IberWQ: A GPU Accelerated Tool for 2D Water Quality Modeling in Rivers and Estuaries
Abstract
:1. Introduction
2. Model Structure and Equations
2.1. Model Structure
2.2. Model Equations
2.3. Numerical Solver
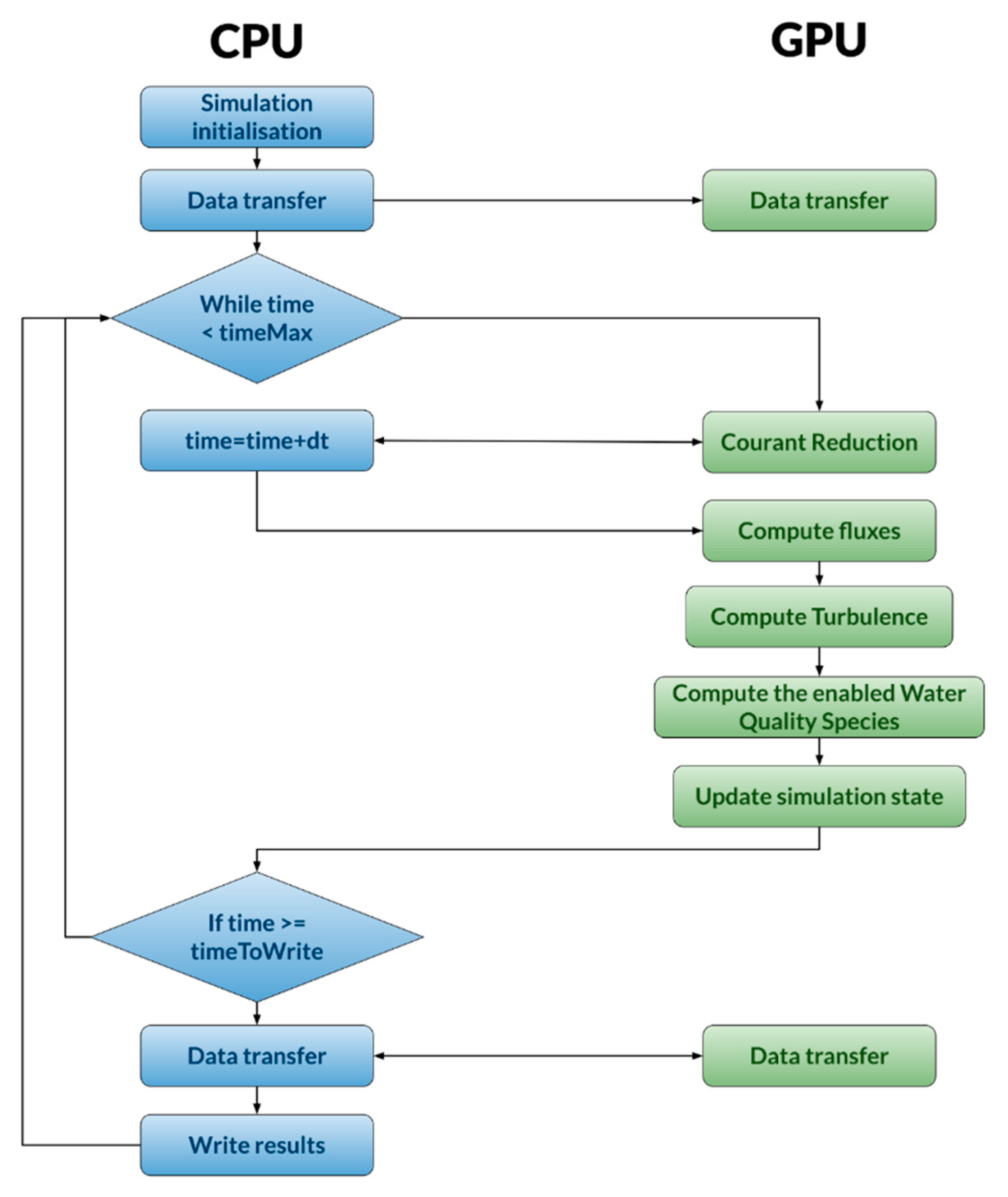
2.4. Parallelization
3. Test Cases
3.1. Faecal Contamination in a Coastal Estuary
3.1.1. Description
3.1.2. Results
3.2. Organic Matter Contamination in an Estuary
3.2.1. Description
3.2.2. Results
3.3. Combined Sewer Overflows in a River Miño Reach
3.3.1. Description
3.3.2. Results
3.4. Effluent Discharge from a Wastewater Treatment Plant
3.4.1. Description
3.4.2. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Reaction Terms
Appendix B. pH model
Appendix C. Model Constants
| Constant | Units | Suggested Values | Description | |
| Min | Max | |||
| mg/mg | 0.7 | 9.0 | Ratio of Nitrogen to Chl-A in phytoplankton | |
| mg/mg | 0.1 | 2.0 | Ratio of Phosphorus to Chl-A in phytoplankton | |
| mg/mg | 14 | 180 | Ratio of Oxygen to Chl-A in phytoplankton | |
| mg/mg | - | - | Ratio of Carbon to Chl-A in phytoplankton | |
| 1/day | 0.04 | 0.8 | Phytoplankton respiration rate | |
| 1/day | 1.0 | 3.0 | Maximum photosynthesis rate | |
| 1/day | 0.05 | 0.5 | Phytoplankton death rate | |
| - | 0.0 | 1.0 | Phytoplankton preference factor for ammonia | |
| m/day | - | - | Phytoplankton settling velocity | |
| mg/L | 0.01 | 0.3 | Nitrogen half-saturation constant for photosynthesis attenuation | |
| mg/L | 0.001 | 0.05 | Phosphorus half-saturation constant for photosynthesis attenuation | |
| mg/L | - | - | Carbon half-saturation constant for photosynthesis attenuation | |
| W/m2 | 0.05 | 0.3 | Light half-saturation constant for photosynthesis attenuation | |
| mg/L | - | - | Oxygen half-saturation constant for respiration attenuation | |
| 1/m | - | - | Light extinction coefficient in water | |
| m/day | - | - | Organic phosphorus settling velocity | |
| m/day | - | - | Inorganic phosphorus settling velocity | |
| 1/day | 0.01 | 0.7 | Organic phosphorus hydrolysis rate at 20 °C | |
| 1/day | 0.01 | 1.0 | Nitrification rate at 20 °C | |
| 1/day | 0.02 | 0.4 | Organic nitrogen hydrolysis rate at 20 °C | |
| 1/day | 0.001 | 0.1 | Denitrification rate at 20 °C | |
| m/day | 0.001 | 0.1 | Organic nitrogen settling velocity | |
| mg/L | Oxygen half-saturation constant for nitrification attenuation | |||
| mg/L | Oxygen half-saturation constant for denitrification attenuation | |||
| 1/day | 0.02 | 3.4 | CBOD degradation rate at 20 °C | |
| m/day | 0.01 | 0.36 | CBOD settling velocity | |
| mg/L | Oxygen half-saturation constant for CBOD degradation attenuation | |||
| kg/m2/day | 0.0 | 0.01 | Sediment oxygen demand rate | |
| mg/mg | - | - | Ratio of oxygen consumed per organic carbon oxidized to inorganic carbon | |
| 1/day | Mancini | Degradation constant for E. coli | ||
Appendix D. Time Series for Test 3





Appendix E. Time Series for Test 4










Appendix F. Data Sources for the Test Cases
| Bathymetry | Bathymetric survey carried out for previous studies. Spatial resolution of 30 m. |
| Effluent Discharge and Concentration | Virtual |
| Streamflow | Annual average flow from river Grande de Xubia. Available from the regional Meteorological Agency MeteoGalicia (www.meteogalicia.gal). |
| Tide | Tidal harmonics obtained from the tidal gauge of Ferrol. Available from Puertos del Estado (www.puertos.es). |
| Bathymetry | Bathymetric survey carried out for previous studies. Spatial resolution of 30 m. |
| Effluent Discharge and Concentration | Sewer network model carried out in previous studies. |
| Streamflow | Annual average flow from river Mero. Available from the regional Meteorological Agency MeteoGalicia (www.meteogalicia.gal). |
| Tide | Tidal harmonics obtained from the tidal gauge of A Coruña. Available from Puertos del Estado (www.puertos.es). |
| Bathymetry | Bathymetric survey carried out in [44]. |
| Effluent Discharge and Concentration | Sewer network model carried out in [46] and [46]. |
| Streamflow | River discharge obtained from a Water Quality Automatic Information System (SAICA) located upstream the river reach under study. Available from the regional water administration Confederación Hidrográfica del Miño-Sil (www.chminosil.es). |
| Bathymetry | Digital terrain model at 2 m resolution, obtained from LiDAR data from the Spanish National Plan of Aerophotogrammetry (PNOA), available from the Spanish National Geographic Institute (www.ign.es). |
| Effluent Discharge and Concentration | Virtual. |
| Streamflow | Annual average flow obtained from the regional water administration Confederación Hidrográfica del Guadalquivir (www.chguadalquivir.es). |
References
- Foundation of Water Research. Urban Pollution Management Manual: A Planning Guide for the Management of Urban Wastewater Discharges During Wet Weather; Foundation of Water Research: Buckinghamshire, UK, 1998. [Google Scholar]
- Robinson, R.B.; Roby, J.C. Concentration-duration-frequency curves for pH in a stream in the great smoky mountains. J. Environ. Eng. 2006, 132, 1600–1605. [Google Scholar] [CrossRef]
- Schwartz, J.S.; Dahle, M.; Bruce Robinson, R. Concentration-duration-frequency curves for stream turbidity: Possibilities for assessing biological impairment. J. Am. Water Resour. Assoc. 2008, 44, 879–886. [Google Scholar] [CrossRef]
- Di Toro, D.; Fitzpatrick, J.; Thomann, R. Documentation for Water Quality Analysis Simulation Program (WASP) and Model Verification Program (MVP); United States Environmental Protection Agency: Washington, DC, USA, 1983.
- Ambrose, R. WASP4, a Hydrodynamic and Water Quality Model: Model Theory, User’s Manual and Programmer’s Guide; United States Environmental Protection Agency: Washington, DC, USA, 1988.
- Chapra, S.C.; Pelletier, G.J.; Tao, H. QUAL2K: A Modeling Framework for Simulating River and Stream Water Quality; Version 2.11: Documentation and Users Manual; Civil and Environmental Engineering Dept., Tufts University: Medford, MA, USA, 2008. [Google Scholar]
- Cole, T.M.; Buchak, E.M. CE-QUAL-W2: A Two-Dimensional, Laterally Averaged, Hydrodynamic and Water Quality Model; Version 2.0: User Manual; US Army Corps of Engineers: Vicksburg, MS, USA, 1995. [Google Scholar]
- Cole, T.M.; Wells, S.A. CE-QUAL-W2: A Two-Dimensional, Laterally Averaged, Hydrodynamic and Water Quality Model; Version 3.5; US Army Engineering and Research Development Center: Vicksburg, MS, USA, 2006. [Google Scholar]
- Kashefipour, S.M.; Lin, B.; Harris, E.; Falconer, R.A. Hydro-environmental modelling for bathing water compliance of an estuarine basin. Water Res. 2002, 36, 1854–1868. [Google Scholar] [CrossRef]
- Kay, D.; Stapleton, C.M.; Wyer, M.D.; McDonald, A.T.; Crowther, J.; Paul, N.; Jones, K.; Francis, C.; Watkins, J.; Wilkinson, J.; et al. Decay of intestinal enterococci concentrations in high-energy estuarine and coastal waters: Towards real-time T 90 values for modelling faecal indicators in recreational waters. Water Res. 2005, 39, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Falconer, R.; Lin, B. Modelling trace metal concentration distributions in estuarine waters. Estuarine Coast. Shelf Sci. 2005, 64, 699–709. [Google Scholar] [CrossRef]
- Gao, G.; Falconer, R.A.; Lin, B. Numerical modelling of sediment–bacteria interaction processes in surface waters. Water Res. 2011, 45, 1951–1960. [Google Scholar] [CrossRef] [Green Version]
- Abu-Bakar, A.; Ahmadian, R.; Falconer, R.A. Modelling the transport and decay processes of microbial tracers in a macro-tidal estuary. Water Res. 2017, 123, 802–824. [Google Scholar] [CrossRef]
- Cea, L.; Bermúdez, M.; Puertas, J.; Bladé, E.; Corestein, G.; Escolano, E.; Conde, A.; Bockelmann-Evans, B.; Ahmadian, R. IberWQ: New simulation tool for 2D water quality modelling in rivers and shallow estuaries. J. Hydroinf. 2016, 18, 816–830. [Google Scholar] [CrossRef] [Green Version]
- Frick, W.E.; Roberts, P.J.W.; Davis, L.R.; Keyes, J.; Baumgartner, D.J.; George, K.P. Dilution Models for Effluent Discharges, 4th ed.; (Visual Plumes); U.S. Environmental Protection Agency, Office of Research and Development: Washington, DC, USA, 2003.
- Doneker, R.L.; Jirka, G.H. CORMIX-GI systems for mixing zone analysis of brine wastewater disposal. Desalination 2001, 139, 263–274. [Google Scholar] [CrossRef]
- Kiesel, J.; Schmalz, B.; Brown, G.L.; Fohrer, N. Application of a hydrological-hydraulic modelling cascade in lowlands for investigating water and sediment fluxes in catchment, channel and reach. J. Hydrol. Hydromech. 2013, 61, 334–346. [Google Scholar] [CrossRef] [Green Version]
- D’Ambrosio, E.; Gentile, F.; de Girolamo, A.M. Assessing the sustainability in water use at the basin scale through water footprint indicators. J. Clean. Prod. 2019, 244, 118847. [Google Scholar] [CrossRef]
- Bladé, E.; Cea, L.; Corestein, G.; Escolano, E.; Puertas, J.; Vázquez-Cendón, E.; Dolz, J.; Coll, A. Iber: herramienta de simulación numérica del flujo en ríos. Rev. Int Metodos Numer. Para Calculo Diseno Ing. 2014, 30, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Michaud, J.P. A Citizien’s Guide to Understanding and Monitoring Lakes and Streams; Washington State Department of Ecology, Publications Office: Olympia, WA, USA, 1991.
- García-Feal, O.; González-Cao, J.; Gómez-Gesteira, M.; Cea, L.; Domínguez, J.M.; Formella, A. An accelerated tool for flood modelling based on Iber. Water (Switzerland) 2018, 10, 1459. [Google Scholar] [CrossRef] [Green Version]
- Chapra, S.C. Surface Water-Quality Modeling; McGraw-Hill: New York, NY, USA, 1997; ISBN 1478608307. [Google Scholar]
- Cea, L.; Puertas, J.; Vázquez-Cendón, M.E. Depth averaged modelling of turbulent shallow water flow with wet-dry fronts. Arch. Comput. Meth. Eng. 2007, 14, 303–341. [Google Scholar] [CrossRef]
- Millero, F.J.; Poisson, A. International one-atmosphere equation of state of seawater. Deep Sea Res. Part A 1981, 28, 625–629. [Google Scholar] [CrossRef]
- Bowie, G.; Mills, W.; Porcella, D.; Campbell, C.; Pagenkopf, J.; Rupp, G.; Johnson, K.; Chan, P.; Gherini, S.; Chamberlin, C. Rates, Constants, and Kinetics Formulations in Surface Water Quality Modeling; EPA: Athens, GA, USA, 1985.
- Cea, L.; Vázquez-Cendón, M.E. Unstructured finite volume discretisation of bed friction and convective flux in solute transport models linked to the shallow water equations. J. Comput. Phys. 2012, 231, 3317–3339. [Google Scholar] [CrossRef]
- Versteeg, H.K.; Malalasekera, W. An Introduction to Computational Fluid Dynamics—The Finite Volume Method; Pearson Education: New York, NY, USA, 2007. [Google Scholar]
- Jasak, H.; Weller, H.G.; Gosman, A.D. High resolution NVD differencing scheme for arbitrarily unstructured meshes. Int. J. Numer. Methods Fluids 1999, 31, 431–449. [Google Scholar] [CrossRef]
- Nvidia Corporation CUDA C Programming Guide. Available online: https://docs.nvidia.com/cuda/pdf/CUDA_C_Programming_Guide.pdf (accessed on 17 December 2019).
- Crespo, A.J.C.; Domínguez, J.M.; Rogers, B.D.; Gómez-Gesteira, M.; Longshaw, S.; Canelas, R.; Vacondio, R.; Barreiro, A.; García-Feal, O. DualSPHysics: Open-source parallel CFD solver based on Smoothed Particle Hydrodynamics (SPH). Comput. Phys. Commun. 2015, 187, 204–216. [Google Scholar] [CrossRef]
- Crossley, A.; Lamb, R.; Waller, S. Fast solution of the Shallow Water Equations using GPU technology. In Proceedings of the British Hydrological Society 3rd International Symposium, Newcastle, UK, 13–19 July 2010. [Google Scholar]
- Vacondio, R.; Dal Palù, A.; Mignosa, P. GPU-enhanced finite volume shallow water solver for fast flood simulations. Environ. Modell. Softw. 2014, 57, 60–75. [Google Scholar] [CrossRef]
- Lacasta, A.; Morales-Hernández, M.; Murillo, J.; García-Navarro, P. An optimized GPU implementation of a 2D free surface simulation model on unstructured meshes. Adv. Eng. Softw. 2014, 78, 1–15. [Google Scholar] [CrossRef]
- Liu, Q.; Qin, Y.; Li, G. Fast Simulation of Large-Scale Floods Based on GPU Parallel Computing. Water 2018, 10, 589. [Google Scholar] [CrossRef] [Green Version]
- NVIDIA Corporation NVIDIA Tesla V100 GPU Architecture. Available online: https://images.nvidia.com/content/volta-architecture/pdf/volta-architecture-whitepaper.pdf (accessed on 7 December 2019).
- Moon, B.; Jagadish, H.V.; Faloutsos, C.; Saltz, J.H. Analysis of the Clustering Properties of Hilbert Space-filling Curve. IEEE Trans. Knowl. Data Eng. 2001, 13, 124–141. [Google Scholar] [CrossRef] [Green Version]
- Nvidia CUB. Available online: https://nvlabs.github.io/cub/ (accessed on 7 December 2019).
- NVIDIA Corporation NVIDIA Turing GPU Architecture. Available online: https://www.nvidia.com/content/dam/en-zz/Solutions/design-visualization/technologies/turing-architecture/NVIDIA-Turing-Architecture-Whitepaper.pdf (accessed on 2 February 2020).
- Cea, L.; Bermúdez, M.; Puertas, J. Uncertainty and sensitivity analysis of a depth-averaged water quality model for evaluation of Escherichia Coli concentration in shallow estuaries. Environ. Modell. Softw. 2011, 26, 1526–1539. [Google Scholar] [CrossRef]
- García-Barcina, J.M.; Oteiza, M.; de la Sota, A. Modelling the faecal coliform concentrations in the Bilbao estuary. Hydrobiologia 2002, 475, 213–219. [Google Scholar] [CrossRef]
- Kashefipour, S.M.; Lin, B.; Falconer, R.A. Modelling the fate of faecal indicators in a coastal basin. Water Res. 2006, 40, 1413–1425. [Google Scholar] [CrossRef] [PubMed]
- Manache, G.; Melching, C.S.; Lanyon, R. Calibration of a continuous simulation fecal coliform model based on historical data analysis. J. Environ. Eng. 2007, 133, 681–691. [Google Scholar] [CrossRef]
- Bode, A.; Álvarez-Ossorio, M.T.; González, N.; Lorenzo, J.; Rodríguez, C.; Varela, M.; Varela, M.M. Seasonal variability of plankton blooms in the Ria de Ferrol (NW Spain): II. Plankton abundance, composition and biomass. Estuarine Coast. Shelf Sci. 2005, 63, 285–300. [Google Scholar] [CrossRef]
- Anta Álvarez, J.; Bermúdez, M.; Cea, L.; Suárez, J.; Ures, P.; Puertas, J. Modelización de los impactos por DSU en el río Miño (Lugo). Ingeniería del agua 2015, 19, 105–116. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters; Wiley: New York, NY, USA, 1996. [Google Scholar]
- Piñeiro, J.; Maestro, I.; Aguirre, F.; Ures, P.; Torres, D.; Anta, J.; Puertas, J.; Suárez, J. Análisis del funcionamiento de un depósito-aliviadero en el sistema de saneamiento unitario en la aglomeración de Lugo. In Proceedings of the Actas de las II Jornadas de Ingeniería del Agua, Barcelona, Spain, 5–6 October 2011. [Google Scholar]












| Model | Run Time (s) | Time per Step (ms) | Millions of Cells per Second | Speedup vs. Iber |
|---|---|---|---|---|
| Iber | 60,545 | 66.8 | 2.2 | 1 |
| Iber+ GPU | 335 | 0.4 | 400.0 | 181 |
| Model | Run Time (s) | Time per Step (ms) | Millions of Cells per Second | Speedup vs. Iber |
|---|---|---|---|---|
| Iber | 31,615 | 28.8 | 1.8 | 1 |
| Iber+ GPU | 522 | 0.5 | 107.1 | 61 |
| Model | Run Time (s) | Time per Step (ms) | Millions of Cells per Second | Speedup vs. Iber |
|---|---|---|---|---|
| Iber | 3054 | 8.6 | 1.0 | 1 |
| Iber+ GPU | 105 | 0.3 | 30.2 | 29 |
| Model | Run Time (s) | Time per Step (ms) | Millions of Cells per Second | Speedup vs. Iber |
|---|---|---|---|---|
| Iber | 44,367 | 146.5 | 0.6 | 1 |
| Iber+ GPU | 482 | 1.6 | 56.9 | 92 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Feal, O.; Cea, L.; González-Cao, J.; Domínguez, J.M.; Gómez-Gesteira, M. IberWQ: A GPU Accelerated Tool for 2D Water Quality Modeling in Rivers and Estuaries. Water 2020, 12, 413. https://doi.org/10.3390/w12020413
García-Feal O, Cea L, González-Cao J, Domínguez JM, Gómez-Gesteira M. IberWQ: A GPU Accelerated Tool for 2D Water Quality Modeling in Rivers and Estuaries. Water. 2020; 12(2):413. https://doi.org/10.3390/w12020413
Chicago/Turabian StyleGarcía-Feal, Orlando, Luis Cea, José González-Cao, José Manuel Domínguez, and Moncho Gómez-Gesteira. 2020. "IberWQ: A GPU Accelerated Tool for 2D Water Quality Modeling in Rivers and Estuaries" Water 12, no. 2: 413. https://doi.org/10.3390/w12020413
APA StyleGarcía-Feal, O., Cea, L., González-Cao, J., Domínguez, J. M., & Gómez-Gesteira, M. (2020). IberWQ: A GPU Accelerated Tool for 2D Water Quality Modeling in Rivers and Estuaries. Water, 12(2), 413. https://doi.org/10.3390/w12020413








