Legionellosis and Recent Advances in Technologies for Legionella Control in Premise Plumbing Systems: A Review
Abstract
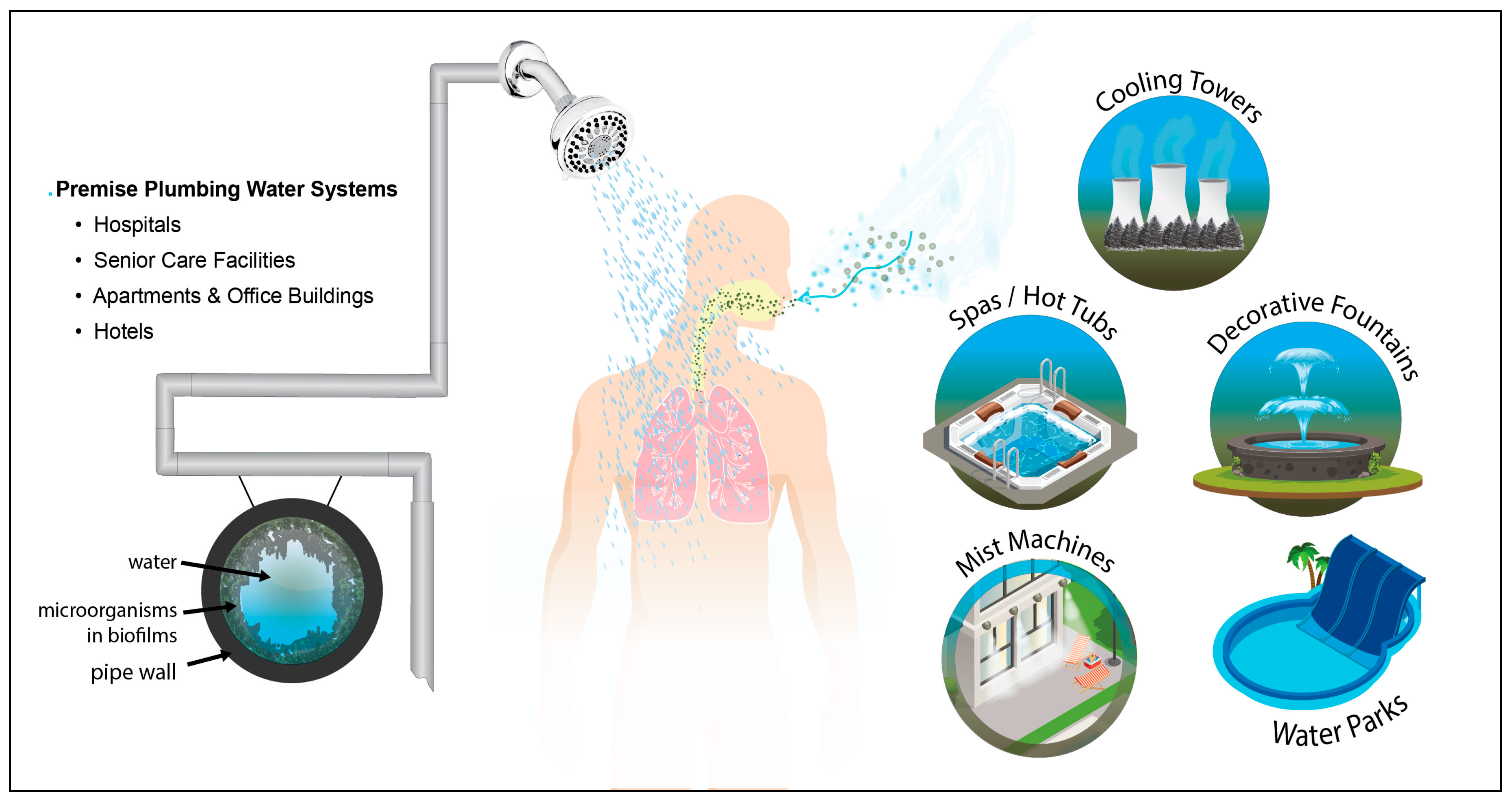
:1. Introduction
2. Legionella and Legionellosis
2.1. Outbreaks of Legionellosis
2.2. Causative Agent of Legionellosis: Legionella
2.3. Species, Serogroups, and Strains of Legionella
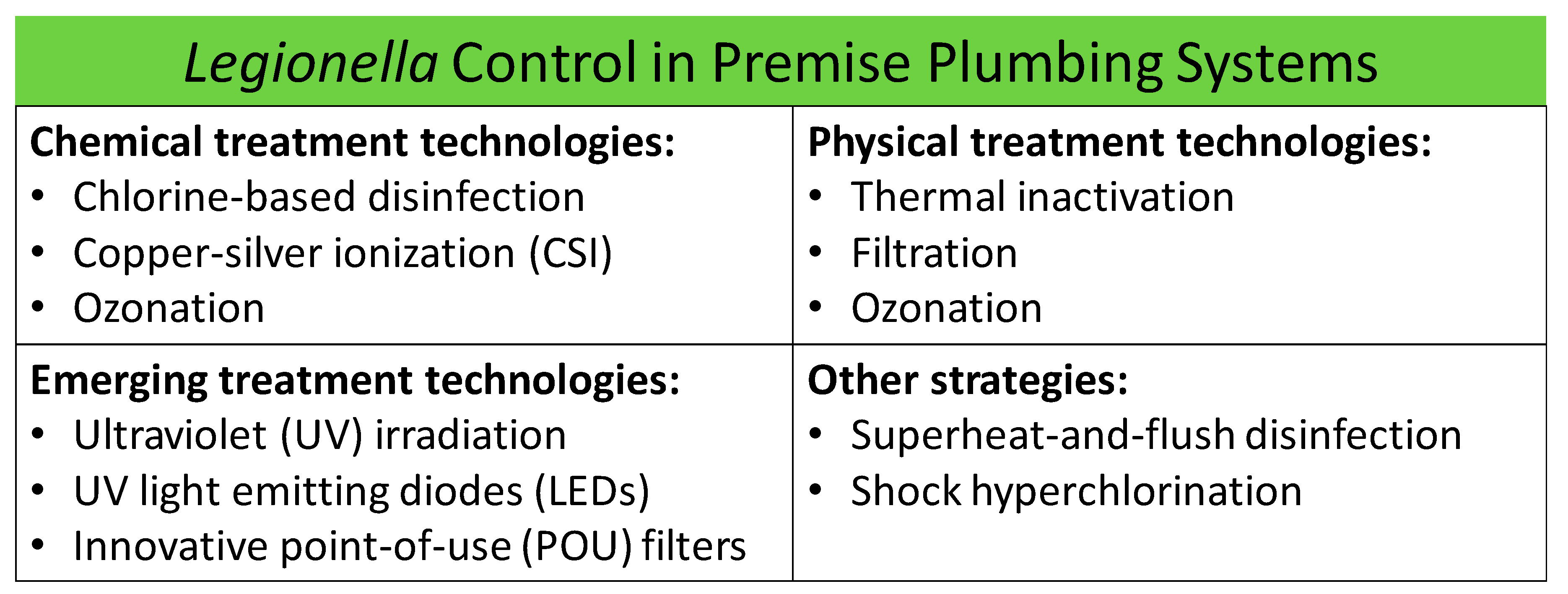
3. Current Technologies for Control of Legionella in Premise Plumbing Systems
3.1. Chemical Treatment Technologies
3.1.1. Chlorine-Based Disinfection
3.1.2. Copper-Silver Ionization (CSI)
3.1.3. Ozonation
3.2. Physical Treatment Technologies
3.2.1. Thermal Inactivation
3.2.2. Media Filtration
3.3. Challenges in Physical and Chemical Treatments
4. Emerging Treatment Technologies
4.1. Ultraviolet (UV) Irradiation
4.2. UV Light Emitting Diodes (LEDs)
4.3. Innovative Point-of-Use (POU) Filters
5. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fraser, D.W.; Tsai, T.R.; Orenstein, W.; Parkin, W.E.; Beecham, H.J.; Sharrar, R.G.; Harris, J.; Mallison, G.F.; Martin, S.M.; McDade, J.E.; et al. Legionnaires’ Disease: Description of an Epidemic of Pneumonia. N. Engl. J. Med. 1978, 297, 1189–1197. [Google Scholar] [CrossRef]
- Benitez, A.J.; Winchell, J.M. Clinical Application of a Multiplex Real-Time PCR Assay for Simultaneous Detection of Legionella Species, Legionella pneumophila, and Legionella pneumophila Serogroup 1. J. Clin. Microbiol. 2013, 51, 348–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerbel, W.; Krause, J.D.; Shelton, B.G.; Springston, J. (Eds.) Recognition, Evaluation, and Control of Legionella in Building Water Systems; American Industrial Hygiene Association: Falls Church, VA, USA, 2015. [Google Scholar]
- Centers for Disease Control and Prevention. Developing a Water Management Program to Reduce Legionella Growth and Spread in Buildings: A Practical Guide to Implementing Industry Standards. 2016. Available online: https://www.cdc.gov/Legionella/WMPtoolkit (accessed on 5 June 2017).
- New York City Department of Health. Title 10, Part 4 of the Official Compilation of Codes, Rules and Regulations of the State of New York, 2016. 2016. Available online: https://www.health.ny.gov/regulations/nycrr/title_10 (accessed on 24 February 2020).
- Centers for Medicaid and Medicare Services. Requirement to Reduce Legionella Risk in Healthcare Facility Water Systems to Prevent Cases and Outbreaks of Legionnaires’ Disease (LD); US Department of Health and Human Services, Centers for Medicaid and Medicare services, Center for Clinical Standards and Quality/Survey and Certification Group: Baltimore, MD, USA, 2017. Available online: https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertificationGenInfo/Policy-and-Memos-to-States-and-Regions.html (accessed on 24 February 2020).
- American Society of Heating, Refrigerating and Air-Conditioning Engineers. Legionellosis: Risk Management for Building Water Systems. ANSI/ASHRAE Standard 188. Atlanta: The Society. 2018. Available online: https://www.ashrae.org/technical-resources/bookstore/ansi-ashrae-standard-188-2018-legionellosis-risk-management-for-building-water-systems (accessed on 24 February 2020).
- Marston, B.J.; Plouffe, J.F.; File, T.M.; Hackman, B.A.; Salstrom, S.J.; Lipman, H.B.; Kolczak, M.S.; Breiman, R.F. Incidence of Community-Acquired Pneumonia Requiring Hospitalization. Arch. Intern. Med. 1997, 157, 1709–1718. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. National Outbreak Reporting System (NORS). 2017. Available online: https://wwwn.cdc.gov/norsdashboard/ (accessed on 24 February 2020).
- Collier, S.A.; Stockman, L.J.; Hicks, L.A.; Garrison, L.E.; Zhou, F.J.; Beach, M.J. Direct Healthcare Costs of Selected Diseases Primarily or Partially Transmitted by Water. Epidemiol. Infect. 2012, 140, 2003–2013. [Google Scholar] [CrossRef] [Green Version]
- Beauté, J. European Legionnaires’ Disease Surveillance Network. Legionnaires’ Disease in Europe, 2011 to 2015. Eurosurveillance 2017, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Communicable Diseases Network Australia. Australia’s Notifiable Disease Status, 2014: Annual Report of the National Notifiable Disease Surveillance System. J. Commun. Dis. 2016, 40, E48–E145. [Google Scholar]
- Oh, Y.; Noga, R.; Shanov, V.; Ryu, H.; Chandra, H.; Yadav, B.; Yadav, J.; Chae, S. Electrically heatable carbon nanotube point-of-use filters for effective separation and in-situ inactivation of Legionella pneumophila. Chem. Eng. J. 2019, 366, 21–26. [Google Scholar] [CrossRef]
- Haley, C.E.; Cohen, M.L.; Halter, J.; Meyer, R.D. Nosocomial Legionnaires’ Disease: A Continuing Common-Source Epidemic at Wadsworth Medical Center. Ann. Intern. Med. 1979, 90, 583–586. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Epidemiologic Notes and Reports: Legionnaires’ Disease Outbreak Associated with a Grocery Store Mist Machine-Louisiana, 1989. Morb. Mortal. Wkly. Rep. 1990, 39, 108–110. [Google Scholar]
- Fenstersheib, M.D.; Miller, M.; Diggins, C.; Liska, S.; Detwiler, L.; Werner, S.B.; Lindquist, D.; Thacker, W.L.; Benson, R.F. Outbreak of Pontiac Fever due to Legionella anisa. Lancet 1990, 336, 35–37. [Google Scholar] [CrossRef]
- Jernigan, D.B.; Hofman, J.; Cetron, M.S.; Genese, C.A.; Nuorti, J.P.; Fields, B.S.; Benson, R.F.; Carter, R.J.; Edelstein, P.H.; Guerrero, I.C.; et al. Outbreak of Legionnaires’ Disease Among Cruise Ship Passengers Exposed to a Contaminated Whirlpool Spa. Lancet 1996, 347, 494–499. [Google Scholar] [CrossRef]
- Van Heijnsbergen, E.; Schalk, J.A.C.; Euser, S.M.; Brandsema, P.S.; de Boer, J.W.; de Roda Husman, A.M. Confirmed and Potential Sources of Legionella Reviewed. Environ. Sci. Technol. 2015, 49, 4797–4815. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, K.A.; Hamilton, M.T.; Johnson, W.; Jjemba, P.; Bukhari, Z.; LeChevallier, M.; Haas, C.N.; Gurian, P.L. Risk-Based Critical Concentrations of Legionella pneumophila for Indoor Residential Water Uses. Environ. Sci. Technol. 2019, 53, 4528–4541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- United States Environmental Protection Agency. Technologies for Legionella Control in Premise Plumbing Systems: Scientific Literature Review; USEPA: Washington, DC, USA, 2016.
- Kirmeyer, G.; Martel, K.; Thompson, G.; Radder, L.; Klement, W.; LeChevallier, M.; Baribeau, H.; Flores, A. Optimizing Chloramine Treatment; Water Research Foundation and AWWA: Denver, CO, USA, 2004. [Google Scholar]
- United States Environmental Protection Agency (USEPA). National Primary Drinking Water Regulations: Stage 2 Disinfectants and Disinfection Byproducts Rule; Final Rule; 71 FR 388. (January 4, 2006); USEPA: Washington, DC, USA, 2006.
- Sarver, E.; Dodson, K.; Scardina, R.P.; Lattyak-Slabaugh, R.; Edwards, M.; Nguyen, C. Copper Pitting in Chlorinated, High-pH Potable Water. J. AWWA 2011, 103, 86–97. [Google Scholar] [CrossRef]
- World Health Organization. Drinking Water Guidelines, 4th ed.; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Rohr, U.; Senger, M.; Selenka, F.; Turley, R.; Wilhelm, M. Four Years of Experience with Silver-Copper Ionization for Control of Legionella in a German University Hospital Hot Water Plumbing System. Clin. Infect. Dis. 1999, 29, 1507–1511. [Google Scholar] [CrossRef]
- Araya, M.; Olivares, M.; Pizarro, F.; Llanos, A.; Figueroa, G.; Uauy, R. Community-based Randomized Double-blind Study of Gastrointestinal Effects and Copper Exposure in Drinking Water. Environ. Health Perspect. 2004, 112, 1068–1073. [Google Scholar] [CrossRef]
- Hong, J.H.; Duncan, S.E.; Dietrich, A.M. Effect of Copper Speciation at Different pH on Temporal Sensory Attributes of Copper. Food Qual. Prefer. 2010, 21, 132–139. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Ultraviolet Disinfection Guidance Manual for the Final Long Term 2 Enhanced Surface Water Treatment Rule; USEPA: Washington, DC, USA, 2006.
- Wright, H.; Gaithuma, D.; Heath, M.; Schulz, C.; Bogan, T.; Cabaj, A.; Schmalweiser, A.; Schmelzer, M.; Finegan-Kelly, J. UV Disinfection Knowledge Base; Water Research Foundation: Denver, CO, USA, 2012. [Google Scholar]
- Vilhunen, S.; Sarkka, J.; Silanpaa, M. Ultraviolet Light-Emitting Diodes in Water Disinfection. Environ. Sci. Pollut. Res. 2009, 16, 439–442. [Google Scholar] [CrossRef]
- Wurtele, M.A.; Kolbe, T.; Lipsz, M.; Kulberg, A.; Weyers, M.; Kneissl, M.; Jekel, M. Application of GaN-based Ultraviolet-C Light Emitting Diodes—UV LEDs—For Water Disinfection. Water Res. 2011, 45, 1481–1489. [Google Scholar] [CrossRef]
- Cameron, D.C.; Grant, M. Institutional Outbreak of Pneumonia. Morb. Mortal. Wkly. Rep. 1965, 14, 265–266. [Google Scholar]
- Glick, T.H.; Gregg, M.B.; Berman, B.; Mallison, G.; Rhodes, W.W.; Kassanoff, I. Pontiac Fever: An Epidemic of Unknown Etiology in a Health Department. Am. J. Epidemiol. 1978, 107, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, A.F.; McDade, J.E.; Patton, C.M.; Bennett, J.V.; Skaliy, P.; Feeley, J.C.; Anderson, D.C.; Potter, M.E.; Newhouse, V.F.; Gregg, M.B.; et al. Pontiac Fever: Isolation of the Etiologic Agent (Legionella Pneumophila) and Demonstration of its Mode of Transmission. Am. J. Epidemiol. 1981, 114, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Phin, N.; Parry-Ford, F.; Harrison, T.; Stagg, H.R.; Zhang, N.; Kumar, K.; Lortholary, O.; Sumla, A.; Abubakar, I. Epidemiology and Clinical Management of Legionnaires’ Disease. Lancet 2014, 14, 1011–1021. [Google Scholar] [CrossRef]
- Michigan Department of Health and Human Services. Legionellosis Outbreak-Genesee County, May 2015–October 2015: Full Analysis; Michigan Department of Health and Human Services: Lansing, CO, USA, 2016. [Google Scholar]
- Bellinger, D.C. Lead Contamination in Flint-An Abject Failure to Protect Public Health. N. Engl. J. Med. 2016, 324, 1101–1103. [Google Scholar] [CrossRef]
- Michigan Department of Health and Human Services. Summary of Legionellosis Outbreak-Genesee County, June 2014–March 2015; Michigan Department of Health and Human Services: Lansing, CO, USA, 2015. [Google Scholar]
- Stout, J.E.; Yu, V.L. Legionellosis. N. Engl. J. Med. 1997, 337, 682–687. [Google Scholar] [CrossRef]
- Hicks, L.A.; Garrison, L.E.; Nelson, G.E. Legionellosis-United States, 2000–2009. Morb. Mortal. Wkly. Rep. 2011, 60, 1083–1086. [Google Scholar]
- Koo, D.T.; Dean, A.G.; Slade, R.W.; Knowles, C.M.; Adams, D.A.; Fortune, W.K.; Hall, P.A.; Fagan, R.F.; Panter-Connah, B.; Holden, H.R.; et al. MMWR Summary of Notifiable Diseases, United States, 1993. Morb. Mortal. Wkly. Rep. 1994, 42, 1–73. [Google Scholar]
- Joseph, C.A.; Ricketts, K.D.; On behalf of the European Working Group for Legionella Infections. Legionnaires’ Disease in Europe 2007–2008. Surveill. Outbreak Rep. 2010, 15, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Chandler, F.W.; Hicklin, M.D.; Blackmon, J.A. Demonstration of the Agent of Legionnaires’ Disease in Tissue. N. Engl. J. Med. 1977, 297, 1218–1220. [Google Scholar] [CrossRef]
- Friedman, S.; Spitalny, K.; Barbaree, J.; Faur, Y.; McKinney, R. Pontaic Fever Outbreak Associated with a Cooling Tower. Am. J. Public Health 1987, 77, 568–572. [Google Scholar] [CrossRef] [Green Version]
- McDade, J.E.; Shepard, C.C.; Fraser, D.W.; Tsai, T.R.; Redus, M.A.; Dowdle, W.R. Isolation of a Bacterium and Demonstration of Its Role in Other Respiratory Disease. N. Engl. J. Med. 1977, 297, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.J.; Steigerwalt, A.G.; Weaver, R.E.; McDade, J.E.; Feeley, J.C.; Mandel, M. Classification of the Legionnaires’ Disease Bacterium: An Interim Report. Curr. Microbiol. 1978, 1, 71–75. [Google Scholar] [CrossRef]
- Brenner, D.J. Deoxyribonucleic Acid Reassociation in the Taxonomy of Enteric Bacteria. Int. J. Syst. Bacteriol. 1972, 23, 298–307. [Google Scholar] [CrossRef] [Green Version]
- Weaver, R.E. Cultural and Staining Characteristics. In Legionnaires’: The Disease, the Bacterium, and Methodology; Centers for Disease Control and Prevention: Atlanta, GA, USA, 1978; pp. 18–21. [Google Scholar]
- Diederen, B.M.W. Legionella spp. and Legionnaires’ Disease. J. Infect. 2008, 56, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Politi, B.D.; Fraser, D.W.; Mallison, G.F.; Mohatt, J.V.; Morris, G.L.; Patton, C.M.; Feeley, J.C.; Telle, R.D.; Bennett, J.V. A Major Focus of Legionnaires’ Disease in Bloomington, Indiana. Ann. Intern. Med. 1979, 90, 587–591. [Google Scholar] [CrossRef]
- Brown, A.; Yu, V.L.; Elder, E.M.; Magnussen, M.H.; Kroboth, F. Nosocomial Outbreak of Legionnaires’ Disease at the Pittsburgh Veterans Administration Medical Center. Trans. Assoc. Am. Physicians 1980, 93, 52–59. [Google Scholar]
- Conwill, D.E.; Werner, S.B.; Dritz, S.K.; Bissett, M.; Coffey, E.; Nygaard, G.; Bradford, L.; Morrison, F.R.; Knight, M.W. Legionellosis: The 1980 San Franciscan Outbreak. Am. Rev. Respir. Dis. 1982, 126, 666–669. [Google Scholar]
- Garbe, P.L.; Davis, B.J.; Weisfeld, J.S.; Markowitz, L.; Miner, P.; Garrity, F.; Barbaree, J.M.; Reingold, A.L. Nosocomial Legionnaires’ Disease: Epidemiologic Demonstration of Cooling Towers as a Source. J. Am. Med. Assoc. 1985, 254, 521–524. [Google Scholar] [CrossRef]
- Brown, C.M.; Nuorti, P.J.; Breiman, R.F.; Hathcock, A.L.; Fields, B.S.; Lipman, H.B.; Lleyellyn, G.C.; Hofman, J.; Cetron, M. A Community Outbreak of Legionnaires’ Disease Linked to Hospital Cooling Towers: An Epidemiological Method to Calculate Dose of Exposure. Int. J. Epidemiol. 1999, 28, 353–359. [Google Scholar] [CrossRef] [Green Version]
- Quinn, C.; Demirjian, A.; Watkins, L.F.; Tomczyk, S.; Lucas, C.; Brown, E.; Kozak-Muiznieks, N.; Benitez, A.; Garrison, L.E.; Kunz, J.; et al. Legionnaires’ Outbreak at a Long-Term Care Facility Caused by a Cooling Tower Using an Automated Disinfection System-Ohio, 2013. J. Environ. Health 2015, 78, 8–13. [Google Scholar]
- Hlady, W.G.; Mullen, R.C.; Mitz, C.S.; Shelton, B.G.; Hopkins, R.S.; Daikos, G.L. Outbreak of Legionnaires’ Disease Linked to a Decorative Fountain by Molecular Epidemiology. Am. J. Epidemiol. 1993, 138, 555–562. [Google Scholar] [CrossRef] [PubMed]
- O’Loughlin, R.E.; Kightlinger, L.; Werpy, M.C.; Brown, E.; Stevens, V.; Hepper, C.; Keane, T.; Venson, R.F.; Fields, B.S.; Moore, M.R. Restaurant Outbreak of Legionnaires’ Disease Associated with a Decorative Fountain: An Environmental and Case-Control Study. BMC Infect. Dis. 2007, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control and Prevention. Legionnaires’ Disease Associated with a Whirlpool Spa Display-Virginia, September-October, 1996. Morb. Mortal. Wkly. Rep. 1997, 46, 336. [Google Scholar]
- Murga, R.; Forster, T.S.; Brown, E.; Pruckler, J.M.; Fields, B.S.; Donlan, R.M. Role of Biofilms in the Survival of Legionella pneumophila in a Model Potable-Water System. Microbiology 2001, 147, 3121–3126. [Google Scholar] [CrossRef] [Green Version]
- Lau, H.Y.; Ashbolt, N.J. The Role of Biofilms and Protozoa in Legionella Pathogenesis: Implications for Drinking Water. J. Appl. Microbiol. 2008, 107, 368–378. [Google Scholar] [CrossRef]
- Declerck, P.; Behets, J.; van Hoef, V.; Ollevier, F. Detection of Legionella spp. and Some of Their Amoeba Hosts in Floating Biofilms from Anthropogenic and Natural Aquatic Environments. Water Res. 2007, 41, 3159–3167. [Google Scholar] [CrossRef]
- Declerck, P.; Behets, J.; Margineanu, A.; van Hoef, V.; Keersmaecker, B.D.; Ollevier, F. Replication of Legionella pneumophila in Biofilms of Water Distribution Pipes. Microbiol. Res. 2009, 164, 593–603. [Google Scholar] [CrossRef]
- Sabria, M.; Yu, V.L. Hospital-acquired Legionellosis: Solutions for a Preventable Solutions. Lancet Infect. Dis. 2002, 2, 368–373. [Google Scholar] [CrossRef]
- Fields, B.S.; Benson, R.F.; Besser, R.E. Legionella and Legionnaires’ Disease: 25 Years of Investigation. Clin. Microbiol. Rev. 2002, 15, 506–526. [Google Scholar] [CrossRef] [Green Version]
- Rowbotham, T.J. Preliminary Report on the Pathogenicity of Legionella pneumophila for Freshwater and Soil Amoebae. J. Clin. Pathol. 1980, 33, 1179–1183. [Google Scholar] [CrossRef] [Green Version]
- Horwitz, M.A. Phagocytosis of the Legionnaires’ Disease Bacterium (Legionella pneumophila) Occurs by a Novel Mechanism: Engulfment within a Pseudopod Coil. Cell 1984, 36, 27–33. [Google Scholar] [CrossRef]
- Rittiga, M.G.; Burmesterb, G.R.; Krauseb, A. Coiling Phagocytosis: When the Zipper James, the Cup is Deformed. Trends Microbiol. 1998, 6, 384–388. [Google Scholar] [CrossRef]
- Horwitz, M.A.; Silverstein, S.C. Legionnaires’ Disease Bacterium (Legionella pneumophila) Multiplies Intracellularly in Human Monocytes. J. Clin. Investig. 1980, 66, 441–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byrne, B.; Swanson, M.S. Expression of Legionella pneumophila Virulence Traits in Response to Growth Conditions. Infect. Immun. 1998, 66, 3029–3034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maiwald, M.; Helbig, J.H.; Luck, P.C. Laboratory Methods for the Diagnosis of Legionella Infections. J. Microbiol. Methods 1998, 33, 59–79. [Google Scholar] [CrossRef]
- IDEXX Laboratories. IDEXX Releases Revolutionary Water Test to Aid in the Fight Against Legionnaires’ Disease. 2016. Available online: https://www.idexx.com/en/about-idexx/news/newsroom-archive/idexx-releases-revolutionary-water-test-aid-fight-against-legionnaires-disease/ (accessed on 24 February 2020).
- Sartory, D.P.; Spies, K.; Lange, B.; Schneider, S.; Langer, B. Evaluation of Most Probable Number Method for the Enumeration of Legionella pneumophila from Potable and Related Water Samples. Lett. Appl. Microbiol. 2017, 64, 271–275. [Google Scholar] [CrossRef]
- Petrisek, R.; Hall, J. Evaluation of a Most Probable Number Method for the Enumeration of Legionella pneumophila from North American Potable and Nonpotable Water Samples. J. Water Health 2018, 16, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Yu, V.L.; Plouffe, J.F.; Pastoris, M.C.; Stout, J.E.; Schousboe, M.; Widmer, A.; Summersgill, J.; File, T.; Heath, C.M.; Paterson, D.L.; et al. Distribution of Legionella Species and Serogroups Isolated by Culture in Patients with Sporadic Community-Acquired Legionellosis: An International Collaborative Survey. J. Infect. Dis. 2002, 186, 127–128. [Google Scholar] [CrossRef] [Green Version]
- Marston, B.J.; Lipman, H.B.; Breiman, R.F. Surveillance for Legionnaires’ Disease: Risk Factors for Morbidity and Mortality. Arch. Intern. Med. 1994, 154, 2417–2422. [Google Scholar] [CrossRef]
- Plouffe, J.; Para, M.; Hackman, B.; Webster, L.; Maher, W. Nosocomial Legionnaires Disease: Difference in Attack Rates Associated with Two Strains of Legionella pneumophila Serogroup 1. In Legionella: Proceedings of the 2nd International Symposium; Thornsberry, C., Balows, A., Feeley, J.C., Jakubowski, W., Eds.; American Society for Microbiology: Washington, DC, USA, 1984. [Google Scholar]
- Cervero-Arago, S.; Rodriguez-Martinez, S.; Puertas-Bennasar, A.; Araujo, R.M. Effect of Common Drinking Water Disinfectants, Chlorine and Heat, on Free Legionella and Amoebae-Associated Legionella. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [Green Version]
- Venkobachar, C.; Iyengar, L.; Rao, A.V.S.P. Mechanism of Disinfection: Effect of Chlorine on Cell Membrane Functions. Water Res. 1977, 11, 727–729. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (USEPA). Federal Register Notice; National Primary Drinking Water Regulations; Disinfectants and Disinfection Byproducts; Final Rule. 63 FR 69390; USEPA: Washington, DC, USA, 1998.
- Orsi, G.B.; Vitali, M.; Marinelli, L.; Ciorba, V.; Tufi, D.; Cimmuto, A.D.; Ursillo, P.; Fabiani, M.; Santis, S.D.; Protano, C.; et al. Legionella Control in the Water System of Antiquated Hospital Buildings by Shock and Continuous Hyperchlorination: 5 Years Experience. BMC Infect. Dis. 2014, 14, 394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Health Protection Surveillance Centre. National guidelines for the control of legionellosis in Ireland, 2009; Report of Legionnaires’ Disease Subcommittee of the Scientific Advisory Committee, Health Protection Surveillance Centre: Dublin, Ireland, 2009. [Google Scholar]
- Loret, J.F.; Robert, S.; Thomas, V.; Cooper, A.J.; McCoy, W.F.; Lévi, Y. Comparison of disinfectants for biofilm, protozoa and Legionella control. IWA J. Water Health 2005, 3, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Grosserode, M.; Wenzel, R.; Pfaller, M.; Helms, C. Continuous hyperchlorination for control of nosocomial Legionella pneumophila pneumonia: A 10-year follow-up of efficacy, environmental effects, and costs. In Legionella: Current Status and Emerging Perspectives; Barbaree, J.M., Breiman, R.F., Dufour, A.P., Eds.; American Society of Microbiology: Washington, DC, USA, 1993; pp. 226–229. [Google Scholar]
- Hassinen, J.; Lundbȁck, M.; Ifwarson, M.; Gedde, U.W. Deterioration of polyethylene pipes exposed to chlorinated water. Polym. Degrad. Stab. 2004, 84, 261–267. [Google Scholar] [CrossRef]
- McGuire, M.J. Eight revolutions in the history of U.S. drinking water disinfection. J. AWWA 2006, 98, 123–149. [Google Scholar] [CrossRef]
- Cooper, I.R.; Hanlon, G.W. Resistance of Legionella pneumophila serotype 1 biofilms to chlorine-based disinfection. J. Hosp. Infect. 2009, 74, 152–159. [Google Scholar] [CrossRef]
- Xing, X.; Wang, H.; Hu, C.; Liu, L. Effects of phosphate-enhanced ozone/biofiltration on formation of disinfection byproducts and occurrence of opportunistic pathogens in drinking water distribution systems. Water Res. 2018, 139, 168–176. [Google Scholar] [CrossRef]
- Fisher, I.; Kastl, G.; Sathasivan, A. New model of chlorine-wall reaction for simulating chlorine concentration in drinking water distribution systems. Water Res. 2017, 125, 427–437. [Google Scholar] [CrossRef]
- Storey, M.V.; Winiecka-Krusnell, J.; Ashbolt, N.J.; Stenström, T.A. The efficacy of heat and chlorine treatment against thermotolerant Acanthamoebae and Legionellae. Scand. J. Infect. Dis. 2004, 36, 656–662. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (USEPA). Alternative Disinfectants and Oxidants Guidance Manual; EPA 815-R-99-014; USEPA: Washington, DC, USA, 1999.
- Lin, Y.E.; Stout, J.E.; Yu, V.L. Controlling Legionella in hospital drinking water: An evidence-based review of disinfection methods. Infect. Control Hosp. Epidemiol. 2011, 32, 166–173. [Google Scholar] [CrossRef] [Green Version]
- Dupuy, M.; Mazoua, S.; Berne, F.; Bodet, C.; Garrec, N.; Herbelin, P.; Ménard-Szczebara, F.; Oberti, S.; Rodier, M.H.; Soreau, S.; et al. Efficiency of Water Disinfectants Against Legionella pneumophila and Acanthamoeba. Water Res. 2011, 45, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; McCann, C.; Hanrahan, J.; Jencson, A.; Joyce, D.; Fyffe, S.; Piescynski, S.; Hawks, R.; Stout, J.E.; Yu, V.L.; et al. Legionella Control by Chlorine Dioxide in Hospital Water Systems. Am. Water Works Assoc. J. 2009, 101, 117–127. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. National Primary Drinking Water Regulations; EPA 816-F-09-004; USEPA: Washington, DC, USA, 2009.
- Aieta, E.M.; Berg, J.D. A review of chlorine dioxide in drinking water treatment. J. AWWA 1986, 78, 62–72. [Google Scholar] [CrossRef]
- Landeen, L.K.; Yahya, M.T.; Gerba, C.P. Efficacy of copper and silver ions and reduced levels of free chlorine in inactivation of Legionella pneumophila. Appl. Environ. Microbiol. 1989, 55, 3045–3050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- United States Environmental Protection Agency (USEPA). The Third Unregulated Contaminants Monitoring rule (UCMR3): Fact Sheet for Assessment Monitoring of List 1 Contaminants; EPA 815-F-12-003; USEPA: Washington, DC, USA, 2012.
- United States Environmental Protection Agency (USEPA). Economic Analysis for the Final Stage 2 Disinfectants and Disinfection Byproducts Rule; EPA 815-R-05-010; USEPA: Washington, DC, USA, 2005.
- Liu, Z.; Stout, J.E.; Tedesco, L.; Boldin, M.; Hwang, C.; Yu, V.L. Efficacy of ultraviolet light in preventing Legionella colonization of a hospital water distribution system. Water Res. 1995, 29, 2275–2280. [Google Scholar] [CrossRef]
- Herath, B.S.; Sathasivan, A. The chloramine stress induces the production of chloramine decaying proteins by microbes in biomass (biofilm). Chemosphere 2020, 124526. [Google Scholar] [CrossRef]
- Marchesi, I.; Ferranti, G.; Bargellini, A.; Marchegiano, P.; Predieri, G.; Stout, J.E.; Borella, P. Monochloramine and Chlorine Dioxide for Controlling Legionella pneumophila Contamination: Biocide Levels and Disinfection By-product Formation in Hospital Water Networks. J. Water Heatlh 2013, 11, 738–747. [Google Scholar] [CrossRef] [Green Version]
- Selbes, M.; Beita-Sandi, W.; Kim, D.; Karanfil, T. The Role of Chloramine Species in NDMA Formation. Water Res. 2018, 140, 100–109. [Google Scholar] [CrossRef] [Green Version]
- United States Environmental Protection Agency (USEPA). Regulatory Determinations for the Third Drinking Water Contaminant Candidate List; Stakeholder Meeting; USEPA: Washington, DC, USA, 2011.
- United States Environmental Protection Agency (USEPA). Integrated Risk Information System (IRIS); N-Nitrosodimethylamine; CASRN 62-75-9; USEPA: Washington, DC, USA, 1993.
- United States Environmental Protection Agency (USEPA). Regional Screening Level (RSL). Summary Table. 2013. Available online: https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables (accessed on 24 February 2020).
- Zhang, X.; Pehkonen, S.O.; Kocherginsky, N.; Ellis, G.A. Copper corrosion in mildly alkaline water with the disinfectant monochloramine. Corros. Sci. 2002, 44, 2507–2528. [Google Scholar] [CrossRef]
- Cunliffe, D.A. Inactivation of Legionella pneumophila by monochloramine. Appl. Bacteriol. 1990, 68, 453–459. [Google Scholar] [CrossRef]
- Liu, Z.; Stout, J.E.; Tedesco, L.; Boldin, M.; Hwang, C.; Diven, W.F.; Yu, V.L. Controlled evaluation of copper-silver ionization in eradicating Legionella pneumophila from a hospital water distribution system. Infect. Dis. 1994, 169, 919–922. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.E.; Vidic, R.D.; Stout, J.E.; Yu, V.L. Individual and Combined Effects of Copper and Silver Ions on Inactivation of Legionella pneumophila. Water Res. 1996, 30, 1905–1913. [Google Scholar] [CrossRef]
- Walraven, N.; Pool, W.; Chapman, C. Efficacy of Copper-Silver Ionisation in Controlling Legionella in Complex Water Distribution Systems and a Cooling Tower: Over 5 Years of Practical Experience. J. Water Process Eng. 2016, 13, 196–205. [Google Scholar] [CrossRef]
- Zevenhuizen, L.P.; Dolfing, J.; Eshuis, E.J.; Scholten-Koerselman, I.J. Inhibitory effects of copper on bacteria related to free ion concentration. Microb. Ecol. 1979, 5, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.E.; Vidic, R.D.; Stout, J.E.; Yu, V.L. Negative effect of high pH on biocidal efficacy of copper and silver ions in controlling Legionella pneumophila. Appl. Environ. Microbiol. 2002, 68, 2711–2715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhan, W.; Sathasivan, A.; Joll, C.; Wai, G.; Heitz, A.; Kristiana, I. Impact of NOM character on copper adsorption by trace ferric hydroxide from iron corrosion in water supply system. Chem. Eng. J. 2012, 200–202, 122–132. [Google Scholar] [CrossRef] [Green Version]
- Triantafyllidou, S.; Lytle, D.; Muhlen, C.; Swertfeger, J. Copper-silver ionization at a US hospital: Interaction of treated drinking water with plumbing materials, aesthetics and other considerations. Water Res. 2016, 102, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Andrews, S.A. Catalysis of copper corrosion products on chlorine decay and HAA formation in simulated distribution systems. Water Res. 2012, 46, 2665–2673. [Google Scholar] [CrossRef]
- Lytle, D.A.; Schock, M.R. Pitting corrosion of copper in waters with high pH and low alkalinity. J. AWWA 2008, 100, 115–129. [Google Scholar] [CrossRef]
- Rhoads, W.; Pruden, A.; Edwards, M.A. Interactive effects of corrosion, copper, and chloramines on Legionella and mycobacteria in hot water plumbing. Environ. Sci. Technol. 2017, 51, 7065–7075. [Google Scholar] [CrossRef]
- Zuma, F.; Lin, J.; Jonnalagadda, S.B. Ozone-initiated Disinfection Kinetics of Escherichia coli in Water. J. Environ. Sci. Health Part A 2009, 44, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Domingue, E.L.; Tyndall, R.L.; Mayberry, W.R.; Pancorbo, O.C. Effects of Three Oxidizing Biocides on Legionella pneumophila Serogroup 1. Appl. Environ. Microbiol. 1988, 54, 741–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanc, D.S.; Carra, P.; Zanetti, G.; Francioli, P. Water Disinfection with Ozone, Copper and Silver Ions, and Temperature Increase to Control Legionella: Seven Years of Experience in a University Teaching Hospital. J. Hosp. Infect. 2005, 60, 69–72. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency (USEPA). Microbial and Disinfection Byproducts Rules Simultaneous Compliance Guidance Manual; EPA 815-R-99-015; USEPA: Washington, DC, USA, 1999.
- United States Environmental Protection Agency (USEPA). Simultaneous Compliance Guidance Manual for the Long Term 2 and Stage 2 DBP Rules; EPA 815-R-07-017; USEPA: Washington, DC, USA, 2007.
- Lee, J.; Kaletunc, G. Evaluation of the Heat Inactivation of Escherichia coli and Lactobacillus plantarum by Differential Scanning Calorimetry. Appl. Environ. Microbiol. 2002, 68, 5379–5386. [Google Scholar] [CrossRef] [Green Version]
- Chapter 29: Plumbing Systems; 2015 International Building Code; International Code Council: Washington, DC, USA, 2015.
- Mouchtouri, V.; Velonakis, E.; Hadjichristodoulou, C. Thermal Disinfection of Hotels, Hospitals, and Athletic Venues Hot Water Distribution Systems Contaminated by Legionella Species. Am. J. Infect. Control 2007, 35, 623–627. [Google Scholar] [CrossRef]
- Sheffer, P.J.; Stout, J.E.; Wagener, M.M.; Muder, R.R. Efficacy of New Point-of-Use Water Filter for Preventing Exposure to Legionella and Waterborne Bacteria. Am. J. Infect. Control 2005, 33, S20–S25. [Google Scholar] [CrossRef]
- Sharma, H. Colonization of Granular Activated Carbon Media Filters by Legionella and Heterotrophic Bacterial Cells. Master’s Thesis, Arizona State University, Tempe, AZ, USA, 2014. [Google Scholar]
- Molloy, S.L.; Ives, R.; Hoyt, A.; Taylor, R.; Rose, J.B. The use of copper and silver in carbon point-of-use filters for the suppression of Legionella throughput in domestic water systems. Appl. Microbiol. 2008, 104, 998–1007. [Google Scholar] [CrossRef]
- Daeschlein, G.; Krüger, W.H.; Selepko, C.; Rochow, M.; Dölken, G.; Kramer, A. Hygienic safety of reusable tap water filters (GermLyser®) with an operating time of 4 or 8 weeks in a haematological oncology transplant unit. BMC Infect. Dis. 2007, 7, 45. [Google Scholar] [CrossRef] [Green Version]
- Marchesi, I.; Marchegiano, P.; Bargellini, A.; Cencetti, S.; Frezza, G.; Miselli, M.; Borella, P. Effectiveness of different methods to control Legionella in the water supply: Ten-year experience in an Italian university hospital. Hosp. Infect. 2011, 77, 47–51. [Google Scholar] [CrossRef]
- Baron, J.L.; Peters, T.; Shafer, R.; MacMurray, B.; Stout, J.E. Field evaluation of a new point-of-use faucet filter for preventing exposure to Legionella and other waterborne pathogens in health care facilities. Am. J. Infect. Control 2014, 42, 1193–1196. [Google Scholar] [CrossRef]
- States, S.; Kuchta, J.; Young, W.; Conley, L.; Ge, J.; Costeloa, M.; Dowling, J.; Wadowsky, R. Controlling Legionella using copper-silver ionization. J. AWWA 1998, 90, 122–129. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Silver in drinking water: Background document for development of WHO Guidelines for Drinking-water Quality; WHO/SDE/WSH/03.04/14; WHO: Geneva, Switzerland, 2003. [Google Scholar]
- Bernard, B.; Gate, G. Epidemic of Obscure Illness- Pontiac, Michigan. Morb. Mortal. Wkly. Rep. 1968, 17, 315–320. [Google Scholar]
- Knudson, G.B. Photoreactivation of UV-Irradiated Legionella pneumophila and Other Legionella Species. Appl. Environ. Microbiol. 1985, 49, 975–980. [Google Scholar] [CrossRef] [Green Version]
- Oguma, K.; Katayama, J.; Mitani, H.; Morita, S.; Hirata, T.; Ohgaki, S. Determination of Pyrimidine Dimers in Escherichia coli and Cryptosporidium parvum During UV Light Inactivation, Photoreactivation, and Dark Repair. Appl. Environ. Microbiol. 2001, 67, 4630–4637. [Google Scholar] [CrossRef] [Green Version]
- Franzin, L.; Cabodi, D.; Fantino, C. Evaluation of the efficacy of ultraviolet irradiation for disinfection of hospital water contaminated by Legionella. Hosp. Infect. 2002, 51, 269. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.K.; Giannetta, E.T.; Getchell-White, S.E.; Durbin, L.J.; Farr, B.M. Ultraviolet Light Disinfection of Hospital Water for Preventing Nosocomial Legionella Infection: A 13-Year Follow-Up. Infect. Control Hosp. Epidemiol. 2003, 24, 580–583. [Google Scholar] [CrossRef]
- Song, K.; Mohseni, M.; Taghipour, F. Application of Ultraviolet Light-Emitting Diodes (UV-LEDs) for Water Disinfection: A Review. Water Res. 2016, 94, 341–349. [Google Scholar] [CrossRef]
- Chevremont, A.C.; Farnet, A.M.; Coulomb, B.; Boudenne, J.L. Effect of Coupled UV-A and UV-C LEDs on Both Microbiological and Chemical Pollution of Urban Wastewaters. Sci. Total Environ. 2012, 426, 304–310. [Google Scholar] [CrossRef]
- Rattanakul, S.; Oguma, K. Inactivation Kinetics and Efficiencies of UV-LEDs against Pseudomonas aerginosa, Legionella pneumophila, and Surrogate Microoragnisms. Water Res. 2018, 130, 31–37. [Google Scholar] [CrossRef]
- Parkinson, J.; Baron, J.L.; Hall, B.; Bos, H.; Racine, P.; Wagener, M.M.; Stout, J.E. Point-of-use filters for prevention of health care–acquired Legionnaires’ disease: Field evaluation of a new filter product and literature review. Am. J. Infect. Control 2019. [Google Scholar] [CrossRef]
| Legionella Strains * | Time to 4-Log Reduction (Min.) | |
|---|---|---|
| 0.2 mg/L Free Chlorine | 0.5 mg/L Free Chlorine | |
| L. pneumophila serogroup 1 lab strain | Not achieved | 8 |
| L. pneumophila serogroup 1 environmental strain | Not achieved | 4 |
| L. pneumophila serogroup 7 lab strain | 9 | 2 |
| L. pneumophila serogroup 8 environmental strain | 20 | 3 |
| L. longbeachae lab strain | 11 | 3 |
| Condition | Number of Positive Legionella Sites (%) | Number of Negative Legionella Sites (%) | p-Value |
|---|---|---|---|
| Before chlorination | 43 (21.1) | 161 (78.9) | <0.001 |
| With continuous hyperchlorination | 23 (5.5) | 393 (94.5) |
| Treatment | Positive Samples | |
|---|---|---|
| Before Treatment | After Treatment | |
| Monochloramine System | ||
| - positive N (%) | 22/22 (100%) | 8/84 (9.5%) |
| - mean CFU/L (range) | 2.2 × 104 (1.0 × 102 – 9.5 × 105) | 3.3 × 102 (25 – 4.9 × 103) |
| Control Sytem | ||
| - positive N (%) | 84/85 (98.8%) | |
| - mean CFU/L (range) | 1.0 × 104 (25 – 1.3 × 106) | |
| Different Parameters for Inactivation of Legionella Pneumophila Serogroup 1 | |||
|---|---|---|---|
| pH | Temperature (°C) | Ozone Concentration (µg/mL) * | Log Inactivation |
| 7.2 | 25 | 0.21 | 2.37 |
| 7.2 | 35 | 0.13 | 2.21 |
| 7.2 | 45 | 0.13 | 2.55 |
| 8.0 | 25 | 0.20 | 2.45 |
| 8.9 | 25 | 0.14 | 3.28 |
| Percent Positivity | CFU/mL | p-Value | |
|---|---|---|---|
| Before ozonation | (66/100) 66% | 10.9 ± 17 | 0.12 |
| After ozonation | (67/120) 56% | 5.2 ± 9.7 |
| Legionella Strain * | Time to 4-Log Reduction (Min) | ||||
|---|---|---|---|---|---|
| 50 °C/R2 | 55 °C/R2 | 60 °C/R2 | 65 °C/R2 | 70 °C/R2 | |
| L. pneumophila serogroup 1 lab strain | 117/0.80 | 10/0.92 | 2/0.90 | 0.8/0.88 | 0.9/0.79 |
| L. pneumophila serogroup 1 environmental strain | 46/0.84 | 8/0.98 | 3/0.83 | 1.4/0.90 | 0.6/0.82 |
| L. pneumophila serogroup 7 lab strain | 40/0.97 | 25/0.96 | 3/0.76 | 0.6/0.87 | 1.2/0.77 |
| L. pneumophila serogroup 8 environmental strain | 68/0.97 | 16/0.89 | 4/0.94 | 0.8/0.90 | 0.7/0.99 |
| L. longbeachae lab strain | 15/0.94 | 2/0.88 | Not achieved | Not achieved | Not achieved |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carlson, K.M.; Boczek, L.A.; Chae, S.; Ryu, H. Legionellosis and Recent Advances in Technologies for Legionella Control in Premise Plumbing Systems: A Review. Water 2020, 12, 676. https://doi.org/10.3390/w12030676
Carlson KM, Boczek LA, Chae S, Ryu H. Legionellosis and Recent Advances in Technologies for Legionella Control in Premise Plumbing Systems: A Review. Water. 2020; 12(3):676. https://doi.org/10.3390/w12030676
Chicago/Turabian StyleCarlson, Kelsie M., Laura A. Boczek, Soryong Chae, and Hodon Ryu. 2020. "Legionellosis and Recent Advances in Technologies for Legionella Control in Premise Plumbing Systems: A Review" Water 12, no. 3: 676. https://doi.org/10.3390/w12030676
APA StyleCarlson, K. M., Boczek, L. A., Chae, S., & Ryu, H. (2020). Legionellosis and Recent Advances in Technologies for Legionella Control in Premise Plumbing Systems: A Review. Water, 12(3), 676. https://doi.org/10.3390/w12030676







