Tracking Sources and Fate of Groundwater Nitrate in Kisumu City and Kano Plains, Kenya
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Water Sampling and Analysis
3. Results and Discussion
3.1. Hydrochemistry
3.2. Spatial Groundwater NO3− Distribution and Its Controlling Factors
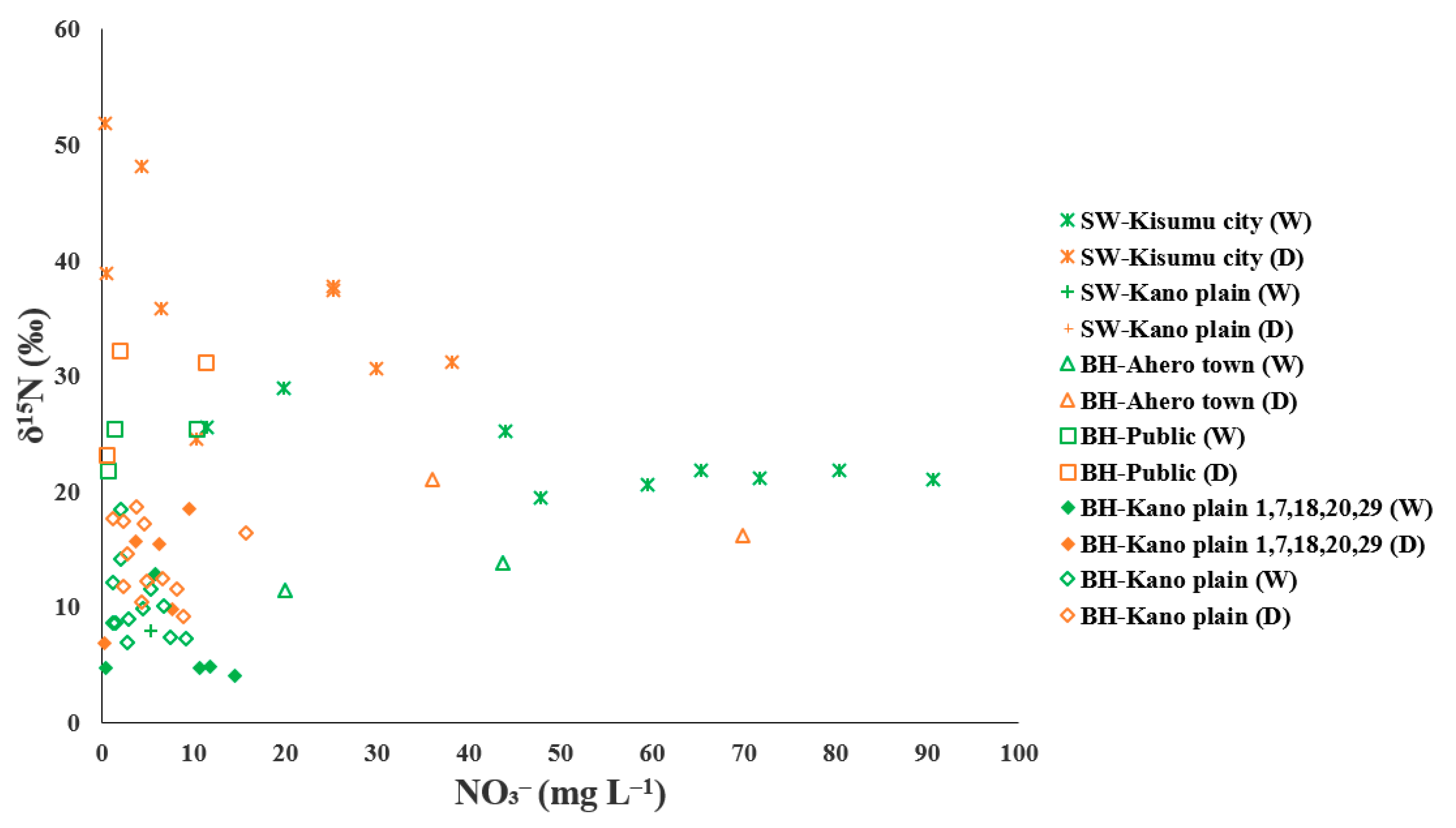
3.3. Use of Multi Isotope and Hydro-Chemical Methods to Track Sources of Groundwater Nitrate Contamination and Removal
4. Conclusions and Recommendations
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- White, R.; Turpie, J.; Gwyneth, L. Greening Africa’s Cities: Enhancing the Relationship between Urbanization, Environmental Assets and Ecosystem Services; World Bank: Washington, DC, USA, 2016; pp. 1–73. [Google Scholar]
- Kendall, C.; Elliott, E.M.; Wankel, S.D. Tracing Anthropogenic Inputs of Nitrogen to Ecosystems. In Stable Isotopes in Ecology and Environmental Science; Michener, R., Lajtha, K., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2007; pp. 375–449. [Google Scholar]
- Schullehner, J.; Hansen, B.; Thygesen, M.; Pedersen, C.B.; Sigsgaard, T. Nitrate in drinking water and colorectal cancer risk: A nationwide population-based cohort study. Int. J. Cancer 2018, 143, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Kenya National Bureau of Statistics (KNBS) 2019, Kenya Population and Housing Census Volume I: Population By County and Sub-County. Available online: http://www.knbs.or.ke (accessed on 7 January 2020).
- Wright, J.A.; Cronin, A.; Okotto-Okotto, J.; Yang, H.; Pedley, S.; Gundry, S.W. A spatial analysis of pit latrine density and groundwater source contamination. Environ. Monit. Assess. 2013, 185, 4261–4272. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, T.; Toure, B.; Li, F. Protect lake victoria through green economy, public participation and good governance. Environ. Sci. Technol. 2012, 46, 10483–10484. [Google Scholar] [CrossRef] [PubMed]
- Okotto-Okotto, J.; Okotto, L.; Price, H.; Pedley, S.; Wright, J. A longitudinal study of long-term change in contamination hazards and shallow well quality in two neighbourhoods of Kisumu, Kenya. Int. J. Environ. Res. Public Health 2015, 12, 4275–4291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aravena, R.; Evans, M.L.; Cherry, J.A. Stable Isotopes of Oxygen and Nitrogen in Source Identification of Nitrate from Septic Systems. Groundwater 1993, 31, 180–186. [Google Scholar] [CrossRef]
- Mengis, M.; Schill, S.; Harris, M.; English, M.; Aravena, R.; Elgood, R.; Maclean, A. Multiple geochemical and isotopic approaches for assesing groundwater nitrate elimination in a riparian zone. Groundwater 1999, 37, 448–457. [Google Scholar] [CrossRef]
- Böttcher, J.; Strebel, O.; Voerkelius, S.; Schmidt, H.L. Using isotope fractionation of nitrate-nitrogen and nitrate-oxygen for evaluation of microbial denitrification in a sandy aquifer. J. Hydrol. 1990, 114, 413–424. [Google Scholar] [CrossRef]
- Widory, D.; Petelet-Giraud, E.; Négrel, P.; Ladouche, B. Tracking the sources of nitrate in groundwater using coupled nitrogen and boron isotopes: A synthesis. Environ. Sci. Technol. 2005, 39, 539–548. [Google Scholar] [CrossRef]
- Widory, D.; Petelet-Giraud, E.; Brenot, A.; Bronders, J.; Tirez, K.; Boeckx, P. Improving the management of nitrate pollution in water by the use of isotope monitoring: the δ 15 N, δ 18 O and δ 11 B triptych. Isotopes Environ. Health Stud. 2013, 49, 29–47. [Google Scholar]
- Seiler, R.L. Combined use of 15N and 18O of nitrate and 11B to evaluate nitrate contamination in groundwater. Appl. Geochemistry 2005, 20, 1626–1636. [Google Scholar] [CrossRef]
- Xue, D.; De Baets, B.; Van Cleemput, O.; Hennessy, C.; Berglund, M.; Boeckx, P. Classification of Nitrate Polluting Activities through Clustering of Isotope Mixing Model Outputs. J. Environ. Qual. 2013, 42, 1486. [Google Scholar] [CrossRef]
- Juma, D.W.; Wang, H.; Li, F. Impacts of population growth and economic development on water quality of a lake: Case study of Lake Victoria Kenya water. Environ. Sci. Pollut. Res. 2014, 21, 5737–5746. [Google Scholar] [CrossRef]
- Mireri, C.; Atekyereza, P.; Kyessi, A.; Mushi, N. Environmental risks of urban agriculture in the Lake Victoria drainage basin: A case of Kisumu municipality, Kenya. Habitat Int. 2007, 31, 375–386. [Google Scholar] [CrossRef] [Green Version]
- Olago, D.O. Constraints and solutions for groundwater development, supply and governance in urban areas in Kenya. Hydrogeol. J. 2019, 27, 1031–1050. [Google Scholar] [CrossRef] [Green Version]
- Oiro, S.O. The hydrogeology and groundwater quality assessment of Kisumu town area in Kenya. Master–s Thesis, Ghent University, Gent, Belgium, 2012. [Google Scholar]
- Xue, D.; Botte, J.; De Baets, B.; Accoe, F.; Nestler, A.; Taylor, P.; Van Cleemput, O.; Berglund, M.; Boeckx, P. Present limitations and future prospects of stable isotope methods for nitrate source identification in surface-and groundwater. Water Res. 2009, 43, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Casciotti, K.L.; Sigman, D.M.; Hastings, M.G.; Böhlke, J.K.; Hilkert, A. Measurement of the oxygen isotopic composition of nitrate in seawater and freshwater using the denitrifier method. Anal. Chem. 2002, 74, 4905–4912. [Google Scholar] [CrossRef]
- Sigman, D.M.; Casciotti, K.L.; Andreani, M.; Barford, C.; Galanter, M.; Böhlke, J.K. A bacterial method for the nitrogen isotopic analysis of nitrate in seawater and freshwater. Anal. Chem. 2001, 73, 4145–4153. [Google Scholar] [CrossRef]
- Tirez, K.; Brusten, W.; Widory, D.; Petelet, E.; Bregnot, A.; Xue, D.; Boeckx, P.; Bronders, J. Boron isotope ratio (δ11B) measurements in Water Framework Directive monitoring programs: Comparison between double focusing sector field ICP and thermal ionization mass spectrometry. J. Anal. At. Spectrom. 2010, 25, 964–974. [Google Scholar] [CrossRef] [Green Version]
- Gaillardet, J.; Allgre, C.J. Boron isotopic compositions of corals: Seawater or diagenesis record? Earth Planet. Sci. Lett. 2018, 136, 665–676. [Google Scholar]
- Ishikawa, T.; Nakamura, E. Suppression of boron volatilization from a hydrofluoric acid solution using a boron-mannitol complex. Anal. Chem. 1990, 62, 2612–2616. [Google Scholar] [CrossRef]
- Spivack, A.; Palmer, M.; Edmond, J. The sedimentary cycle of the boron isotopes. Geochim. Cosmochim. Acta 1987, 51, 1939–1949. [Google Scholar] [CrossRef]
- Spivack, A.J.; Edmond, J.M. Determination of Boron Isotope Ratios by Thermal Ionization Mass Spectrometry of the Dicesium Metaborate Cation. Anal. Chem. 1986, 58, 31. [Google Scholar] [CrossRef]
- Gonfiantini, R.; Tonarini, S.; Gröning, M.; Adorni-Braccesi, A.; Al-Ammar, A.S.; Astner, M.; Bächler, S.; Barnes, R.M.; Bassett, R.L.; Cocherie, A.; et al. Intercomparison of boron isotope and concentration measurements. Part II: Evaluation of results. Geostand. Newsl. 2003, 27, 41–57. [Google Scholar] [CrossRef]
- Allen, D.M.; Suchy, M. Geochemical evolution of groundwater on Saturna Island, British Columbia. Can. J. Earth Sci. 2001, 38, 1059–1080. [Google Scholar] [CrossRef]
- Barathi, M.; Kumar, A.S.K.; Rajesh, N. Impact of fluoride in potable water – An outlook on the existing defluoridation strategies and the road ahead. Coord. Chem. Rev. 2019, 387, 121–128. [Google Scholar] [CrossRef]
- Piper, A. A Graphical Procedure in the Geochemical Interpretation of Water Analysis. Trans. Am. Geophys. Union 1944, 25, 914–923. [Google Scholar] [CrossRef]
- Ako, A.A.; Shimada, J.; Hosono, T.; Kagabu, M.; Ayuk, A.R.; Nkeng, G.E.; Eyong, G.E.T.; Takounjou, A.L.F. Spring water quality and usability in the Mount Cameroon area revealed by hydrogeochemistry. Environ. Geochem. Health 2012, 34, 615–639. [Google Scholar] [CrossRef]
- Nyilitya, B.; Mureithi, M.S.; Boeckx, P. Tracking sources of excess nitrate discharge in Lake Victoria, Kenya for improved Nitrogen use efficiency in the catchment. In Proceedings of the 2016 International Nitrogen Initiative Conference, “Solutions to improve nitrogen effieciency for the world”, Melbourne, Australia, 4–8 December 2016. [Google Scholar]
- Shanyengana, E.S.; Seely, M.K.; Sanderson, R.D. Major-ion chemistry and ground-water salinization in ephemeral floodplains in some arid regions of Namibia. J. Arid Environ. 2004, 57, 211–223. [Google Scholar] [CrossRef]
- Kamtchueng, B.T.; Fantong, W.Y.; Wirmvem, M.J.; Tiodjio, R.E.; Takounjou, A.F.; Ndam Ngoupayou, J.R.; Kusakabe, M.; Zhang, J.; Ohba, T.; Tanyileke, G.; et al. Hydrogeochemistry and quality of surface water and groundwater in the vicinity of Lake Monoun, West Cameroon: approach from multivariate statistical analysis and stable isotopic characterization. Environ. Monit. Assess. 2016, 188, 524. [Google Scholar] [CrossRef]
- Mayer, B.; Bollwerk, S.M.; Mansfeldt, T.; Hütter, B.; Veizer, J. The oxygen isotope composition of nitrate generated by nitrification in acid forest floors. Geochim. Cosmochim. Acta 2001, 65, 2743–2756. [Google Scholar] [CrossRef]
- Mayer, B.; Van Breemen, N.; Howarth, R.W.; Seitzinger, S.; Billen, G.; Lajtha, K.; Nadelhoffer, K.J.; Van Dam, D.; Hetling, L.J.; Nosal, M.; et al. Sources of nitrate in rivers draining sixteen watersheds in the northeastern U.S.: Isotopic constraints. Biogeochemistry 2002, 57–58, 171–197. [Google Scholar] [CrossRef]
- Fukada, T.; Hiscock, K.M.; Dennis, P.F.; Grischek, T. A dual isotope approach to identify denitrification in groundwater at a river-bank infiltration site. Water Res. 2003, 37, 3070–3078. [Google Scholar] [CrossRef]
- Sacchi, E.; Acutis, M.; Bartoli, M.; Brenna, S.; Delconte, C.A.; Laini, A.; Pennisi, M. Origin and fate of nitrates in groundwater from the central Po plain: Insights from isotopic investigations. Appl. Geochem. 2013, 34, 164–180. [Google Scholar] [CrossRef]
- Altman, S.J.; Parizek, R.R. Dilution of Nonpoint-Source Nitrate in Groundwater. J. Environ. Qual. 1995, 24, 707–718. [Google Scholar] [CrossRef]
- Koba, K.; Tokuchi, N.; Wada, E.; Nakajima, T.; Iwatsubo, G. Intermittent denitrification: The application of a 15N natural abundance method to a forested ecosystem. Geochim. Cosmochim. Acta 1997, 61, 5043–5050. [Google Scholar] [CrossRef]
- Wyffels, S.; Boeckx, P.; Pynaert, K.; Verstraete, W.; Van Cleemput, O. Sustained nitrite accumulation in a membrane-assisted bioreactor (MBR) for the treatment of ammonium-rich wastewater. J. Chem. Technol. Biotechnol. 2003, 78, 412–419. [Google Scholar] [CrossRef]
- Philips, S.; Wyffels, S.; Sprengers, R.; Verstraete, W. Oxygen-limited autotrophic nitrification/denitrification by ammonia oxidisers enables upward motion towards more favourable conditions. Appl. Microbiol. Biotechnol. 2002, 59, 557–566. [Google Scholar]
- Pynaert, K.; Wyffels, S.; Sprengers, R.; Boeckx, P.; Van Cleemput, O.; Verstraete, W. Oxygen-limited nitrogen removal in a lab-scale rotating biological contactor treating an ammonium-rich wastewater. Water Sci. Technol. 2002, 45, 357–363. [Google Scholar] [CrossRef]
- Komor, S.C. Boron Contents and Isotopic Compositions of Hog Manure, Selected Fertilizers, and Water in Minnestoa. J. Environ. Qual. 1997, 26, 1212–1222. [Google Scholar] [CrossRef]
- Widory, D.; Kloppmann, W.; Chery, L.; Bonnin, J.; Rochdi, H.; Guinamant, J.L. Nitrate in groundwater: An isotopic multi-tracer approach. J. Contam. Hydrol. 2004, 72, 165–188. [Google Scholar] [CrossRef]







| Boreholes | WHO Limit | Number Exceeded WHO | Shallow Wells | WHO Limit | Number Exceeded WHO | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wet | Dry | p Value | Wet | Dry | p Value | |||||||||||||
| Min | Mean | Max | Min | Mean | Max | Min | Mean | Max | Min | Mean | Max | |||||||
| Physico-Chemical Parameters | ||||||||||||||||||
| pH (-) | 7 | 7.6 ± 0.7 | 10.1 | 6.1 | 7.4 ± 0.6 | 9.6 | 0.23 | 6.5–8.5 | 4 | 6.3 | 7.1 ± 0.7 | 8.6 | 6.2 | 7.0 ± 0.6 | 8.2 | 0.70 | 6.5–8.5 | 10 |
| Temp (°C) | 25.3 | 28.0 ± 2.2 | 37.6 | 25.7 | 28.1 ± 1.9 | 36 | 0.84 | - | 0 | 25.1 | 26.4 ± 0.9 | 28.2 | 24.5 | 26.2 ± 1.0 | 28.5 | 0.43 | - | 0 |
| EC (µS cm−1) | 295 | 1091 ± 390 | 2520 | 400 | 1052 ± 383 | 2562 | 0.71 | - | 0 | 248 | 821 ± 282 | 1420 | 290 | 785 ± 3.02 | 1427 | 0.74 | - | 0 |
| DO (mg O2 L−1) | 1.4 | 3.1 ± 1.4 | 6.6 | 2 | 3.9 ± 1.2 | 6.8 | 0.01 | - | 0 | 1.2 | 2.9 ± 1.5 | 6.2 | 1.6 | 4.1 ± 2.0 | 9.8 | 0.05 | - | 0 |
| Cl‒ (mg L−1) | 1.6 | 22 ± 21 | 80.1 | 0.1 | 29.4 ± 39.7 | 156 | 0.29 | 250 | 0 | 1.7 | 32.0 ± 22.8 | 75.5 | 2.8 | 41.5 ± 34.3 | 103 | 0.33 | 250 | 0 |
| SO42− (mg L−1) | 0.9 | 37 ± 41 | 212 | 0.4 | 49.1 ± 67.7 | 360 | 0.33 | 250 | 0 | 1.4 | 31 ± 17 | 56.3 | 9 | 37 ± 17.5 | 67.2 | 0.38 | 250 | 0 |
| NO3− (mg L−1) | <0.04 | 5.8 ± 8.8 | 43.7 | <0.04 | 6.7 ± 12.5 | 69.9 | 0.74 | 50 | 1 | <0.04 | 33.5 ± 32.4 | 90.6 | 0.04 | 10.9 ± 13.0 | 38.2 | 0.02 | 50 | 6 |
| NO2− (mg L−1) | <0.04 | 0.02 ± 0.01 | 0.06 | <0.04 | 1.4 ± 0.9 | 3.2 | <0.0001 | 0.2 | 27 | <0.04 | 0.04 ± 0.04 | 0.15 | <0.04 | 0.6 ± 0.8 | 2.2 | 0.006 | 0.2 | 6 |
| HCO3− (mg L−1) | 2.4 | 93 ± 46 | 167 | 20.2 | 98.5 ± 36.4 | 172 | 0.67 | 500 | 0 | 16.6 | 47 ± 38 | 109 | 4.9 | 57.2 ± 43.8 | 122 | 0.61 | 500 | 0 |
| Na+ (mg L−1) | 33.6 | 190 ± 92 | 452 | 29.8 | 186 ± 98 | 461 | 0.86 | 250 | 0 | 41 | 112 ± 7 5 | 311 | 31.1 | 98.3 ± 63.0 | 236 | 0.60 | 250 | 0 |
| K+ (mg L−1) | 4.9 | 23.6 ± 15.5 | 66.5 | 3.5 | 23.3 ± 14.7 | 67.9 | 0.90 | 250 | 0 | 1.3 | 22 ± 15 | 55 | 3.1 | 23.8 ± 17.8 | 62.1 | 0.75 | 250 | 0 |
| Ca2+ (mg L−1) | 1.11 | 23.9 ± 16.6 | 74.7 | 1.2 | 23.2 ± 13.3 | 60 | 0.85 | 75 | 0 | 2.5 | 26.6 ± 14.2 | 51.7 | 7.7 | 24.7 ± 12.2 | 42.4 | 0.70 | 75 | 0 |
| Mg2+ (mg L−1) | 0.1 | 6.1 ± 7.1 | 31.3 | 0.04 | 7.4 ± 7.8 | 30 | 0.46 | 50 | 0 | 0.4 | 8.0 ± 6.8 | 30 | 1.8 | 7.1 ± 3.8 | 12.9 | 0.68 | 50 | 0 |
| NH4+ (mg L−1) | <0.01 | 0.03 ± 0.02 | 0.14 | <0.01 | 0.03 ± 0.02 | 0.09 | 0.84 | - | 0 | <0.01 | 0.7 ± 2.8 | 11.4 | <0.01 | 1.1 ± 3.9 | 14.1 | 0.74 | - | 0 |
| B (µg L−1) | 16 | 24.5 ± 7.5 | 34 | - | 0 | 20 | 23 ± 4.2 | 26 | - | 0 | ||||||||
| F‒ (mg L−1) | 0.7 | 4.1 ± 2.6 | 10.9 | 1.5 | 31 | 0.3 | 1.6 ± 2.0 | 8.0 | 1.5 | 6 | ||||||||
| Isotopic Parameters | ||||||||||||||||||
| δ15N (‰) | 4.1 | 12.6 ± 6.2 | 25.8 | 6.9 | 15.5 ± 6.0 | 32.2 | 0.06 | - | 8 | 19.5 ± 5.8 | 28.9 | 12.4 | 33.6 ± 11.5 | 51.8 | 0.0004 | - | ||
| δ18O (‰) | −2.4 | 9 ± 5.4 | 20.8 | −1.7 | 11.1 ± 5.5 | 24.1 | 0.13 | - | 7.5 | 13.3 ± 4.0 | 19.8 | 12 | 18.0 ± 4.8 | 29.3 | 0.0138 | - | ||
| δ11B (‰) | 23 | 30.3 ± 6.3 | 36 | - | 16 | 25 ± 12.7 | 34 | - | ||||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nyilitya, B.; Mureithi, S.; Boeckx, P. Tracking Sources and Fate of Groundwater Nitrate in Kisumu City and Kano Plains, Kenya. Water 2020, 12, 401. https://doi.org/10.3390/w12020401
Nyilitya B, Mureithi S, Boeckx P. Tracking Sources and Fate of Groundwater Nitrate in Kisumu City and Kano Plains, Kenya. Water. 2020; 12(2):401. https://doi.org/10.3390/w12020401
Chicago/Turabian StyleNyilitya, Benjamin, Stephen Mureithi, and Pascal Boeckx. 2020. "Tracking Sources and Fate of Groundwater Nitrate in Kisumu City and Kano Plains, Kenya" Water 12, no. 2: 401. https://doi.org/10.3390/w12020401
APA StyleNyilitya, B., Mureithi, S., & Boeckx, P. (2020). Tracking Sources and Fate of Groundwater Nitrate in Kisumu City and Kano Plains, Kenya. Water, 12(2), 401. https://doi.org/10.3390/w12020401





