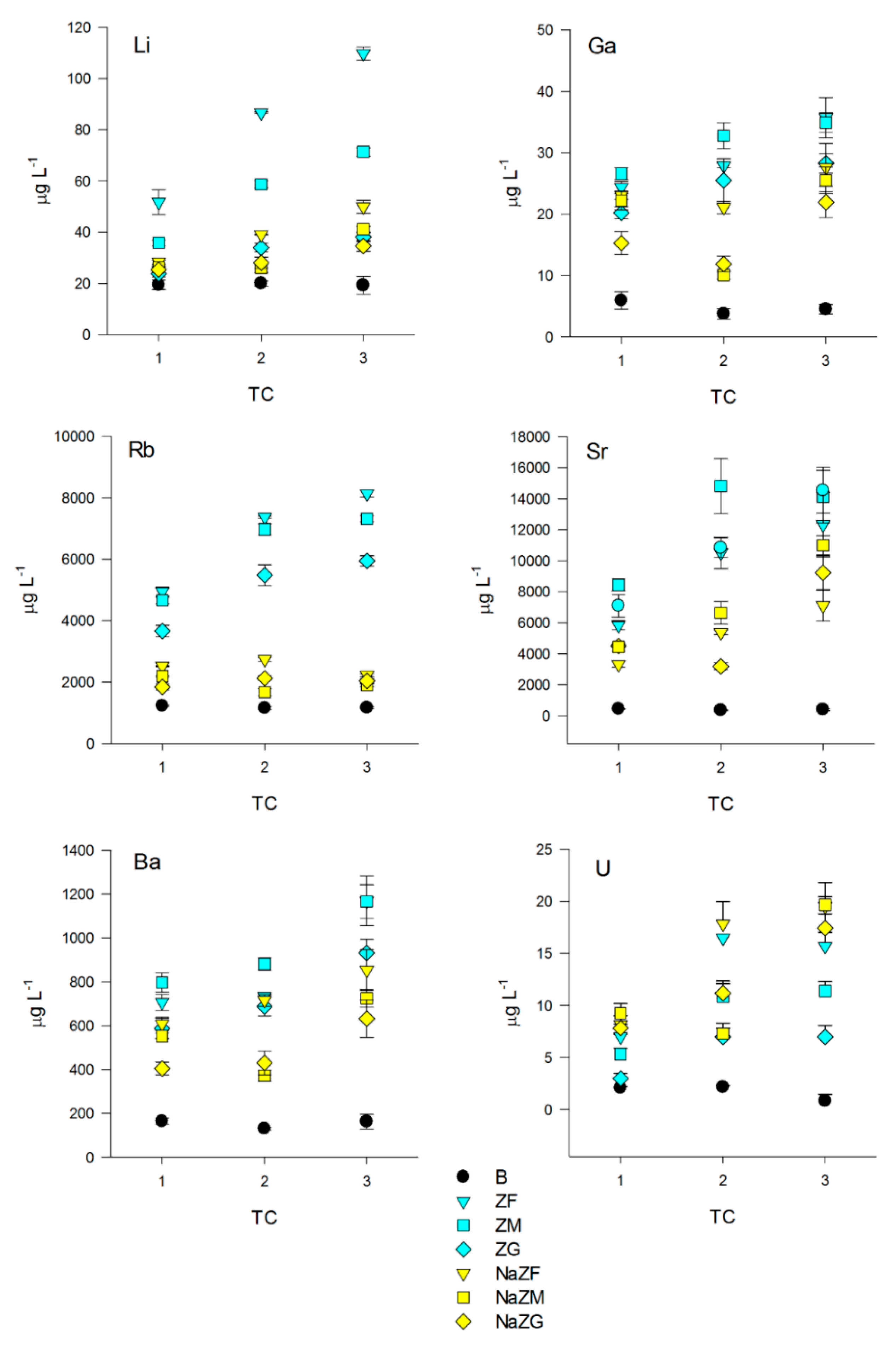
Other elements and some HMs anyway showed more interesting behaviors. For instance, concentration of Li remained unchanged in the B samples, but we found a direct correlation between the number of TCs in all samples treated with both natural or NaZTs (p < 0.05) (Figure 6) with a probable slight effect of particle size since finer grain size generally reached higher Li release (p < 0.05). We also observed a tendency for a higher release of Li from ZT at a natural state with respect to NaZTs, even if this trend was not always confirmed (i.e., NaZG released more Li than ZG). Li was consequently always inversely correlated with NH4+ in these treatments (p < 0.05), suggesting that while NH4+ was being adsorbed, also some Li was being released in solution.
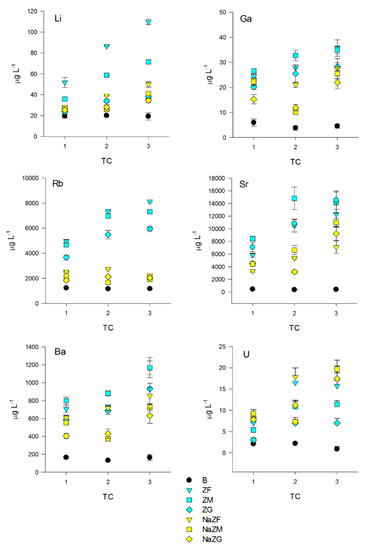
Figure 6.
Dynamics of Li, Ga, Rb, Sr, Ba, and U among the three treatment cycles (TC) of the pig-slurry treated with natural ZTs in three grain sizes (ZF = grain size 0.1–0.7 mm, ZM = grain size 0.7–2.0 mm and ZG = grain size 2.0–5.0 mm) and Na-homoionic NaZTs in three grain sizes (NaZF = grain size 0.1–0.7 mm, NaZM = grain size 0.7–2.0 mm, and NaZG = grain size 2.0–5.0 mm)and in the blank samples (without the addition of ZTs).
Another interesting trend was observed for Ga, where no variations in the B samples with respect to the starting concentrations occurred, but a significant increase after the treatments with both natural and NaZTs was observed (p < 0.05) (Figure 6). The release of Ga was higher in the natural forms (ZF, ZM, and ZG) in comparison to all the three NaZTs (p < 0.05), with higher release from the finer grain sizes (p < 0.05). As for Li, a direct correlation between Ga and the number of TC and an inverse correlation with NH4+ content was found (p < 0.05).
Significant trends were also observed for Rb and Sr with respect to the PS initial values (Figure 6). Rb concentration remained constant in the B sample after the three TCs (p > 0.05), while a significant increase was recorded in ZF, ZM, and ZG, as well as in the NaZTs (p < 0.05), even if of a lower entity. The PS resulted more enriched in Rb at each TC with the higher enrichment for ZF and ZM, indicating a significant effect of grain size (p < 0.05). Concerning the NaZTs, the entity of Rb enrichment of the PS after each treatment was lower with respect to the natural forms, but still significant (p < 0.05) and with also a slight significant effect of grain size (finer grain size = higher release) (p < 0.05). Rb is vicariant of K and, thus, can be likely present as trace exchangeable cation in the zeolites being part of the volcanic tuff. The Na-enrichment has clearly reduced the amount of exchangeable Rb, leading to a relatively lower transfer of this element into the PS after the treatments with NaZTs.
Sr showed a very similar trend if compared with Rb, where an increase in the treated PS was recorded after ZT at natural state, and NaZTs were used (p < 0.05). As for Rb, the increase in Sr was higher in samples treated with ZT at natural state than in that treated with the NaZTs, but, in this case, with no clear effect of grain size (p > 0.05). Sr is vicariant of Ca, and hence it has been plausibly released by cation exchange processes during the treatment.
Regarding Ba, concentrations in solution increased in the treatments where any kind of ZT was employed with respect to the initial values (p > 0.05), while concentrations in the B sample remained unchanged (p > 0.05) (Figure 6). The higher release was obtained with natural ZTs with respect to the NaZTs. Concentration in the PS increased almost constantly at each TC with no effects of grain size (p > 0.05). Ba was present at significant amounts in the employed volcanic tuff (data from X-Ray fluorescence are provided in the Supplementary Material “S1”), and it is known to be a potentially exchangeable ion by chabazite [59].
Significant enrichment in the PS was also individuated for uranium (U) in all ZT treated samples with respect to the former PS (p < 0.05) (Figure 6). Concentrations of U remained unchanged in the B samples, but it increased by up to 18.5 µg L−1 when ZTs were employed. The observed trend indicated a positive correlation with the number of performed cycles. From the previous analysis performed by the authors on the same rock sample (unpublished results), the amount of U of this volcanic tuff was 8.379 mg kg−1; it was thus evident that some of the U contained in the former volcanic trachytic glass was transferred into the PS during the treatments.
4. Conclusions
Thanks to the performed experiments, it was possible to evaluate the variations in chemical composition that the PS underwent through sequential treatments with natural and Na-exchanged chabazite-rich volcanic tuffs.
Generally, both kind of ZTs profoundly influenced the chemistry of the PS after performing three sequential treatments, with strong significant differences between the natural ZTs and the Na-homoionic ZTs, but without any consistent significant effect of grain size within the three tested particle size (0.1–0.7, 0.7–2.0, and 2.0–5.0 mm).
The pH of the PS raised slightly towards sub-alkaline to alkaline values during the treatments, while the EC was significantly reduced, thanks to the ion sorption processes by the zeolite-rich tuffs.
The Na-homoionic ZTs were more efficient in terms of NH4+ removal from the PS, reaching up to almost 60% reduction of the initial content after three treatment cycles, notwithstanding the extremely high initial NH4+ content of the PS. However, the release of Na+ by Na-homoionic ZTs might impair the quality of the PS, especially if it is later used as fertilizer since the Na+ content can be increased by up to 500%. This aspect must be taken into consideration by considering alternatives in the pre-treatments of the zeolites, like creating K-forms instead of Na-forms.
Additionally, the augmented sorption capacity of the Na-forms of ZTs led to stronger competition with K+ ions, which were subtracted from the PS during the process and thus further reduced its fertilization value. The amount of P decreased probably because of precipitation of mineral phases; however, the treatments with NaZTs decreased the potential P reduction. In terms of trace elements (HMs and non-HMs), the treatments with ZTs significantly increased concentrations of Li, Ba, Rb, Sr, Ga, and U in the PS, while no consistent and significant effects were encountered for Ti, V, Cr, Mn, Fe, Ni, Cu, and Zn.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4441/12/2/310/s1. Supplementary Materials S1 Results from X-Ray Fluorescence analysis performed on the solid zeolitite samples before and after the treatments with pig-slurry.
Author Contributions
Conceptualization, G.F. and B.F.; Laboratory experiment execution and sample analysis, G.F., G.G., V.M.; Investigation, G.F.; Data curation, G.F., D.D.G.; Writing–Original Draft Preparation, G.F.; Supervision, M.C., B.F.; Project Administration, B.F.; Funding acquisition: M.C., B.F., G.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by FerraraBio SRL (www.ferrarabio.it).
Acknowledgments
The authors gratefully thank Tassinari Renzo for ICP-MS analysis and Daniele Malferrari for laboratory assistance during ISE measurements. We gratefully thank Vittorio Scarpa for his precious help during the experimental execution and sample analysis.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Chemical composition of the studied pig-slurry (PS) before and after the first treatment cycle with natural ZTs (ZF, ZM, and ZG) and Na-exchanged ZTs (NaZF, NaZM, and NaZG) and in blank samples (without ZTs, B). Standard deviation within brackets (three replicates). Different letters (a,b,c,d,e) indicate significant differences obtained from ANOVA and Tukey HSD tests. * Electrical conductivity is reported in mS cm−1.
Table A1.
Chemical composition of the studied pig-slurry (PS) before and after the first treatment cycle with natural ZTs (ZF, ZM, and ZG) and Na-exchanged ZTs (NaZF, NaZM, and NaZG) and in blank samples (without ZTs, B). Standard deviation within brackets (three replicates). Different letters (a,b,c,d,e) indicate significant differences obtained from ANOVA and Tukey HSD tests. * Electrical conductivity is reported in mS cm−1.
| Parameter | Initial PS | B | ZF | ZM | ZG | NaZF | NaZM | NaZG |
|---|---|---|---|---|---|---|---|---|
| pH | 7.14 (0.03) a | 7.25 (0.02) b | 7.18 (0.02) a | 7.18 (0.05) a | 7.13 (0.01) a | 7.17 (0.01) a | 7.15 (0.03) a | 7.16 (0.01) a |
| EC * | 34.1 (0.5) e | 32.7 (1.4) d | 31.8 (0.7) b,c,d | 30.7 (0.3) a,b,c | 31.9 (0.2) c,d | 31.4 (0.3) a,b,c,d | 30.0 (0.2) a | 31.0 (0.2) c,d |
| Major Elements mg L−1 | ||||||||
| NH4 | 3690 (200) b | 3670 (270) b | 3290 (150) a,b | 3210 (170) a | 3290 (190) a,b | 3070 (110) a | 2910 (110) a | 3030 (80) a |
| Na | 304 (8) a | 326 (6) a,b | 351 (5) b,c | 361 (10) c | 372 (10) c | 1017 (35) d | 1165 (15) e | 958 (57) d |
| Mg | 65.0 (2.5) a | 33.3 (0.7) a,b | 38.1 (0.4) b | 40.2 (1.5) b | 39.8 (2.3) b | 39.9 (1.8) b | 38.5 (0.6) b | 35.1 (2.6) a,b |
| P | 215 (14) d | 167 (4) b,c | 142 (10) a,b,c | 140 (8) a,b | 139 (7) a | 169 (12) c | 155 (8) a,b,c | 144 (12) a,b,c |
| K | 1691 (11) b,c | 1775 (31) c | 1780 (89) c | 1799 (58) c | 1847 (44) d | 1433 (59) a | 1419 (26) a | 1585 (29) b |
| Ca | 170 (26) a | 199 (19) a | 333 (6) b,c | 351 (27) c | 327 (16) b,c | 288 (18) b | 319 (6) b,c | 282 (29) b |
| Heavy Metals µg L−1 | ||||||||
| Ti | 763 (46) c,d | 611 (17) a,b | 803 (58) d | 689 (54) b,c | 595 (19) a,b | 688 (11) b,c | 593 (25) a,b | 528 (25) a |
| V | 24.0 (1.8) a | 25.8 (1.1) a,b | 44.2 (1.7) c,d | 36.6 (0.8) b | 28.7 (1.0) a | 46.5 (3.8) d | 39.0 (2.9) b,c | 31.1 (1.4) a,b |
| Cr | 61.6 (3) a | 74.5 (8.0) a | 71.4 (5.0) a | 65.7 (12.7) a | 74.8 (5.4) a | 76.5 (9.1) a | 75.7 (5.7) a | 70.5 (7.9) a |
| Mn | 1044 (332) a | 1088 (100) a,b | 1432 (135) a,b | 1426 (356) a,b | 1333 (19) a,b | 1606 (102) b | 1521 (113) a,b | 1072 (119) a,b |
| Fe | 4678 (763) a | 5846 (475) a,b | 7546 (430) c | 6865 (751) b | 6387 (85) a,b,c | 6872 (89) b,c | 6541 (581) b,c | 5888 (329) a,b |
| Ni | 170 (12) a | 231 (62) a,b | 296 (48) b | 259 (99) b | 257 (10) b | 256 (11) b | 269 (15) b | 239 (20) b |
| Cu | 815 (265) a | 1189 (98) b | 1241 (63) b | 1142 (169) a,b | 1184 (32) b | 1351 (43) b | 1366 (69) b | 1271 (115) b |
| Zn | 2138 (891) a | 3155 (344) a,b | 3444 (317) b | 3010 (522) a,b | 3101 (53) a,b | 3739 (170) b | 3407 (243) b | 3113 (198) a,b |
| Ga | 6.33 (0.29) a | 5.92 (1.42) a | 24.3 (1.0) d | 26.6 (1.0) e | 20.2 (1.0) c | 23.0 (0.6) c,d | 22.2 (1.5) c,d | 15.3 (1.9) b |
| U | 2.00 (0.62) a | 2.08 (0.14) a | 7.00 (0.25) b,c | 5.33 (0.58) b | 3.00 (0.50) a | 8.17 (0.88) c,d | 9.27 (0.93) d | 7.83 (0.38) c,d |
| Other Trace Elements µg L−1 | ||||||||
| Li | 20.6 (3.7) a | 19.4 (1.8) a | 51.7 (4.9) d | 35.8 (1.2) c | 23.7 (1.2) a,b | 28.4 (0.8) b | 26.4 (0.5) b | 25.3 (1.0) b |
| Rb | 1205 (22) a | 1222 (19) a | 4946 (148) f | 4664 (105) f | 3663 (189) e | 2515 (11) d | 2185 (19) c | 1841 (98) b |
| Sr | 429 (13) a | 446 (26) a | 5843 (284) d | 8451 (396) f | 7098 (726) e | 3317 (182) b | 4508 (110) c | 4440 (280) c |
| Ba | 178 (12) a | 164 (14) a | 707 (37) d | 797 (37) e | 588 (46) c | 608 (30) c | 553 (13) c | 405 (29) b |

Table A2.
Chemical composition of the studied pig-slurry (PS) after the second treatment cycle with natural ZTs (ZF, ZM, and ZG) and Na-exchanged ZTs (NaZF, NaZM, and NaZG) and in blank samples (without ZTs, B). Standard deviation within brackets (three replicates). Different letters (a,b,c,d,e,f) indicate significant differences obtained from ANOVA and Tukey HSD tests. * Electrical conductivity is reported in mS cm−1.
Table A2.
Chemical composition of the studied pig-slurry (PS) after the second treatment cycle with natural ZTs (ZF, ZM, and ZG) and Na-exchanged ZTs (NaZF, NaZM, and NaZG) and in blank samples (without ZTs, B). Standard deviation within brackets (three replicates). Different letters (a,b,c,d,e,f) indicate significant differences obtained from ANOVA and Tukey HSD tests. * Electrical conductivity is reported in mS cm−1.
| Parameter | B | ZF | ZM | ZG | NaZF | NaZM | NaZG |
|---|---|---|---|---|---|---|---|
| pH | 7.75 (0.01) d | 7.53 (0.02) a,b | 7.56 (0.02) a,b | 7.51 (0.01) a | 7.63 (0.04) c | 7.60 (0.02) b,c | 7.57 (0.02) b |
| EC * | 32.2 (0.3) b | 29.6 (0.6) a | 28.9 (0.7) a | 29.2 (0.2) a | 29.3 (0.1) a | 28.8 (0.3) a | 29.0 (0.9) a |
| Major Elements mg L−1 | |||||||
| NH4 | 3490 (190) d | 2770 (70) c | 2710 (130) b,c | 2720 (110) b,c | 2400 (90) a,b | 2190 (26) a | 2330 (28) a |
| Na | 343 (17) a | 401 (5) a | 468 (130) a | 405 (11) a | 1759 (64) c | 1000 (70) b | 1603 (105) c |
| Mg | 21.3 (7.2) a | 72.7 (19) c | 48.4 (6.8) b,c | 50.3 (4.5) b,c | 37.8 (3.6) b | 31.6 (2.2) a,b | 38.4 (2.6) b |
| P | 148 (21) b | 133 (42) a,b | 91.9 (5.9) a | 105 (15) a,b | 110 (8) a,b | 118 (8) a,b | 77.3 (14) a |
| K | 2144 (83) d | 2101 (28) d | 2144 (52) d | 2126 (54) d | 888 (39) a | 1317 (93) c | 1056 (42) b |
| Ca | 141 (10) a | 350 (43) d | 368 (56) d | 284 (26) c,d | 243 (7) c | 186 (13) a,b | 238 (24) b,c |
| Heavy Metals µg L−1 | |||||||
| Ti | 510 (58) c | 731 (89) d | 388 (46) a,b | 419 (52) a,b | 475 (42) b | 427 (30) a,b | 308 (61) a |
| V | 21.9 (0.9) a | 48.4 (0.1) d | 38.6 (3.3) b,c | 29.9 (3.6) a | 46.5 (2.7) c,d | 27.2 (1.9) a | 28.8 (5.4) a |
| Cr | 81.5 (4.7) b | 60.9 (6.0) a | 56.5 (6.1) a | 66.6 (7.3) a | 61.3 (6.0) a | 83.3 (5.9) b | 60.0 (3.5) a |
| Mn | 626 (80) a | 910 (87) a | 542 (158) a | 764 (92) a | 824 (361) a | 948 (67) a | 741 (206) a |
| Fe | 5244 (65) a | 6223 (273) a | 4667 (666) a | 5020 (241) a | 5350 (794) a | 5202 (366) a | 4646 (735) a |
| Ni | 180 (14) a | 277 (14) b,c | 300 (32) c | 236 (14) b | 188 (7) a | 167 (12) a | 178 (7) a |
| Cu | 1102 (33) b | 826 (80) a,b | 703 (93) a | 916 (47) a,b | 925 (77) b | 1094 (77) b | 911 (192) a,b |
| Zn | 2688 (36) a,b | 2315 (293) a,b | 1785 (261) a | 2452 (17) a,b | 3033 (456) a,b | 3311 (233) a,b | 5045 (2993) b |
| Ga | 3.75 (0.87) a | 27.9 (0.4) d | 32.8 (2.1) e | 25.5 (3.5) c,d | 21.1 (1.0) c | 10.1 (0.7) b | 11.9 (1.3) b |
| U | 2.17 (0.14) a | 16.8 (0.5) d | 10.9 (1.3) c | 7.0 (1.3) b | 17.9 (2.1) d | 7.3 (0.5) b | 11.2 (1.2) c |
| Other Trace Elements µg L−1 | |||||||
| Li | 20.0 (1.0) a | 86.7 (0.3) f | 58.7 (1.4) e | 33.9 (1.7) c | 39.1 (0.2) d | 26.0 (1.8) b | 28.0 (2.2) b |
| Rb | 1150 (42) a | 7368 (40) f | 6970 (183) f | 5487 (337) e | 2736 (64) d | 1668 (117) b | 2128 (80) c |
| Sr | 357 (27) a | 10518 (1020) d | 14813 (1765) e | 10838 (626) d | 5375 (147) c | 3192 (224) b | 6638 (734) c |
| Ba | 131 (6) a | 734 (6) c | 881 (29) d | 687 (41) c | 716 (27) c | 372 (26) b | 430 (54) b |

Table A3.
Chemical composition of the studied pig-slurry (PS) after the third treatment cycle with natural ZTs (ZF, ZM, and ZG) and Na-exchanged ZTs (NaZF, NaZM, and NaZG) and in blank samples (without ZTs, B). Standard deviation within brackets (three replicates). Different letters (a,b,c,d,e,f) indicate significant differences obtained from ANOVA and Tukey HSD tests. * Electrical conductivity is reported in mS cm−1.
Table A3.
Chemical composition of the studied pig-slurry (PS) after the third treatment cycle with natural ZTs (ZF, ZM, and ZG) and Na-exchanged ZTs (NaZF, NaZM, and NaZG) and in blank samples (without ZTs, B). Standard deviation within brackets (three replicates). Different letters (a,b,c,d,e,f) indicate significant differences obtained from ANOVA and Tukey HSD tests. * Electrical conductivity is reported in mS cm−1.
| Parameter | B | ZF | ZM | ZG | NaZF | NaZM | NaZG |
|---|---|---|---|---|---|---|---|
| pH | 8.12 (0.04) c | 7.82 (0.05) a,b,c | 7.77 (0.01) a | 7.76 (0.02) a | 7.90 (0.02) c | 7.88 (0.03) b,c | 7.82 (0.02) a,b |
| EC * | 31.3 (0.2) c | 28.0 (0.7) b | 27.7 (0.5) a,b | 27.7 (0.2) a,b | 27.2 (0.4) a,b | 26.7 (0.4) a | 28.0 (0.1) b |
| Major Elements mg L−1 | |||||||
| NH4 | 3660 (60) d | 2440 (40) c | 2380 (90) c | 2330 (80) c | 1750 (80) b | 1510 (80) a | 1800 (100) b |
| Na | 365 (17) a | 434 (13) b | 435 (12) b | 472 (12) b | 2383 (136) c,d | 2537 (111) d | 2004 (88) c |
| Mg | 17.3 (1.6) a | 60.5 (2.7) b,c | 60.2 (4.3) b,c | 69.1 (9.8) c | 43.6 (5.0) b | 51.2 (12.4) b,c | 57.2 (11.3) b,c |
| P | 124 (4) b | 64.1 (11.4) a | 75.9 (7.1) a | 81.6 (13.5) a,b | 86.5 (17.5) a,b | 82.3 (21.3) a,b | 103 (31) a,b |
| K | 1911 (41) d | 1787 (43) c | 1778 (35) c | 1848 (35) c,d | 516 (37.6) a | 426 (17.8) a | 745 (70.7) b |
| Ca | 163 (26) a | 367 (68) b | 298 (39) b | 321 (38) b | 310 (47) b | 313 (46) b | 363 (21) b |
| Heavy Metals µg L−1 | |||||||
| Ti | 434 (15) a,b | 796 (221) c | 598 (12) b,c | 363 (43) a | 453 (55) a,b | 363 (58) a | 400 (95) a,b |
| V | 18.9 (0.9) a | 52.1 (5.5) d,e | 42.8 (2.3) c,d | 27.3 (2.4) a | 53.2 (4.4) e | 41.1 (3.4) c | 32.3 (3.5) b,c |
| Cr | 51.4 (3.2) a | 52.9 (3.9) a | 49.6 (1.0) a | 48.3 (3.0) a | 50.2 (4.8) a | 43.9 (6.0) a | 42.7 (4.4) a |
| Mn | 771 (129) a | 1152 (247) a | 911 (49) a | 719 (168) a | 781 (180) a | 811 (229) a | 1021 (101) a |
| Fe | 6260 (534) a | 9047 (2028) a | 7626 (534) a | 4613 (369) a | 5358 (134) a | 4529 (561) a | 4779 (590) a |
| Ni | 150 (34) a | 208 (25) a | 190 (22) a | 202 (36) a | 191 (18) a | 172 (23) a | 197 (10) a |
| Cu | 1251 (63) b | 825 (145) a | 832 (40) a | 743 (79) a | 856 (190) a | 831 (122) a | 951 (129) a,b |
| Zn | 4438 (1339) b | 2596 (423) a | 2534 (14) a | 2034 (71) a | 2854 (735) a,b | 2148 (284) a | 2439 (334) a |
| Ga | 4.50 (0.75) a | 35.7 (3.3) d | 34.9 (1.6) c,d | 28.3 (1.6) b,c | 27.6 (3.9) b | 25.5 (2.2) b | 21.9 (2.5) b |
| U | 0.83 (0.63) a | 15.7 (1.4) d | 11.4 (0.9) c | 7.00 (1.09) b | 19.5 (0.9) e | 19.7 (2.1) e | 17.4 (1.4) d |
| Other trace Elements µg L−1 | |||||||
| Li | 19.2 (3.5) a | 110 (2.6) f | 71.4 (1.6) e | 38.1 (1.7) b,c | 49.9 (2.6) d | 41.2 (1.2) c | 34.5 (2.1) b |
| Rb | 1164 (32) a | 8141 (117) f | 7315 (100) e | 5949 (166) d | 2229 (45) c | 1896 (42) b | 2034 (68) b,c |
| Sr | 404 (76) a,b | 12328 (2082) c | 14143 (1703) c | 14544 (1479) c | 7106 (993) b | 9230 (1088) b | 11003 (624) b,c |
| Ba | 163 (33) a | 1169 (113) d | 1167 (77) d | 931 (62) c | 854 (93) c | 725 (41) b,c | 632 (86) b |
References
- Steinfeld, H.; Food and Agriculture Organization of the United Nations; Livestock, E.; Firm, D. Livestock’s Long Shadow: Environmental Issues and Options; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006; ISBN 9251055718. [Google Scholar]
- Longhurst, R.D.; Roberts, A.H.C.; O’Connor, M.B. Farm dairy effluent: A review of published data on chemical and physical characteristics in New Zealand. N. Z. J. Agric. Res. 2000, 43, 7–14. [Google Scholar] [CrossRef]
- Pagliari, P.H.; Laboski, C.A.M. Investigation of the Inorganic and Organic Phosphorus Forms in Animal Manure. J. Environ. Qual. 2012, 41, 901–910. [Google Scholar] [CrossRef]
- Bolan, N.S.; Khan, M.A.; Donaldson, J.; Adriano, D.C.; Matthew, C. Distribution and bioavailability of copper in farm effluent. Sci. Total Environ. 2003, 309, 225–236. [Google Scholar] [CrossRef]
- Hickey, C.W.; Quinn, J.M.; Davies-Colley, R.J. Effluent Characteristics of Dairy Shed Oxidation Ponds and Their Potential Impacts on Rivers. N. Z. J. Mar. Freshw. Res. 1989, 23, 569–584. [Google Scholar] [CrossRef]
- Hooda, P.S.; Edwards, A.C.; Anderson, H.A.; Miller, A. A review of water quality concerns in livestock farming areas. Sci. Total Environ. 2000, 250, 143–167. [Google Scholar] [CrossRef]
- Martinez, J.; Guiziou, F.; Peu, P.; Gueutier, V. Influence of treatment techniques for pig slurry on methane emissions during subsequent storage. Biosyst. Eng. 2003, 85, 347–354. [Google Scholar] [CrossRef]
- Leytem, A.B.; Dungan, R.S.; Bjorneberg, D.L.; Koehn, A.C. Emissions of Ammonia, Methane, Carbon Dioxide, and Nitrous Oxide from Dairy Cattle Housing and Manure Management Systems. J. Environ. Qual. 2011, 40, 1383–1394. [Google Scholar] [CrossRef] [PubMed]
- Jongbloed, A.W.; Lenis, N.P. Environmental concerns about animal manure. J. Anim. Sci. 1998, 76, 2641–2648. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.S.; Horne, D.J.; Currie, L.D. Growth and chemical composition of legume-based pasture irrigated with dairy farm effluent. N. Z. J. Agric. Res. 2004, 47, 85–93. [Google Scholar] [CrossRef]
- Guan, T.Y.; Holley, R.A. Pathogen Survival in Swine Manure Environments and Transmission of Human Enteric Illness—A Review. J. Environ. Qual. 2003, 32, 383–392. [Google Scholar] [CrossRef]
- Ross, C.; Donnison, A. Campylobacter and farm dairy effluent irrigation. N. Z. J. Agric. Res. 2003, 46, 255–262. [Google Scholar] [CrossRef]
- Martinez, J.; Dabert, P.; Barrington, S.; Burton, C. Livestock waste treatment systems for environmental quality, food safety, and sustainability. Bioresour. Technol. 2009, 100, 5527–5536. [Google Scholar] [CrossRef] [PubMed]
- Bernet, N.; Béline, F. Challenges and innovations on biological treatment of livestock effluents. Bioresour. Technol. 2009, 100, 5431–5436. [Google Scholar] [CrossRef]
- Faccini, B.; Di Giuseppe, D.; Malferrari, D.; Coltorti, M.; Abbondanzi, F.; Campisi, T.; Laurora, A.; Passaglia, E. Ammonium-exchanged zeolitite preparation for agricultural uses: From laboratory tests to large-scale application in ZeoLIFE project prototype. Period. Mineral. 2015, 84, 303–321. [Google Scholar]
- De Smedt, C.; Someus, E.; Spanoghe, P. Potential and actual uses of zeolites in crop protection. Pest Manag. Sci. 2015, 71, 1355–1367. [Google Scholar] [CrossRef] [PubMed]
- Passaglia, E. Zeoliti Naturali, Zeolititi e Loro Applicazioni; Arvan: Mira-Venezia, Italy, 2008; ISBN 9788887801194. [Google Scholar]
- Leyva-Ramos, R.; Monsivais-Rocha, J.E.; Aragon-Piña, A.; Berber-Mendoza, M.S.; Guerrero-Coronado, R.M.; Alonso-Davila, P.; Mendoza-Barron, J. Removal of ammonium from aqueous solution by ion exchange on natural and modified chabazite. J. Environ. Manage. 2010, 91, 2662–2668. [Google Scholar] [CrossRef]
- Inglezakis, V.J. The concept of “capacity” in zeolite ion-exchange systems. J. Colloid Interface Sci. 2005, 281, 68–79. [Google Scholar] [CrossRef]
- Lei, L.; Li, X.; Zhang, X. Ammonium removal from aqueous solutions using microwave-treated natural Chinese zeolite. Sep. Purif. Technol. 2008, 58, 359–366. [Google Scholar] [CrossRef]
- Lin, L.; Lei, Z.; Wang, L.; Liu, X.; Zhang, Y.; Wan, C.; Lee, D.-J.; Tay, J.H. Adsorption mechanisms of high-levels of ammonium onto natural and NaCl-modified zeolites. Sep. Purif. Technol. 2013, 103, 15–20. [Google Scholar] [CrossRef]
- Canli, M.; Abali, Y.; Bayca, S.U. Removal of methylene blue by natural and ca and k-exchanged zeolite treated with hydrogen peroxide. Physicochem. Probl. Miner. Process. 2013, 49, 481–496. [Google Scholar]
- Colombani, N.; Di Giuseppe, D.; Faccini, B.; Ferretti, G.; Mastrocicco, M.; Coltorti, M. Estimated Water Savings in an Agricultural Field Amended With Natural Zeolites. Environ. Process. 2016, 3, 617–628. [Google Scholar] [CrossRef]
- Di Giuseppe, D.; Ferretti, G.; Faccini, B.; Blasi, E.; Passeri, N.; Bianchini, G.; Coltorti, M. Is it possible to cultivate corn in a sustainable way using a quarry waste? Period. Mineral. 2016, 85, 179–183. [Google Scholar]
- Ferretti, G.; Di Giuseppe, D.; Natali, C.; Faccini, B.; Bianchini, G.; Coltorti, M. C-N elemental and isotopic investigation in agricultural soils: Insights on the effects of zeolitite amendments. Chem. Erde-Geochem. 2017, 77, 45–52. [Google Scholar] [CrossRef]
- Eslami, M.; Khorassani, R.; Coltorti, M.; Malferrari, D.; Faccini, B.; Ferretti, G.; Di Giuseppe, D.; Fotovat, A.; Halajnia, A. Leaching behaviour of a sandy soil amended with natural and NH4+ and K+ saturated clinoptilolite and chabazite. Arch. Agron. Soil Sci. 2018, 64, 1142–1151. [Google Scholar] [CrossRef]
- Gholamhoseini, M.; Ghalavand, A.; Khodaei-Joghan, A.; Dolatabadian, A.; Zakikhani, H.; Farmanbar, E. Zeolite-amended cattle manure effects on sunflower yield, seed quality, water use efficiency and nutrient leaching. Soil Tillage Res. 2013, 126, 193–202. [Google Scholar] [CrossRef]
- Colombani, N.; Di Giuseppe, D.; Faccini, B.; Ferretti, G.; Mastrocicco, M.; Coltorti, M. Inferring the interconnections between surface water bodies, tile-drains and an unconfined aquifer-aquitard system: A case study. J. Hydrol. 2016, 537, 86–95. [Google Scholar] [CrossRef]
- Reháková, M.; Čuvanová, S.; Dzivák, M.; Rimár, J.; Gaval’Ová, Z. Agricultural and agrochemical uses of natural zeolite of the clinoptilolite type. Curr. Opin. Solid State Mater. Sci. 2004, 8, 397–404. [Google Scholar] [CrossRef]
- Ferretti, G.; Keiblinger, K.M.; Di Giuseppe, D.; Faccini, B.; Colombani, N.; Zechmeister-Boltenstern, S.; Coltorti, M.; Mastrocicco, M. Short-Term Response of Soil Microbial Biomass to Different Chabazite Zeolite Amendments. Pedosphere 2018, 28, 277–287. [Google Scholar] [CrossRef]
- Ferretti, G.; Faccini, B.; Vittori Antisari, L.; Di Giuseppe, D.; Coltorti, M. 15N Natural Abundance, Nitrogen and Carbon Pools in Soil-Sorghum System Amended with Natural and NH4+-Enriched Zeolitites. Appl. Sci. 2019, 9, 4524. [Google Scholar] [CrossRef]
- Zaman, M.; Nguyen, M.L.; Matheson, F.; Blennerhassett, J.D.; Quin, B.F. Can soil amendments (zeolite or lime) shift the balance between nitrous oxide and dinitrogen emissions from pasture and wetland soils receiving urine or urea-N? Aust. J. Soil Res. 2007, 45, 543–553. [Google Scholar] [CrossRef]
- Bundan, L.; Majid, N.M.A.; Ahmed, O.H.; Jiwan, M.; Kundat, F.R. Ammonia volatilization from urea at different levels of zeolite. Int. J. Phys. Sci. 2011, 6, 7717–7720. [Google Scholar]
- Bernardi, A.C.C.; Mota, E.P.; Cardosa, R.D. Ammonia Volatilization from Soil, Dry- Matter Yield, and Nitrogen Levels of Italian Ryegrass. Commun. Soil Sci. Plant Anal. 2014, 45, 153–162. [Google Scholar] [CrossRef]
- Kučić, D.; Kopčić, N.; Briški, F. Zeolite and potting soil sorption of CO2 and NH3 evolved during co-composting of grape and tobacco waste. Chem. Pap. 2013, 67, 1172–1180. [Google Scholar] [CrossRef]
- Ferretti, G.; Keiblinger, K.M.; Zimmermann, M.; Di Giuseppe, D.; Faccini, B.; Colombani, N.; Mentler, A.; Zechmeister-Boltenstern, S.; Coltorti, M.; Mastrocicco, M. High resolution short-term investigation of soil CO2, N2O, NOx and NH3 emissions after different chabazite zeolite amendments. Appl. Soil Ecol. 2017, 119, 138–144. [Google Scholar] [CrossRef]
- Faccini, B.; Di Giuseppe, D.; Ferretti, G.; Coltorti, M.; Colombani, N.; Mastrocicco, M. Natural and NH4+-enriched zeolitite amendment effects on nitrate leaching from a reclaimed agricultural soil (Ferrara Province, Italy). Nutr. Cycl. Agroecosyst. 2018, 110, 327–341. [Google Scholar] [CrossRef]
- Vezzoli, L.; Conticelli, S.; Innocenti, F.; Landi, P.; Manetti, P.; Palladino, D.M.; Trigilla, R. Stratigraphy of the Latera Volcanic Complex: Proposals for a new nomenclature. Period. Mineral. 1987, 56, 89–110. [Google Scholar]
- Passaglia, E.; Vezzalini, G. Crystal chemistry of diagenetic zeolites in volcanoclastic deposits of Italy. Contrib. Mineral. Petrol. 1985, 90, 190–198. [Google Scholar] [CrossRef]
- Malferrari, D.; Laurora, A.; Brigatti, M.F.; Coltorti, M.; Di Giuseppe, D.; Faccini, B.; Passaglia, E.; Vezzalini, M.G. Open-field experimentation of an innovative and integrated zeolitite cycle: Project definition and material characterization. Rend. Lincei 2013, 24, 141–150. [Google Scholar] [CrossRef]
- Huang, H.; Xiao, X.; Yan, B.; Yang, L. Ammonium removal from aqueous solutions by using natural Chinese (Chende) zeolite as adsorbent. J. Hazard. Mater. 2010, 175, 247–252. [Google Scholar] [CrossRef]
- Luedecke, C.; Hermanowicz, S.; Jenkins, D. Precipitation of ferric phosphate in activated sludge: A chemical model and its verification. Water Pollut. Res. Control Bright. 1988, 1988, 325–337. [Google Scholar]
- Yan, H.; Shih, K. Effects of calcium and ferric ions on struvite precipitation: A new assessment based on quantitative X-ray diffraction analysis. Water Res. 2016, 95, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Paul, J.W.; Beauchamp, E.G. Relationship between volatile fatty acids, total ammonia, and pH in manure slurries. Biol. Wastes 1989, 29, 313–318. [Google Scholar] [CrossRef]
- Chan, M.T.; Selvam, A.; Wong, J.W.C. Reducing nitrogen loss and salinity during “struvite” food waste composting by zeolite amendment. Bioresour. Technol. 2016, 200, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Lebedynets, M.; Sprynskyy, M.; Sakhnyuk, I.; Zbytniewski, R.; Golembiewski, R.; Buszewski, B. Adsorption of Ammonium Ions onto a Natural Zeolite: Transcarpathian Clinoptilolite. Adsorpt. Sci. Technol. 2004, 22, 731–741. [Google Scholar] [CrossRef]
- Kithome, M.; Paul, J.W.; Lavkulich, L.M.; Bomke, A.A. Kinetics of Ammonium Adsorption and Desorption by the Natural Zeolite Clinoptilolite. Soil Sci. Soc. Am. J. 1998, 62, 622–629. [Google Scholar] [CrossRef]
- Velthof, G.; Kuikman, P.; Oenema, O. Nitrous oxide emission from animal manures applied to soil under controlled conditions. Biol. Fertil. Soils 2003, 37, 221–230. [Google Scholar] [CrossRef]
- Jha, V.K.; Hayashi, S. Modification on natural clinoptilolite zeolite for its NH4+ retention capacity. J. Hazard. Mater. 2009, 169, 29–35. [Google Scholar] [CrossRef]
- Kotoulas, A.; Agathou, D.; Triantaphyllidou, I.; Tatoulis, T.; Akratos, C.; Tekerlekopoulou, A.; Vayenas, D. Zeolite as a Potential Medium for Ammonium Recovery and Second Cheese Whey Treatment. Water 2019, 11, 136. [Google Scholar] [CrossRef]
- Nguyen, M.L.; Tanner, C.C. Ammonium removal from wastewaters using natural New Zealand zeolites. N. Z. J. Agric. Res. 1998, 41, 427–446. [Google Scholar] [CrossRef]
- Leyva-Ramos, R.; Aguilar-Armenta, G.; Gonzalez-Gutierrez, L.V.; Guerrero-Coronado, R.M.; Mendoza-Barron, J. Ammonia exchange on clinoptilolite from mineral deposits located in Mexico. J. Chem. Technol. Biotechnol. 2004, 79, 651–657. [Google Scholar] [CrossRef]
- Cheng, Q.; Li, H.; Xu, Y.; Chen, S.; Liao, Y.; Deng, F.; Li, J. Study on the adsorption of nitrogen and phosphorus from biogas slurry by NaCl-modified zeolite. PLoS ONE 2017, 12, e0176109. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, J.; Jiang, Y. Crystallization and precipitation of phosphate from swine wastewater by magnesium metal corrosion. Sci. Rep. 2015, 5, 16601. [Google Scholar] [CrossRef] [PubMed]
- Ouki, S.K.; Kavannagh, M. Performance of natural zeolites for the treatment of mixed metal-contaminated effluents. Waste Manag. Res. 1997, 15, 383–394. [Google Scholar] [CrossRef]
- Malamis, S.; Katsou, E. A review on zinc and nickel adsorption on natural and modified zeolite, bentonite and vermiculite: Examination of process parameters, kinetics and isotherms. J. Hazard. Mater. 2013, 252–253, 428–461. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.; El-Bishtawi, R. Removal of Lead and Nickel Ions Using Zeolite Tuff. J. Chem. Technol. Biotechnol. 1997, 69, 27–34. [Google Scholar] [CrossRef]
- Pansini, M.; Colella, C.; De Gennaro, M. Chromium removal from water by ion exchange using zeolite. Desalination 1991, 83, 145–157. [Google Scholar] [CrossRef]
- Barrer, R.M.; Davies, J.A.; Rees, L.V.C. Thermodynamics and thermochemistry of cation exchange in chabazite. J. Inorg. Nucl. Chem. 1969, 31, 219–232. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).