Biodiversity of Calanoida Copepoda in Different Habitats of the North-Western Red Sea (Hurghada Shelf)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling and Analysis
2.3. Species Identification and Dissection
2.4. Statistical Analysis
3. Results
3.1. Physico-Chemical Parameters
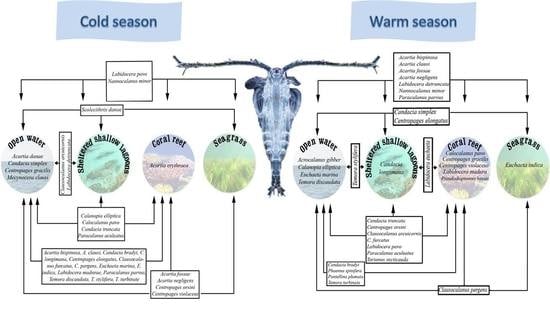
3.2. Dynamics of Species in Different Habitats
3.3. Seasonal Variation
3.4. Multiple correlation analysis
3.5. Multiple Regression Analysis
3.6. Cluster Analysis
3.7. Principal Component Analysis (PCA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hilmi, N.; Safa, A.; Reynaud, S.; Allemand, D. Coral Reefs and Tourism in Egypt’s Red Sea. Top. Middle East. N. Afr. Econ. 2012, 14, 416–434. [Google Scholar]
- Abo-Taleb, H.A.; Gharib, S.M. Seasonal distribution of epipelagic copepods at the different habitats in the north- western Red Sea, Egypt. Egypt. J. Aquat. Biol. Fish. 2018, 22, 139–157. [Google Scholar] [CrossRef][Green Version]
- Shimode, S.; Toda, T.; Kikuchi, T. Spatio-temporal changes in diversity and community structure of planktonic copepods in Sagami Bay, Japan. Mar. Biol. 2006, 148, 581–597. [Google Scholar] [CrossRef]
- Ferdous, Z.; Muktadir, A.K.M. A Review: Potentiality of zooplankton as bioindicator. Am. J. Appl. Sci. 2009, 6, 1815–1819. [Google Scholar] [CrossRef]
- Abu El-Regal, M.A.; El-Wazeer, A.; Abou Elnaga, Z.S.; Amr, A.A. Composition and spatio-temporal distribution of zooplankton community in the Egyptian Red Sea coast at Hurghada. Egypt. J. Aquat. Biol. Fish. 2018, 22, 1–12. [Google Scholar] [CrossRef]
- Ashour, M.; Abo-Taleb, H.A.; Abou-Mahmoud, M.M.; El-Feky, M.M.M. Effect of the integration between plankton natural productivity and environmental assessment of irrigation water, El-Mahmoudia Canal, on aquaculture potential of Oreochromis niloticus. Turk. J. Fish. Aquat. Sci. 2018, 18, 1163–1175. [Google Scholar] [CrossRef]
- Cornils, A.; Sieger, R.; Mizdalski, E.; Schumacher, S.; Grobe, H.; Schnack-Schiel, S.B. Copepod species abundance from the Southern Ocean and other regions (1980–2005)—A legacy. Earth Sys. Sci. Data 2018, 10, 1457–1471. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Matsuno, K.; Homma, T. Spatial changes in the vertical distribution of calanoid copepods down to great depths in the North Pacific. Zool. Stud. 2015, 54, 13. [Google Scholar] [CrossRef]
- Hernández-León, S.; Ikeda, T. A global assessment of mesozooplankton respiration in the ocean. J. Plankton Res. 2005, 27, 153–158. [Google Scholar] [CrossRef]
- Kobari, T.; Steinberg, D.K.; Ueda, A.; Tsuda, A.; Silver, M.W.; Kitamura, M. Impacts of ontogenetically migrating copepods on downward carbon flux in the western Subarctic Pacific Ocean. Deep Sea Res. Part II: Top. Stud. Oceanogr. 2008, 55, 1648–1660. [Google Scholar] [CrossRef]
- Moku, M.; Kawaguchi, K.; Watanabe, H.; Ohno, A. Feeding habits of three dominant myctophid fishes, Diaphus theta, Stenobrachius leucopsarus and S. nannochir, in the Subarctic and transitional waters of the Western North Pacific. Mar. Ecol. Prog. Ser. 2000, 207, 129–140. [Google Scholar] [CrossRef]
- Chiffings, A.W. Arabian Seas. In A global representative system of Marine Protected Areas, Central Indian Ocean, Arabian Seas, East Africa and East Asian Seas; Kelleher, G., Bleakley, C., Wells, S., Eds.; Great Barrier Reef Marine Park Authority: Washington, DC, USA, 1995; Volume 3, pp. 39–70. [Google Scholar]
- Delalo, E.P. Distribution of the zooplankton biomass in the Red Sea and the Gulf of Aden, winter 1961-1962. Okeanol. Issled. 1966, 15, 131–139. [Google Scholar]
- Halim, Y. Plankton of the Red Sea. Oceanogr. Mar. Biol.-Ann. Rev. 1969, 7, 231–275. [Google Scholar]
- Weikert, H. The oxygen minimum layer in the Red Sea: Ecological implications of the zooplankton occurrence in the area of the Atlantis-II Deep. Meeresforsch.-Rep. Mar. Res. 1980, 28, 1–9. [Google Scholar]
- Weikert, H. The vertical distribution of zooplankton in relation to habitat zones in the area of the Atlantis II Deep, Central Red Sea. Mar. Ecol. Prog. Ser. Oldend. 1982, 8, 129–143. [Google Scholar] [CrossRef]
- Schmidt, H.E. The vertical distribution and diurnal migration of some zooplankton in the Bay of Eilat (Red Sea). Helgol. Wiss. Meeresunters. 1973, 24, 333–340. [Google Scholar] [CrossRef][Green Version]
- Almeida Prado-Por, M.S.; Por, F.D. First data on the Calanoida (Copepoda) of the Northern Gulf of Aqaba (Red Sea). Rapp. Comm. Int. Mer Méditerr. 1981, 27, 173–174. [Google Scholar]
- Almeida Prado-Por, M.S. The diversity and dynamics of Calanoida (Copepoda) in the northern Gulf of Eilat (Aqaba), Red Sea. Oceanol. Acta 1983, 6, 139–145. [Google Scholar]
- Almeida Prado-Por, M.S. Distribution of the Calanoid Copepoda along the Gulf of Elat (Aqaba), Red Sea. Rapp. Comm. Int. Mer Méditerr. 1985, 29, 249–252. [Google Scholar]
- Almeida Prado-Por, M.S. A dial cycle of vertical distribution of the Calanoidea (Crustacea: Copepoda) in the northern Gulf of Aqaba (Elat). A propos des migrations Lessepsiennes. Bull. Inst. Océan. Monaco 1990, 7, 109–116. [Google Scholar]
- Rice, E.W.; Baird, R.B.; Eaton, A.D.; Clesceri, L.S. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017; p. 4-144-153. [Google Scholar]
- Santhanam, R.; Srinivasan, A. A Manual of Marine Zooplankton; Oxford & IBH Publishing: New Delhi, India, 1994; p. 160. [Google Scholar]
- Sars, G.O. The fresh-water Entomostraca of the Cape Province (Union of South Africa). Part III: Copepoda. Ann. S. Afr. Mus. 1927, 25, 85–149. [Google Scholar]
- Newell, G.E.; Newell, R.C. Marine Plankton: A Practical Guide; Hutchinson Educational Ltd. Press: London, UK, 1963; p. 221. [Google Scholar]
- Rose, M. Faune de France 26: Copépodes pélagiques; Paul Lechevalier: Paris, France, 1933; p. 374. [Google Scholar]
- Mori, T. The Pelagic Copepoda from the Neighbouring Waters of Japan; Soyo Company: Tokyo, Japan, 1964; p. 150. [Google Scholar]
- González, J.G.; Bowman, T.E. Planktonic copepods from Bahia Fosforescente, Puerto Rico, and adjacent waters. Proc. US Natl. Mus. 1965, 117, 241–304. [Google Scholar] [CrossRef]
- Silas, E.G.; Pillai, P.P. The calanoid copepod family Pontellidae from the Indian Ocean. J. Mar. Biol. Assoc. India. 1973, 15, 771–858. [Google Scholar]
- Bradford-Grieve, J.M. The marine fauna of New Zealand: Pelagic calanoid Copepoda: Megacalanidae, Calanidae, Paracalanidae, Mecynoceridae, Eucalanidae, Spinocalanidae, Clausocalanidae. New Zeal. Ocean. Inst. Mem. 1994, 102, 1–60. [Google Scholar]
- Boltovskoy, D.; Correa, N.; Boltovskoy, A. Diversity and endemism in cold waters of the South Atlantic: Contrasting patterns in the plankton and the benthos. Sci. Mar. 2005, 69, 17–26. [Google Scholar] [CrossRef]
- Abo-Taleb, H.A. Key to the Red Sea Labidocera (Crustacea; Calanoida: Pontellidae) copepods, the distribution of the species in various habitats, with special reference to two new records, and a historical correction. Egypt. J. Aquat. Res. 2019, 45, 1–7. [Google Scholar] [CrossRef]
- Heneash, A.; Ashour, M.; Matar, M. Effect of Un-live Microalgal diet, Nannochloropsis oculata and Arthrospira (Spirulina) platensis, Comparing to Yeast on Population of Rotifer, Brachionus plicatilis. Mediterr. Aquac. J. 2015, 7, 48–54. [Google Scholar] [CrossRef]
- Ashour, M.; Elshobary, M.E.; El-Shenody, R.; Kamil, A.-W.; Abomohra, A.E.-F. Evaluation of a native oleaginous marine microalga Nannochloropsis oceanica for dual use in biodiesel production and aquaculture feed. Biomass Bioenergy 2019, 120, 439–447. [Google Scholar] [CrossRef]
- Ashour, M.; Kamel, A. Enhance growth and biochemical composition of Nannochloropsis oceanica, cultured under nutrient limitation, using commercial agricultural fertilizers. J. Ma. Sci. Res. Dev. 2017, 7, 233. [Google Scholar]
- El-Shenody, R.A.; Ashour, M.; Ghobara, M.M.E. Evaluating the chemical composition and antioxidant activity of three Egyptian seaweeds Dictyota dichtoma, Turbinaria decurrens, and Laurencia obtuse. Braz. J. food Technol. 2019, 22, e2018203. [Google Scholar] [CrossRef]
- Elshobary, M.; El-Shenody, R.; Ashour, M.; Zabed, H.M.; Qi, X. Antimicrobial and antioxidant characterization of bioactive components from Chlorococcum minutum, a newly isolated green microalga. Food Biosci. J. 2020. (Accepted). [Google Scholar] [CrossRef]
- Sharawy, Z.Z.; Ashour, M.; Abbas, E.; Ashry, O.; Helal, M.; Nazmi, H.; Kelany, M.; Kamel, A.; Hassaan, M.; Rossi, W.J.; et al. Effects of dietary marine microalgae, Tetraselmis suecica, on production, gene expression, protein markers and bacterial count of Pacific white shrimp Litopenaeus vannamei. Aquac. Res. 2020, 1–13. [Google Scholar] [CrossRef]
- Nour, M.A.; Zaki, M.A.; Ashour, M.; Kamel, A.; Taha, S.M.; Omar, E. Effect of different Nannochloropsis oculata diets (cultured under different nitrogen and phosphorus regime) on growth and biochemical composition of rotifers Brachionus plicatilis. In Proceedings of the 12th International Congress on Microbial Interaction and Applications of Beneficial Microbes, Munich, Germany, 17–18 July 2017. [Google Scholar] [CrossRef]
- Omar, E.; Zaki, M.; Ashour, M.; Kamel, A.; Taha, S.M.; Nour, M.A. Effect of nutrient limitations on growth and biochemical composition of outdoor cultured Chaetoceros calcitrans. In Proceedings of the 12th International Congress on Microbial Interaction and Applications of Beneficial Microbes, Munich, Germany, 17–18 July 2017. [Google Scholar] [CrossRef]
- Nour, A.M.; Ashour, M.; Kamil, A.; Taha, S.M.; Zaki, M.A.; Omar, E. The use of artificial fertilizers for culture of marine microalgae: 1. Growth and biochemical constituents of Nannochloropsis oculata. In Proceedings of the World Congress and Expo on Applied Microbiology, Frankfurt, Germany, 18–20 August 2015. [Google Scholar] [CrossRef]
- El-Wazzan, E.; Kamel, A.; Ashour, M.; Abdel Razek, F.A. Effect of microalgal diet on larval growth and survival of the carpet shell clam, Tapes deccussatus. In Proceedings of the 3rd International Conference on Aquatic Resources: Prospects and Challenges (ICAR, 09), Alexandria, Egypt, 17–20 November 2009. [Google Scholar]
- Nour, A.M.; Ashour, M.; Kamil, A.; Taha, S.M.; Zaki, M.A.; Omar, E. The use of artificial fertilizers for culture of marine microalgae: 2. Growth and biochemical constituents of Chaetoceros calcitrans. In Proceedings of the World Congress and Expo on Applied Microbiology, Frankfurt, Germany, 18–20 August 2015. [Google Scholar] [CrossRef]
- Beckmann, W. Mesozooplankton distribution on a transect from the Gulf of Aden to the Central Red Sea during the winter monsoon. Oceanol. Acta 1984, 7, 87–102. [Google Scholar]
- Abou Zaid, M.M.A.; El Raey, M.; Ezz, S.M.A.; Aziz, N.E.A.; Abo-Taleb, H.A. Diversity of copepoda in a stressed eutrophic bay (El-Mex Bay), Alexandria, Egypt. Egypt. J. Aquat. Res. 2014, 40, 143–162. [Google Scholar] [CrossRef]
- Sameoto, D.D. Influence of the biological and physical environment on the vertical distribution of mesozooplankton and micronekton in the Eastern Tropical Pacific. Mar. Biol. 1986, 93, 263–279. [Google Scholar] [CrossRef]
- Devreker, D.; Souissi, S.; Seuront, L. Development and mortality of the first naupliar stages of Eurytemora affinis (Copepoda, Calanoida) under different conditions of salinity and temperature. J. Exp. Mar. Biol. Ecol. 2004, 303, 31–46. [Google Scholar] [CrossRef]
- Holste, L.; Peck, M.A. The effects of temperature and salinity on egg production and hatching success of Baltic Acartia tonsa (Copepoda: Calanoida): A laboratory investigation. Mar. Biol. 2005, 148, 1061–1070. [Google Scholar] [CrossRef]
- Farrag, M.M.; El-Naggar, H.A.; Abou-Mahmoud, M.A.; Alabssawy, A.N.; Ahmed, H.O.; Abo-Taleb, H.A.; Kostas, K. Marine Biodiversity Patterns off Alexandria Area, Southeastern Mediterranean Sea, Egypt. Environ. Monit. Ass. 2019, 191, 367–394. [Google Scholar] [CrossRef]
- Abo-Taleb, H.A.; Al-Maghraby, M.A.; El Raey, M.; Aboul Ezz, S.M.; Abdel Aziz, N.E.; Abou Zaid, M.M. Mapping the Different Planktonic Groups at One of the Egyptian Bays along Mediterranean Coast. Oceanogr. Fish Open Access J. 2018, 6, 5. [Google Scholar] [CrossRef]
- Aboul Ezz, S.M.; Abdel Aziz, N.E.; Abou Zaid, M.M.; El Raey, M.; Abo-Taleb, H.A. Environmental assessment of El-Mex Bay, Southeastern Mediterranean by using Rotifera as a plankton bio-indicator. Egypt. J. Aquat. Res. 2014, 40, 43–57. [Google Scholar] [CrossRef]
- Karlsson, K.; Puiac, S.; Winder, M. Life-history responses to changing temperature and salinity of the Baltic Sea copepod Eurytemora affinis. Mar. Biol. 2018, 165, 1–30. [Google Scholar] [CrossRef]
- Srinui, K.; Ohtsuka, S.; Metillo, E.B.; Nishibori, M. A new species of Acartia (Copepoda, Calanoida) from the Philippines based on morphological and molecular analyses. Zookeys 2019, 814, 71–94. [Google Scholar] [CrossRef]
- Abo-Taleb, H.A.; El Raey, M.; Zaid, M.A.; Ezz, S.A.; Aziz, N.A. study of the physico-chemical conditions and evaluation of the changes in eutrophication-related problems in El-Mex Bay. Afr. J. Environ. Sci. Technol. 2015, 9, 354–364. [Google Scholar]
- Soliman, H.A.; Fatma, A.A.; AbouZeid, A.E.; Ashour, M. Optimum growth conditions of three isolated diatoms species; Skeletonema costatum, Chaetoceros calcitrans and Detonula confervacea and their utilization as feed for marine penaeid shrimp larvae. EJAR 2010, 36, 161–183. [Google Scholar]
- Soliman, H.A.; Fatma, A.A.; AbouZeid, A.E.; Ashour, M. Population growth rate, fecundity, filtration and ingestion rate of marine rotifer Brachionus plicatilis fed with motile and immotile microalgae. EJAR 2008, 34, 426–439. [Google Scholar]
- Ashour, M. Marine Microalgae: Aquaculture and Biodiesel Production, 1st ed.; LAP Lambert Academic Publisher: Saarbrücken, Germany, 2015; pp. 1–240. [Google Scholar]
- Reid, P.C.; Edwards, M. Long-term changes in the pelagos, benthos and fisheries of the North Sea. Senck. Marit. 2001, 31, 107–115. [Google Scholar] [CrossRef]
- Chust, G.; Vogt, M.; Benedetti, F.; Nakov, T.; Villéger, S.; Aubert, A.; Vallina, S.M.; Righetti, D.; Not, F.; Biard, T.; et al. Mare incognitum: A glimpse into future plankton diversity and ecology research. Front. Mar. Sci. 2017, 10, 4–68. [Google Scholar]
- Khalil, M.T.; El-Rakman, N.S.A. Abundance and diversity of surface zooplankton in the Gulf of Aqaba, Red Sea. Egypt. J. Plankton Res. 1997, 19, 927–936. [Google Scholar]
- Al-Najjar, T. The Seasonal Dynamics and Grazing Control of Phyto- and Mesozooplankton in the Northern Gulf of Aqaba. Ph.D. Thesis, University of Bremen, Bremen, Germany, 2000. [Google Scholar]
- El-Serehy, H.; Abdel-Rahman, N. Distribution patterns of planktonic copepods crustaceans in the coral reefs and sandy areas along the Gulf of Aqaba, Red Sea, Egypt. Egypt. J. Biol. 2004, 6, 126–135. [Google Scholar]
- Chen, S.J. An indoor culture study of Euierpe acutifrons from Xiamen Bay. J. Xiamen Fish. Coll. 1988, 12, 339–345. [Google Scholar]
- Li, C.; Luo, X.; Huang, X.; Gu, B. Influences of temperature on development and survival, reproduction and growth of a calanoid copepod (Pseudodiaptomus dubia). Sci. World, J. 2009, 9, 866–879. [Google Scholar] [CrossRef]
- Gusmão, L.F.M.; McKinnon, A.D. Sex ratios, intersexuality and sex change in copepods. J. Plankton Res. 2009, 31, 1101–1117. [Google Scholar] [CrossRef]
- Abu El-Regal, M. Abundance and diversity of coral reef fish larvae at Hurghada, Egyptian Red Sea. Egypt. J. Aquat. Biol. Fish. 2008, 12, 17–33. [Google Scholar] [CrossRef][Green Version]
- Beck, M.W.; Heck, K.L.; Able, K.W.; Childers, D.L.; Eggleston, D.B.; Gillanders, B.M.; Halpern, B.; Hays, C.G.; Hoshino, K.; Minello, T.J.; et al. The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates. Bioscience 2001, 51, 633–641. [Google Scholar] [CrossRef]
- Williams, D.M.; Dixon, P.; English, S. Cross-shelf distribution of copepods and fish larvae across the central Great Barrier Reef. Mar. Biol. 1988, 99, 577–589. [Google Scholar]
- Cushing, D.H. Plankton production and year-class strength in fish populations: An update of the match/mismatch hypothesis. Adv. Mar. Biol. 1990, 26, 249–293. [Google Scholar]
- Wilson, G.G. A description of the early juvenile colour patterns of eleven Lethrinus species (Pisces: Lethrinidae) from the Great Barrier Reef, Australia. Rec. Aust. Mus. 1998, 50, 55–83. [Google Scholar] [CrossRef]
- Nakamura, Y.; Shibuno, T.; Lecchini, D.; Watanabe, Y. Habitat selection by emperor fish larvae. Aquat. Biol. 2009, 6, 61–65. [Google Scholar] [CrossRef]
- Atema, J.; Kingsford, M.J.; Gerlach, G. Larval reef fish could use odour for detection, retention and orientation to reefs. Mar. Ecol. Prog. Ser. 2002, 241, 151–160. [Google Scholar] [CrossRef]
- Glynn, P.W. Ecology of a Caribbean coral reef. The Porites reef-flat biotope: Part II. Plankton community with evidence for depletion. Mar. Biol. 1973, 22, 1–21. [Google Scholar] [CrossRef]
- Johannes, R.E.; Gerber, R. Import and export of net plankton by an Eniwetok coral reef community. Gt. Barrier Reef Comm. (Brisbane, Aust.) 1974, 1, 97–104. [Google Scholar]
- Hamner, W.M.; Jones, M.S.; Carleton, J.H.; Hauri, I.R.; Williams, D.M. Zooplankton, planktivorous fish, and water currents on a windward reef face: Great Barrier Reef, Australia. Bull. Mar. Sci. 1988, 42, 459–479. [Google Scholar]
- Cowen, R.K.; Gawarkiewicz, G.; Pineda, J.; Thorrold, S.R.; Werner, F.E. Population connectivity in marine systems an overview. Oceano. 2007, 20, 14–21. [Google Scholar] [CrossRef]
- Cornils, A.; Niehoff, B.; Richter, C.; Al-Najjar, T.; Schnack-Schiel, S.B. Seasonal abundance and reproduction of clausocalanid copepods in the northern Gulf of Aqaba (Red Sea). J. Plankton Res. 2006, 29, 57–70. [Google Scholar] [CrossRef]
- Ashjian, C.J.; Wishner, K.F. Temporal persistence of copepod species groups in the Gulf Stream. Deep Sea Res. Part I: Oceanogr. Res. Pap. 1993, 40, 483–516. [Google Scholar] [CrossRef]
- Matsuno, K.; Yamaguchi, A.; Hirawake, T.; Nishino, S.; Inoue, J.; Kikuchi, T. Reproductive success of Pacific copepods in the Arctic Ocean and the possibility of changes in the Arctic ecosystem. Polar Biol 2015, 38, 1075–1079. [Google Scholar] [CrossRef]
- Owre, H.B. Plankton of the Florida current. Part VIII. A list of the copepoda. Bull. Mar. Sci. 1962, 12, 489–495. [Google Scholar]
- Conway, D.V.P.; White, R.G.; Hugues-Dit-Ciles, J.; Gallienne, C.P.; Robins, D.B. Guide to the Coastal and Surface Zooplankton of the South-Western Indian Ocean; DEFRA Darwin Initiative Zooplankton Programme; Occasional Publication of the Marine Biological Association of the United Kingdom, No. 15: Plymouth, UK, 2003; p. 354. [Google Scholar]
- Grice, G.D.; Hulsemann, K. Abundance, vertical distribution and taxonomy of calanoid copepods at selected stations in the northeast Atlantic. Proc. Zool. Soc. Lond. 1965, 146, 213–262. [Google Scholar] [CrossRef]
- Raymont, J.E. Plankton and Productivity in the Oceans. Volume II zooplankton; Pergamon Press: New York, NY, USA, 1983; p. 496. [Google Scholar]
- Vervoort, W. Notes on the Biogeography and Ecology of Free-Living, Marine Copepoda. In Biogeography and Ecology in Antarctica; van Mieghem, J., van Oye, P., Eds.; Springer: Dordrecht, The Netherland, 1965; Volume 15, pp. 381–400. [Google Scholar]
- Atkinson, A.; Sinclair, J.D. Zonal distribution and seasonal vertical migration of copepod assemblages in the Scotia Sea. Polar Biol. 2000, 23, 46–58. [Google Scholar] [CrossRef]








| Habitat Types | Stations | Depth (m) | Temperature °C | Salinity g/l (ppt) | DO mg/l | pH | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | W | C | W | C | W | C | W | |||
| Seagrass (SG) | SG1 | 6 | 29.10 | 30.90 | 41.03 | 41.61 | 7.68 | 6.22 | 7.70 | 7.40 |
| SG2 | 5 | 29.50 | 32.50 | 41.20 | 41.00 | 7.62 | 6.25 | 7.30 | 7.50 | |
| SG3 | 6.5 | 29.00 | 31.00 | 41.30 | 42.00 | 7.40 | 6.00 | 7.50 | 7.00 | |
| Av ± SD | 5.83 ± 0.76 | 29.20 ± 0.26 | 31.47 ± 0.9 | 41.18 ± 0.14 | 41.54 ± 0.50 | 7.57 ± 0.15 | 6.16 ± 0.14 | 7.5 ± 0.20 | 7.30 ± 0.26 | |
| Coral Reef (CR) | CR1 | 12 | 28.70 | 31.00 | 41.45 | 41.78 | 7.29 | 6.15 | 8.10 | 8.10 |
| CR2 | 9 | 28.50 | 30.00 | 41.35 | 41.88 | 7.34 | 6.16 | 8.40 | 8.30 | |
| CR3 | 13 | 28.40 | 29.90 | 41.11 | 41.76 | 7.45 | 6.24 | 8.00 | 8.20 | |
| Av± SD | 11.33 ± 2.08 | 28.53 ± 0.15 | 30.30 ± 0.61 | 41.30 ± 0.17 | 41.81 ± 0.06 | 7.36 ± 0.08 | 6.18 ± 0.05 | 8.17 ± 0.21 | 8.20 ± 0.10 | |
| Sheltered Shallow Lagoon (SSL) | SSL1 | 2.8 | 28.00 | 34.00 | 39.20 | 42.60 | 7.23 | 6.13 | 7.90 | 8.40 |
| SSL2 | 2 | 28.10 | 36.50 | 35.20 | 44.00 | 6.90 | 5.50 | 8.00 | 9.00 | |
| SSL3 | 5 | 28.30 | 32.56 | 40.00 | 42.20 | 7.24 | 6.13 | 8.00 | 8.40 | |
| Av± SD | 3.27 ± 1.55 | 28.13 ± 0.15 | 34.35 ± 1.99 | 38.13 ± 2.57 | 42.93 ± 0.95 | 7.12 ± 0.19 | 5.92 ± 0.36 | 7.97 ± 0.06 | 8.60 ± 0.35 | |
| Open Deep Water (ODW) | ODW1 | 29 | 28.50 | 30.10 | 41.01 | 41.23 | 7.89 | 6.25 | 8.10 | 8.20 |
| ODW2 | 31 | 29.00 | 29.80 | 41.07 | 40.32 | 7.59 | 6.32 | 7.80 | 8.00 | |
| ODW3 | 56 | 28.00 | 30.20 | 41.12 | 40.22 | 8.05 | 6.85 | 7.90 | 8.00 | |
| Av± SD | 38.67 ± 15.04 | 28.50 ± 0.50 | 30.03 ± 0.21 | 41.07 ± 0.06 | 40.59 ± 0.56 | 7.84 ± 0.23 | 6.47 ± 0.33 | 7.93 ± 0.15 | 8.07 ± 0.12 | |
| Acartiidae Acartia bispinosa Carl 1907 Acartia clausi Giesbrecht 1889 Acartia danae Giesbrecht 1889 Acartia erythraea Giesbrecht 1889 Acartia fossae Gurney 1927 Acartia negligens Dana 184 | Clausocalanoidae Clausocalanus arcuicornis (Dana 1849) Clausocalanus furcatus (Brady 1883) Clausocalanus pergens Farran 1926 | Pontellidae Calanopia elliptica (Dana 1846; 1849) Labidocera detruncata (Dana 1849) Labidocera euchaeta (Dana 1846) Labidocera madura Scott A.1909 Labidocera pavo Giesbrecht 1889 Pontellina plumata (Dana 1849) |
| Calanidae Nannocalanus minor (Claus 1863) | Euchaetidae Euchaeta indica Wolfenden 1905 Euchaeta marina (Prestandrea 1833) | Pseudodiaptomidae Pseudodiaptomus hessei (Mrázek 1894) |
| Candaciidae Candacia bradyi Scott A. 1902 Candacia longimana (Claus 1863) Candacia simplex (Giesbrecht 1889) Candacia truncata (Dana 1849) | Paracalanidae Acrocalanus gibber Giesbrecht 1888 Calocalanus pavo (Dana 1852) Mecynocera clausi Thompson I.C. 1888 Paracalanus aculeatus Giesbrecht 1888 Paracalanus parvus (Claus 1863) | Scolecitrichidae Scolecithrix danae (Lubbock 1856) |
| Temoridae Temora discaudata Giesbrecht 1889 Temora stylifera (Dana 1849) Temora turbinata (Dana 1849) | ||
| Centropagoidae Centropages elongatus Giesbrecht 1896 Centropages gracilis (Dana 1849) Centropages orsini Giesbrecht 1889 Centropages violaceus (Claus 1863) | Phaennidae Phaenna spinifera Claus 1863 | Tortanidae Tortanus recticauda (Giesbrecht, 1889) |
| Habitat types | Stations | Cold Season | Warm Season | ||
|---|---|---|---|---|---|
| Diversity sp. No. | Density Org./m3 | Diversity sp. No. | Density Org./m3 | ||
| Seagrass (SG) | SG1 | 7 | 111 | 11 | 889 |
| SG2 | 7 | 148 | 11 | 963 | |
| SG3 | 7 | 112 | 11 | 892 | |
| Total | 7 | 124 | 11 | 915 | |
| Coral Reef (CR) | CR1 | 7 | 127 | 14 | 1233 |
| CR2 | 10 | 209 | 12 | 1387 | |
| CR3 | 20 | 367 | 15 | 1276 | |
| Total | 25 | 234 | 24 | 1299 | |
| Sheltered Shallow Lagoon (SSL) | SSL1 | 6 | 157 | 12 | 1099 |
| SSL2 | 8 | 145 | 13 | 1063 | |
| SSL3 | 6 | 338 | 14 | 689 | |
| Total | 9 | 213 | 19 | 950 | |
| Open Deep Water (ODW) | ODW1 | 19 | 316 | 17 | 1400 |
| ODW2 | 17 | 236 | 13 | 1065 | |
| ODW3 | 27 | 387 | 16 | 1470 | |
| Total | 31 | 313 | 26 | 1312 | |
| Total | 32 | 221 | 34 | 1119 | |
| Habitats | Cold Season | Warm Season | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Labidocera pavo | Nannocal-anus minor | Acartia bispinosa | Acartia clausi | Acartia fossae | Acartia negligens | Labidocera detruncata | Nannocal-anus minor | Paracalanus parvus | |
| SG | 8.33 ± 4.50a | 4.00 ± 0.00a | 13.3 ± 1.5a | 17.3 ± 1.5a | 13.0 ± 2.0a | 2.0 ± 1.0a | 1.3 ± 0.6a | 22.0 ± 3.0b | 4.3 ± 1.5a |
| CR | 4.00 ± 4.00a | 5.33 ± 2.30a | 18.3 ± 2.3a | 18.3 ± 24.8a | 11.3 ± 16.3a | 4.3 ± 3.5a | 1.3 ± 2.3a | 18.3 ± 2.3b | 0.7 ± 0.6a |
| SSL | 10.0 ± 10.14a | 3.00 ± 4.35a | 33.3 ± 25.5a | 13.7 ± 8.7a | 11.7 ± 11.1a | 2.3 ± 1.5a | 1.7 ± 2.1a | 26.7 ± 14.6b | 0.3 ± 0.6a |
| ODW | 11.33 ± 6.65a | 7.33 ± 8.50a | 28. ± 8.9a | 22.7 ± 10.6a | 5.3 ± 2.3a | 1.7 ± 2.1a | 1.3 ± 2.3a | 52.0 ± 8.9a | 10.7 ± 16.7a |
| mean ± SD | 8.42 ± 6.45a | 4.92 ± 4.52 | 23.3 ± 14.2 | 18 ± 12.6 | 10.3 ± 9.0 | 2.6 ± 2.2 | 1.4 ± 1.7 | 29.8 ± 15.7 | 4.0 ± 8.4 |
| Seasons | Habitats | Range | Average ± SD |
|---|---|---|---|
| Cold season | SG | 76–82 | 79 ± 31.2 |
| CR | 72–140 | 106 ± 52.73 | |
| SSL | 73–284 | 178.5 ± 119.63 | |
| ODW | 101–478 | 289.5 ± 221.04 | |
| Warm season | SG | 705–837 | 771 ± 439.02 |
| CR | 1047–1292 | 1169.5 ± 662.96 | |
| SSL | 589–1166 | 877.5 ± 549.69 | |
| ODW | 360–1165 | 762.5 ± 534.59 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abo-Taleb, H.; Ashour, M.; El-Shafei, A.; Alataway, A.; Maaty, M.M. Biodiversity of Calanoida Copepoda in Different Habitats of the North-Western Red Sea (Hurghada Shelf). Water 2020, 12, 656. https://doi.org/10.3390/w12030656
Abo-Taleb H, Ashour M, El-Shafei A, Alataway A, Maaty MM. Biodiversity of Calanoida Copepoda in Different Habitats of the North-Western Red Sea (Hurghada Shelf). Water. 2020; 12(3):656. https://doi.org/10.3390/w12030656
Chicago/Turabian StyleAbo-Taleb, Hamdy, Mohamed Ashour, Ahmed El-Shafei, Abed Alataway, and Mahmoud M. Maaty. 2020. "Biodiversity of Calanoida Copepoda in Different Habitats of the North-Western Red Sea (Hurghada Shelf)" Water 12, no. 3: 656. https://doi.org/10.3390/w12030656
APA StyleAbo-Taleb, H., Ashour, M., El-Shafei, A., Alataway, A., & Maaty, M. M. (2020). Biodiversity of Calanoida Copepoda in Different Habitats of the North-Western Red Sea (Hurghada Shelf). Water, 12(3), 656. https://doi.org/10.3390/w12030656