Removal of High-Strength Ammonia Nitrogen in Biofilters: Nitrifying Bacterial Community Compositions and Their Effects on Nitrogen Transformation
Abstract
:1. Introduction
- (1)
- , transformed by ammonia-oxidizing bacteria (AOB) and ammonia-oxidizing archaea (AOA).
- (2)
- , transformed by nitrite-oxidizing bacteria (NOB).
2. Materials and Methods
2.1. Bioreactor Construction and Basic Physicochemical Properties of Filter Media
2.2. Bioreactor Operation and Sampling
2.3. Synthetic Wastewater Composition
2.4. Analyses
2.4.1. Water Sample Collection and Water Quality Analysis
2.4.2. Filter Material Extraction and High-Throughput Sequencing (HTS) Analysis
2.4.3. Statistical Analyses
3. Results
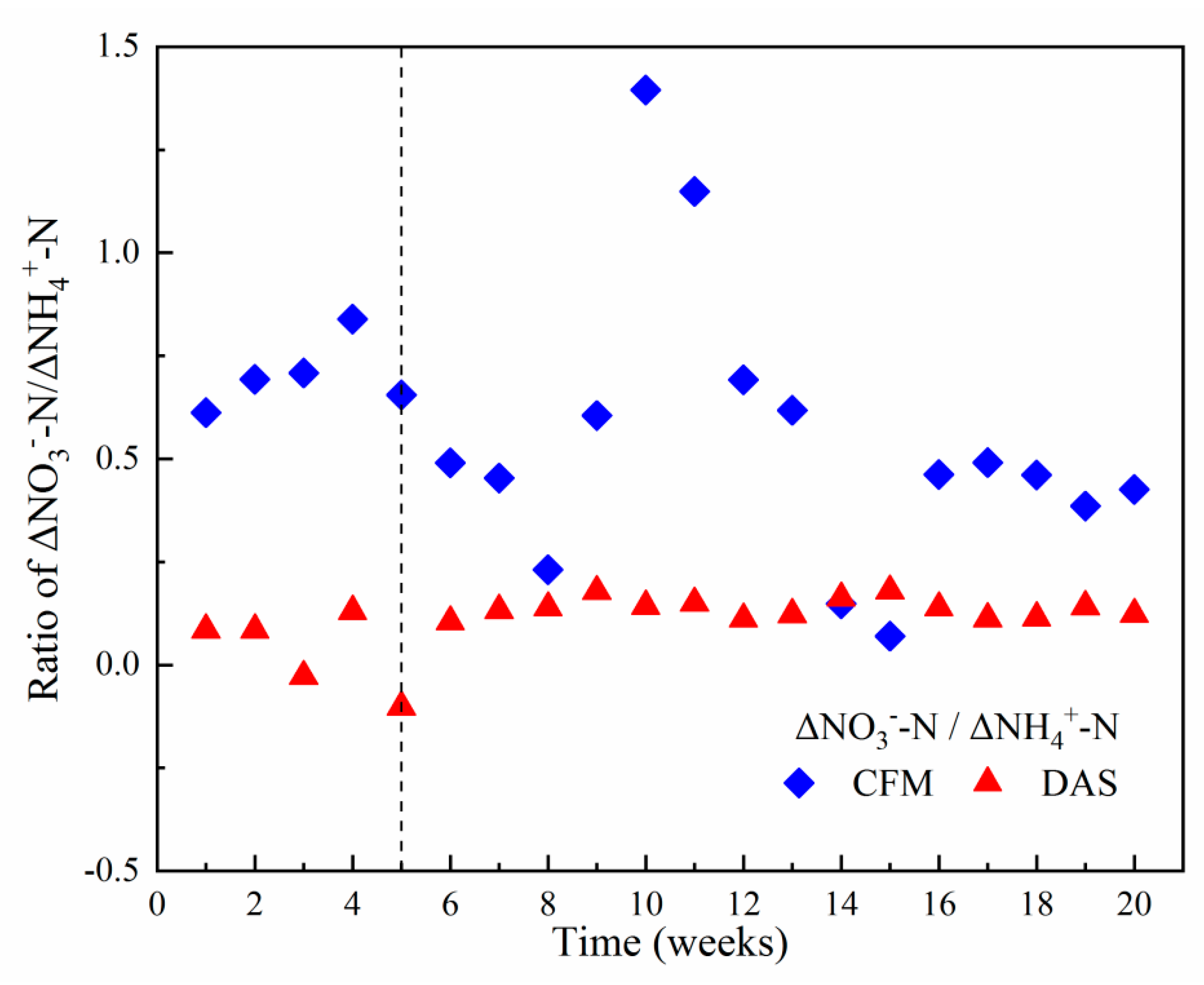
3.1. Wastewater Treatment Performance
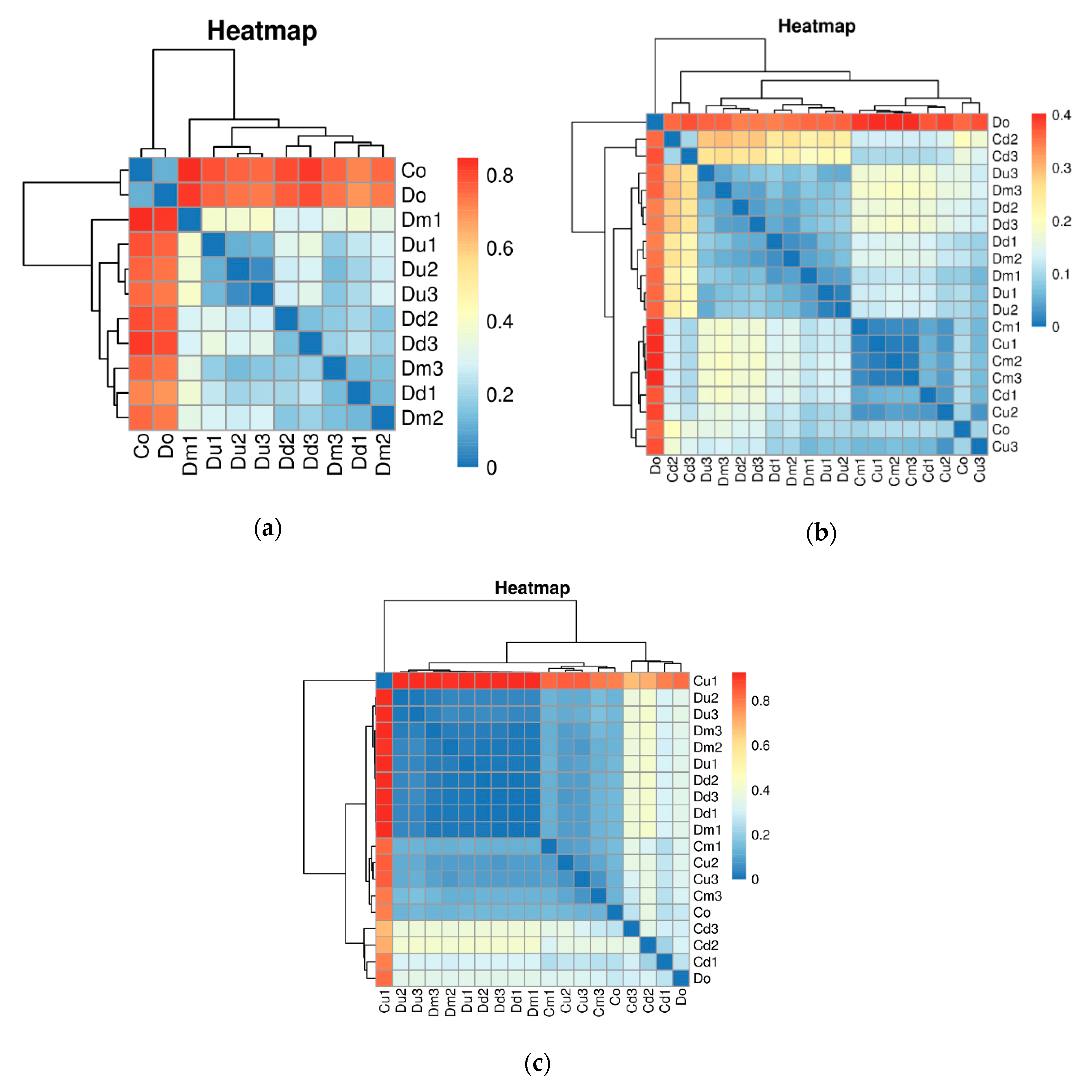
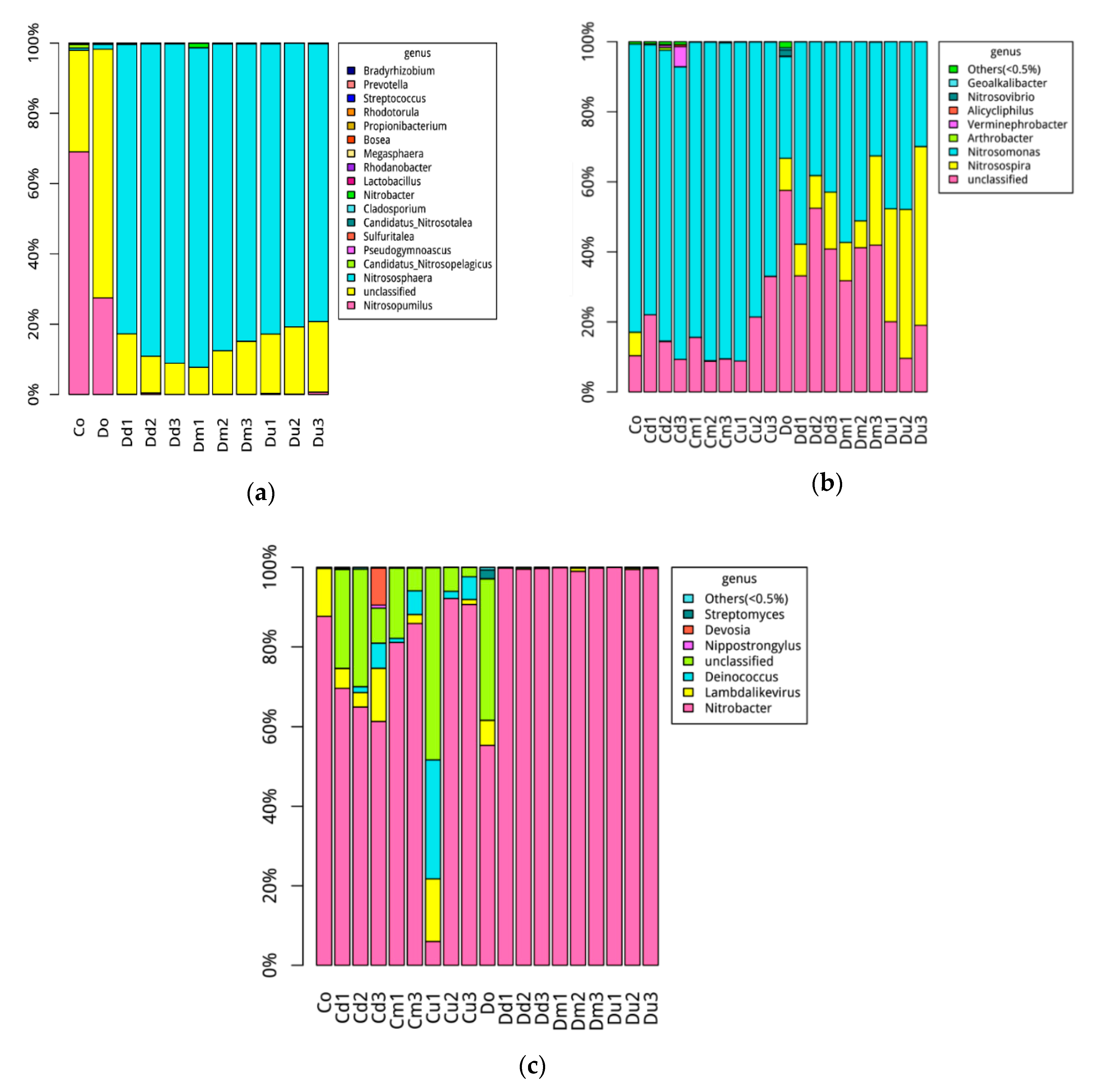
3.2. Nitrifying Bacteria Community Composition Distributions
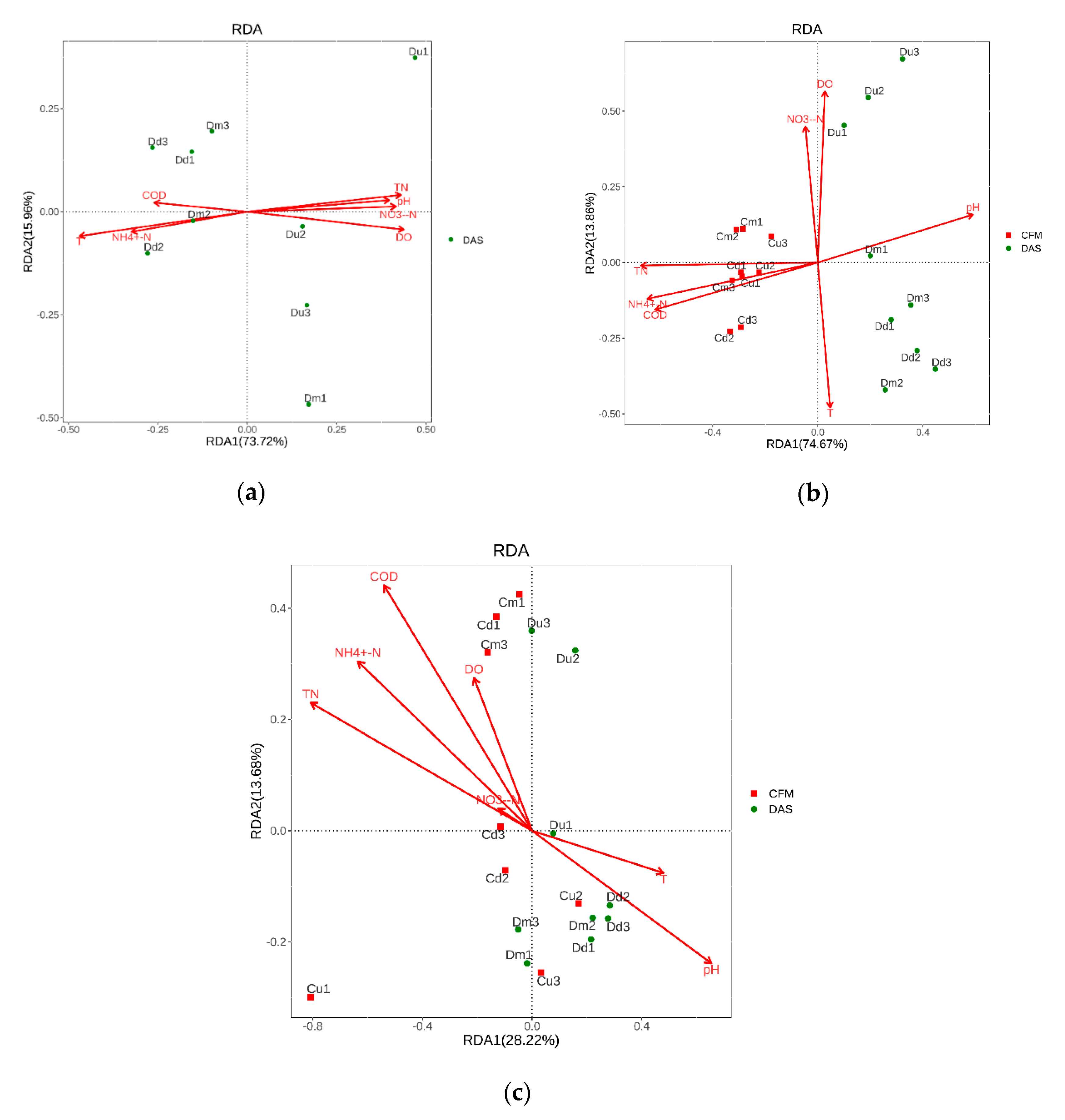
3.3. Correlation between Environmental Variables and Nitrifying Bacteria Structures
3.4. Pearson Relationship between the Nitrifying Bacterial Genera and Nitrogen Removal
4. Discussion
4.1. Effective COD and Nitrogen Removal
4.2. Compositions of Nitrifying Bacterial Communities and Its Relationship with Environment Factors
4.3. Response of Dominant Genera to Nitrogen Removal and Transformation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kizito, S.; Wu, S.; Kipkemoi Kirui, W.; Lei, M.; Lu, Q.; Bah, H.; Dong, R. Evaluation of slow pyrolyzed wood and rice husks biochar for adsorption of ammonium nitrogen from piggery manure anaerobic digestate slurry. Sci. Total Environ. 2015, 505, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Eryuruk, K.; Tezcan Un, U.; Bakır Ogutveren, U. Electrochemical treatment of wastewaters from poultry slaughtering and processing by using iron electrodes. J. Clean. Prod. 2018, 172, 1089–1095. [Google Scholar] [CrossRef]
- Dias, D.F.C.; Passos, R.G.; Rodrigues, V.A.J.; de Matos, M.P.; Santos, C.R.S.; von Sperling, M. Performance evaluation of a natural treatment system for small communities, composed of a UASB reactor, maturation ponds (baffled and unbaffled) and a granular rock filter in series. Environ. Technol. 2018, 39, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Winkler, M.K.; Straka, L. New directions in biological nitrogen removal and recovery from wastewater. Curr. Opin. Biotechnol. 2019, 57, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Callery, O.; Healy, M.G.; Rognard, F.; Barthelemy, L.; Brennan, R.B. Evaluating the long-term performance of low-cost adsorbents using small-scale adsorption column experiments. Water Res. 2016, 101, 429–440. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wang, X.; Yang, F.; Kong, M.; Peng, F.; Chao, J.; Gao, Y.; Wu, D.; Zhu, Y.; Zhang, Y. Nitrogen removal performance and ammonia- and nitrite-oxidizing bacterial community analysis of a novel industrial waste-based biofilter. Chem. Eng. J. 2016, 299, 156–166. [Google Scholar] [CrossRef]
- Al Nakouzi, N.; Le Moulec, S.; Albigès, L.; Wang, C.; Beuzeboc, P.; Gross-Goupil, M.; de La Motte Rouge, T.; Guillot, A.; Gajda, D.; Massard, C.; et al. Cabazitaxel Remains Active in Patients Progressing After Docetaxel Followed by Novel Androgen Receptor Pathway Targeted Therapies. Eur. Urol. 2015, 68, 228–235. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.; Zhang, P.; Yang, L.; Xu, H.A.; Xi, G. Adsorption characteristics of a novel ceramsite for heavy metal removal from stormwater runoff. Chin. J. Chem. Eng. 2018, 26, 96–103. [Google Scholar] [CrossRef]
- Cao, W.; Wang, Y.; Sun, L.; Jiang, J.; Zhang, Y. Removal of nitrogenous compounds from polluted river water by floating constructed wetlands using rice straw and ceramsite as substrates under low temperature conditions. Ecol. Eng. 2016, 88, 77–81. [Google Scholar] [CrossRef]
- Wu, H.; Fan, J.; Zhang, J.; Ngo, H.H.; Guo, W.; Liang, S.; Lv, J.; Lu, S.; Wu, W.; Wu, S. Intensified organics and nitrogen removal in the intermittent-aerated constructed wetland using a novel sludge-ceramsite as substrate. Bioresour. Technol. 2016, 210, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zou, F.; Fang, X.; Tsang, D.C.W.; Poon, C.S.; Leng, Z.; Baek, K. A novel type of controlled low strength material derived from alum sludge and green materials. Constr. Build. Mater. 2018, 165, 792–800. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, Y.; Zhao, X.; Kumar, J.L. High rate nitrogen removal in an alum sludge-based intermittent aeration constructed wetland. Environ. Sci. Technol. 2012, 46, 4583–4590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.; Zhao, Y.; Rymszewicz, A. Robust biological nitrogen removal by creating multiple tides in a single bed tidal flow constructed wetland. Sci. Total Environ. 2014, 470, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhao, X.; Zhao, Y. Achieving high-rate autotrophic nitrogen removal via Canon process in a modified single bed tidal flow constructed wetland. Chem. Eng. J. 2014, 237, 329–335. [Google Scholar] [CrossRef]
- Chen, H.; Jin, W.; Liang, Z.; Abomohra, A.E.F.; Zhou, X.; Tu, R.; Han, S. Abundance and diversity of ammonia-oxidizing archaea in a biological aerated filter process. Ann. Microbiol. 2017, 67, 405–416. [Google Scholar] [CrossRef]
- Wongkiew, S.; Hu, Z.; Chandran, K.; Lee, J.W.; Khanal, S.K. Nitrogen transformations in aquaponic systems: A review. Aquacult. Eng. 2017, 76, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Tian, J.; Yu, C.; Liu, J.; Ye, C.; Zhou, X.; Chen, L. Performance of an Ultraviolet Mutagenetic Polyphosphate-Accumulating Bacterium PZ2 and Its Application for Wastewater Treatment in a Newly Designed Constructed Wetland. Appl. Biochem. Biotechnol. 2017, 181, 735–747. [Google Scholar] [CrossRef]
- Nsenga Kumwimba, M.; Meng, F. Roles of ammonia-oxidizing bacteria in improving metabolism and cometabolism of trace organic chemicals in biological wastewater treatment processes: A review. Sci. Total Environ. 2019, 659, 419–441. [Google Scholar] [CrossRef]
- Meng, J.; Li, J.; Li, J.; Antwi, P.; Deng, K.; Wang, C.; Buelna, G. Nitrogen removal from low COD/TN ratio manure-free piggery wastewater within an upflow microaerobic sludge reactor. Bioresour. Technol. 2015, 198, 884–890. [Google Scholar] [CrossRef]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Waste Water; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Ma, J.; Wu, H.; Wang, Y.; Qiu, G.; Fu, B.; Wu, C.; Wei, C. Material inter-recycling for advanced nitrogen and residual COD removal from bio-treated coking wastewater through autotrophic denitrification. Bioresour. Technol. 2019, 289, 121616. [Google Scholar] [CrossRef]
- Yue, X.; Yu, G.; Lu, Y.; Liu, Z.; Li, Q.; Tang, J.; Liu, J. Effect of dissolved oxygen on nitrogen removal and the microbial community of the completely autotrophic nitrogen removal over nitrite process in a submerged aerated biological filter. Bioresour. Technol. 2018, 254, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, X.; Zhang, H.; Wu, H. Enhanced nitrogen removal of low C/N domestic wastewater using a biochar-amended aerated vertical flow constructed wetland. Bioresour. Technol. 2017, 241, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Katayama, Y.; Gu, J.D. More wide occurrence and dominance of ammonia-oxidizing archaea than bacteria at three Angkor sandstone temples of Bayon, Phnom Krom and Wat Athvea in Cambodia. Int. Biodeterior. Biodegrad. 2017, 117, 78–88. [Google Scholar] [CrossRef]
- Nsenga Kumwimba, M.; Lotti, T.; Senel, E.; Li, X.; Suanon, F. Anammox-based processes: How far have we come and what work remains? A review by bibliometric analysis. Chemosphere 2020, 238, 124627. [Google Scholar] [CrossRef] [PubMed]
- Martens-Habbena, W.; Berube, P.M.; Urakawa, H.; de la Torre, J.R.; Stahl, D.A. Ammonia oxidation kinetics determine niche separation of nitrifying Archaea and Bacteria. Nature 2009, 461, 976–979. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Norton, J.M.; Stark, J.M.; Reeve, J.R.; Habteselassie, M.Y. Ammonia-oxidizing bacteria are more responsive than archaea to nitrogen source in an agricultural soil. Soil. Biol. Biochem. 2016, 96, 4–15. [Google Scholar] [CrossRef] [Green Version]
- Roy, D.; McEvoy, J.; Blonigen, M.; Amundson, M.; Khan, E. Seasonal variation and ex-situ nitrification activity of ammonia oxidizing archaea in biofilm based wastewater treatment processes. Bioresour. Technol. 2017, 244, 850–859. [Google Scholar] [CrossRef]
- Liu, Z.; Xie, H.; Hu, Z.; Zhang, J.; Zhang, J.; Sun, H.; Lan, W. Role of Ammonia-Oxidizing Archaea in Ammonia Removal of Wetland Under Low-Temperature Condition. Water Air Soil Pollut. 2017, 228, 356. [Google Scholar] [CrossRef]
- Gao, J.F.; Luo, X.; Wu, G.X.; Li, T.; Peng, Y.Z. Quantitative analyses of the composition and abundance of ammonia-oxidizing archaea and ammonia-oxidizing bacteria in eight full-scale biological wastewater treatment plants. Bioresour. Technol. 2013, 138, 285–296. [Google Scholar] [CrossRef]
- Limpiyakorn, T.; Furhacker, M.; Haberl, R.; Chodanon, T.; Srithep, P.; Sonthiphand, P. amoA-encoding archaea in wastewater treatment plants: A review. Appl. Microbiol. Biotechnol. 2013, 97, 142–539. [Google Scholar] [CrossRef]
- Yan, L.; Li, Z.; Wang, G.; Gao, Y.; Wang, Y.; Gu, J.D.; Wang, W. Diversity of ammonia-oxidizing bacteria and archaea in response to different aeration rates during cattle manure composting. Ecol. Eng. 2016, 93, 46–54. [Google Scholar] [CrossRef]
- Hu, D.; Zhou, Z.; Niu, T.; Wei, H.; Dou, W.; Jiang, L.M.; Lv, Y. Co-treatment of reject water from sludge dewatering and supernatant from sludge lime stabilization process for nutrient removal: A cost-effective approach. Sep. Purif. Technol. 2017, 172, 357–365. [Google Scholar] [CrossRef]
- Wells, G.F.; Park, H.D.; Yeung, C.H.; Eggleston, B.; Francis, C.A.; Criddle, C.S. Ammonia-oxidizing communities in a highly aerated full-scale activated sludge bioreactor: Betaproteobacterial dynamics and low relative abundance of Crenarchaea. Environ. Microbiol. 2009, 11, 2310–2328. [Google Scholar] [CrossRef] [PubMed]
- Terada, A.; Sugawara, S.; Yamamoto, T.; Zhou, S.; Koba, K.; Hosomi, M. Physiological characteristics of predominant ammonia-oxidizing bacteria enriched from bioreactors with different influent supply regimes. Biochem. Eng. J. 2013, 79, 153–161. [Google Scholar] [CrossRef]
- Vandekerckhove, T.G.L.; Kerckhof, F.M.; De Mulder, C.; Vlaeminck, S.E.; Boon, N. Determining stoichiometry and kinetics of two thermophilic nitrifying communities as a crucial step in the development of thermophilic nitrogen removal. Water Res. 2019, 156, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Pelissari, C.; Guivernau, M.; Vinas, M.; Garcia, J.; Velasco-Galilea, M.; Souza, S.S.; Sezerino, P.H.; Avila, C. Effects of partially saturated conditions on the metabolically active microbiome and on nitrogen removal in vertical subsurface flow constructed wetlands. Water Res. 2018, 141, 185–195. [Google Scholar] [CrossRef]
- Wang, L.K.; Zeng, G.M.; Yang, Z.H.; Luo, L.L.; Xu, H.Y.; Huang, J. Operation of partial nitrification to nitrite of landfill leachate and its performance with respect to different oxygen conditions. Biochem. Eng. J. 2014, 87, 62–68. [Google Scholar] [CrossRef]


| Index | Values |
|---|---|
| chemical oxygen demand (COD) | 174.4 ± 59.9 (mg·L−1) |
| ammonia nitrogen (NH4+-N) | 117.0 ± 28.9 (mg·L−1) |
| nitrate nitrogen (NO3−-N) | 1.5 ± 0.7 (mg·L−1) |
| Total nitrogen (TN) | 131.1 ± 26.9 (mg·L−1) |
| pH | 8.0 ± 0.2 |
| Air temperature | 25.1 ± 7.7 °C |
| Water temperature | 23.5 ± 6.3 °C |
| total phosphorus (TP) | 22.8 ± 4.0 (mg·L−1) |
| Filter | Genus | Eff. COD | Eff. TN | Eff. NH4+-N | Eff. NO3−-N | |
|---|---|---|---|---|---|---|
| CFM | Nitrosomonas | R | 0.066 | −0.111 | −0.065 | −0.285 |
| p-Value | 0.866 | 0.776 | 0.867 | 0.458 | ||
| Nitrobacter | R | 0.328 | 0.139 | 0.485 | 0.115 | |
| p-Value | 0.427 | 0.742 | 0.224 | 0.786 | ||
| Lambdalikevirus | R | −0.638 | −0.097 | −0.188 | −0.250 | |
| p-Value | 0.088 | 0.819 | 0.656 | 0.551 | ||
| Deinococcus | R | −0.443 | 0.222 | −0.687 | 0.294 | |
| p-Value | 0.271 | 0.598 | 0.060 | 0.480 | ||
| DAS | Nitrososphaera | R | 0.439 | −0.441 | 0.569 | −0.672 * |
| p-Value | 0.237 | 0.234 | 0.110 | 0.048 | ||
| Nitrosomonas | R | 0.538 | 0.336 | 0.435 | −0.135 | |
| p-Value | 0.135 | 0.377 | 0.242 | 0.729 | ||
| Nitrosospira | R | −0.764 * | 0.384 | −0.807 ** | 0.808 ** | |
| p-Value | 0.016 | 0.307 | 0.009 | 0.008 | ||
| Nitrobacter | R | 0.144 | −0.032 | 0.092 | −0.069 | |
| p-Value | 0.711 | 0.935 | 0.814 | 0.860 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, F.; Gao, Y.; Zhu, X.; Pang, Q.; Wang, L.; Xu, W.; Yu, J.; Gao, P.; Huang, J.; Cui, Y. Removal of High-Strength Ammonia Nitrogen in Biofilters: Nitrifying Bacterial Community Compositions and Their Effects on Nitrogen Transformation. Water 2020, 12, 712. https://doi.org/10.3390/w12030712
Peng F, Gao Y, Zhu X, Pang Q, Wang L, Xu W, Yu J, Gao P, Huang J, Cui Y. Removal of High-Strength Ammonia Nitrogen in Biofilters: Nitrifying Bacterial Community Compositions and Their Effects on Nitrogen Transformation. Water. 2020; 12(3):712. https://doi.org/10.3390/w12030712
Chicago/Turabian StylePeng, Fuquan, Yuexiang Gao, Xiang Zhu, Qingqing Pang, Longmian Wang, Wenwen Xu, Jianghua Yu, Pengcheng Gao, Jingxian Huang, and Yibin Cui. 2020. "Removal of High-Strength Ammonia Nitrogen in Biofilters: Nitrifying Bacterial Community Compositions and Their Effects on Nitrogen Transformation" Water 12, no. 3: 712. https://doi.org/10.3390/w12030712
APA StylePeng, F., Gao, Y., Zhu, X., Pang, Q., Wang, L., Xu, W., Yu, J., Gao, P., Huang, J., & Cui, Y. (2020). Removal of High-Strength Ammonia Nitrogen in Biofilters: Nitrifying Bacterial Community Compositions and Their Effects on Nitrogen Transformation. Water, 12(3), 712. https://doi.org/10.3390/w12030712




