The Effect of Irrigation Treatment on the Growth of Lavender Species in an Extensive Green Roof System
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Physical-Hydraulic Properties of Substrate
2.3. Experimental Design and Irrigation Treatments
2.4. Plant Growth Biometrics
2.5. Meteorological Conditions
2.6. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Physical-Hydraulic Properties of Substrate
3.2. Symptoms Induced by Water Stress
3.3. Plant Growth
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Azunre, G.A.; Amponsah, O.; Peprah, C.; Takyi, S.A.; Braimah, I. A review of the role of urban agriculture in the sustainable city discourse. Cities 2019, 93, 104–119. [Google Scholar] [CrossRef]
- Mougeot, L.J.A. Urban. Agriculture: Definition, Presence, Potentials and Risks, and Policy Challenges; Cities Feeding People Series Report 31; International Development Research Centre (IDRC): Ottawa, ON, Canada, 2000; 62p. [Google Scholar]
- Marques-Perez, I.; del Rio, B.S.G. Identifying Functionality of Peri-Urban Agricultural Systems: A Case Study. In Urban Agriculture; Samer, M., Ed.; InTech: London, UK, 2016; pp. 61–88. [Google Scholar]
- Artmann, M.; Sartison, K. The Role of Urban Agriculture as a Nature-Based Solution: A Review for Developing a Systemic Assessment Framework. Sustainability 2018, 10, 1–32. [Google Scholar] [CrossRef]
- Dunnett, N.; Kingsbury, N. Planting Green Roofs and Living Walls; Timber Press: Portland, OR, USA, 2004; 254p. [Google Scholar]
- Dubbeling, M.; Orsini, F.; Gianquinto, G. The Status and Challenges of Rooftop Agriculture. In Rooftop Urban Agriculture; Orsini, F., Dubbeling, M., de Zeeuw, H., Gianquinto, G., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 3–8. [Google Scholar]
- Besir, A.B.; Cuce, E. Green roofs and facades: A comprehensive review. Renew. Sustain. Energy Rev. 2018, 82, 915–939. [Google Scholar] [CrossRef]
- Paraskevopoulou, A.; Mitsios, I.; Fragkakis, I.; Nektarios, P.; Ntoulas, N.; Londra, P.; Papafotiou, M. The growth of Arthrocnemum macrostachyum and Halimione portulacoides in an extensive green roof system under two watering regimes. Agric. Agric. Proc. 2015, 4, 242–249. [Google Scholar] [CrossRef]
- Savi, T.; Boldrin, D.; Marin, M.; Love, V.L.; Andri, S.; Trtiah, M.; Nardini, A. Does shallow substrate improve water status of plants growing on green roofs? Testing the paradox in two sub-Mediterranean shrubs. Ecol. Eng. 2015, 84, 292–300. [Google Scholar] [CrossRef]
- Du, P.; Arndt, S.K.; Farrell, C. Is plant survival on green roofs related to their drought response, water use or climate or origin? Sci. Total Environ. 2019, 667, 25–32. [Google Scholar] [CrossRef]
- Papafotiou, M.; Pergalioti, N.; Tassoula, L. Growth of Native Aromatic Xerophytes in an Extensive Mediterranean Green Roof as Affected by Substrate Type and Depth and Irrigation Frequency. HortScience 2013, 48, 1327–1333. [Google Scholar] [CrossRef]
- Papafotiou, M.; Pergalioti, N.; Massas, I.; Kargas, G. Effect of Substrate Type and Depth and the Irrigation Frequency on Growth of Semiwoody Mediterranean Species in Green Roofs. Acta Hortic. 2013, 990, 481–486. [Google Scholar] [CrossRef]
- Tassoula, L.; Papafotiou, M.; Liakopoulos, G.; Kargas, G. Growth of the Native Xerophyte Convolvulus cneorum L. on an Extensive Mediterranean Green Roof under Different Substrate Types and Irrigation Regimens. HortScience 2015, 50, 1118–1124. [Google Scholar] [CrossRef]
- Papafotiou, M.; Tassoula, L.; Liakopoulos, G.; Kargas, G. Effect of substrate type and irrigation frequency on growth of Mediterranean xerophytes on green roofs. Acta Hortic. 2016, 1108, 309–315. [Google Scholar] [CrossRef]
- Papafotiou, M.; Tassoula, L.; Kefalopoulou, R. Effect of substrate type and irrigation frequency on growth of Pallenis maritima on an urban extensive green roof at the semi-arid Mediterranean region. Acta Hortic. 2017, 1189, 275–278. [Google Scholar] [CrossRef]
- Tassoula, L.; Papafotiou, M.; Fouskaki, M. Growth of the halophyte Atriplex halimus on a green roof at the semi-arid Mediterranean region as affected by substrate type and irrigation regime. Acta Hortic. 2017, 1189, 287–290. [Google Scholar] [CrossRef]
- Baltzoi, P.; Fotia, K.; Kyrkas, D.; Nikolaou, K.; Paraskevopoulou, A.T.; Accogli, A.R.; Karras, G. Low water–demand plants for landscaping and agricultural cultivations—A review regarding local species of Epirus/Greece and Apulia/Italy. Agric. Agric. Proc. 2015, 4, 250–260. [Google Scholar] [CrossRef]
- Wolf, D.; Lundholm, J.T. Water uptake in green roof microcosms: Effects of plant species and water availability. Ecol. Eng. 2008, 33, 179–186. [Google Scholar] [CrossRef]
- Vestrella, A.; Biel, C.; Savè, A.; Bartoli, F. Mediterranean Green Roof Simulation in Caldes de Montbui (Barcelona): Thermal and Hydrological Performance Test of Frankenia laevis L., Dymondia margaretae Compton and Iris lutescens Lam. Appl. Sci. 2018, 8, 2497. [Google Scholar] [CrossRef]
- Bartlett, M.K.; Klein, T.; Jansen, S.; Choat, B.; Sack, L. The correlations and sequence of plant stomatal, hydraulic, and wilting responses to drought. PNAS 2016, 113, 13098–13103. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, T.J.; Atkinson, C.J. Stomatal behavior in water stressed plants. In Stress Responses in Plants: Adaptation and Acclimation Mechanisms; Alscher, R.G., Cumming, J.R., Eds.; Wiley-Liss: New York, NY, USA, 1990; pp. 241–264. [Google Scholar]
- Arve, L.E.; Torre, S.; Olsen, J.E.; Tanino, K.K. Stomatal Responses to Drought Stress and Air Humidity. In Abiotic Stress in Plants—Mechanisms and Adaptations; Arun, K.S., Venkateswarlu, B., Eds.; Intech: Rijeka, Croatia, 2011; pp. 279–280. [Google Scholar]
- Pirasteh-Anosheh, H.; Saed-Moucheshi, A.; Pakniyat, H.; Pessarakli, M. Stomatal responses to drought stress. In Water Stress and Crop. Plants: A Sustainable Approach, 1st ed.; Ahmad, P., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2016; Volume 1, pp. 24–40. [Google Scholar]
- Torres-Ruiz, J.M.; Diaz-Espejo, A.; Morales-Sillero, A.; Martín- Palomo, M.J.; Mayr, S.; Beikircher, B.; Fernandez, J.E. Shoot hydraulic characteristics, plant water status and stomatal response in olive trees under different soil water conditions. Plant. Soil 2013, 373, 77–87. [Google Scholar] [CrossRef]
- Casson, S.A.; Hetherington, A.M. Environmental regulation of stomatal development. Curr. Opinion Plant. Biol. 2010, 13, 90–95. [Google Scholar] [CrossRef]
- De Boodt, M.; Verdonck, O. The physical properties of the substrates in horticulture. Acta Hortic. 1972, 26, 37–44. [Google Scholar] [CrossRef]
- The Plant List, Version 1.1. 2013. Available online: http://www.theplantlist.org/1.1/browse/A/Lamiaceae/Lavandula/#statistics (accessed on 18 March 2020).
- Upson, T. The taxonomy of the genus Lavandula, L. In Lavender The genus Lavandula; Lis-Balchin, M., Ed.; Taylor & Francis: London, UK, 2002; pp. 2–34. [Google Scholar]
- Lis-Balchin, M. General introduction to the genus Lavandula. In Lavender The genus Lavandula; Lis-Balchin, M., Ed.; Taylor & Francis: London, UK, 2002; p. 1. [Google Scholar]
- Haines, W.B. Studies in the physical properties of soils. V. The hysteresis effect in capillary properties and the modes of moisture distribution associated therewith. J. Agric. Sci. 1930, 20, 97–116. [Google Scholar] [CrossRef]
- Van Genuchten, M.T.; Leij, F.J.; Yates, S.R. The RETC Code for Quantifying the Hydraulic Functions of Unsaturated Soils; U.S.D.A. (U.S. Department of Agriculture, Agricultural Research Service): Riverside, CA, USA, 1991. [Google Scholar]
- Mualem, Y. A new model for predicting the hydraulic conductivity of unsaturated porous media. Water Resour. Res. 1976, 12, 513–522. [Google Scholar] [CrossRef]
- Van Genuchten, M.T. A closed-form equation for predicting the hydraulic conductivity of unsaturated soils. Soil Sci. Soc. Am. J. 1980, 44, 892–898. [Google Scholar] [CrossRef]
- Klute, A.; Dirksen, C. Methods of Soil Analysis, Part 1, Physical and Mineralogical Methods; Klute, A., Ed.; American Society of Agronomy: Madison, WI, USA, 1986; pp. 694–700. [Google Scholar]
- IERSD-NOA. Monthly Bulletin; Institute of Environmental Research and Sustainable Development of the National Observatory of Athens: Penteli, Greece, 2016; Available online: https://meteo.gr/Monthly_Bulletins.cfm (accessed on 7 January 2020).
- W.M.O. Guide to Meteorological Instruments and Methods of Observation; WMO-No. 8 (updated 2017); World Meteorological Organization (W.M.O.): Geneva, Switzerland, 2014; 1166p. [Google Scholar]
- Raviv, M.; Lieth, J.H. Soilless Culture Theory and Practice; Elsevier BV: London, UK, 2008; p. 587. [Google Scholar]
- Naaz, R.; Bussières, P. Particle Sizes Related to Physical Properties of Peat-Based Substrates. Acta Hortic. 2011, 893, 971–978. [Google Scholar] [CrossRef]
- Londra, P.A. Simultaneous determination of water retention curve and unsaturated hydraulic conductivity of substrates using a steady-state laboratory method. HortScience 2010, 45, 1106–1112. [Google Scholar] [CrossRef]
- Londra, P.A.; Paraskevopoulou, A.T.; Psychoyou, M. Evaluation of water-air balance of various substrates on begonia growth. HortScience 2012, 47, 1153–1158. [Google Scholar] [CrossRef]
- Londra, P.A.; Psychoyou, M.; Valiantzas, J.D. Evaluation of substrate hydraulic properties amended by urea-formaldehyde resin foam. HortScience 2012, 47, 1375–1381. [Google Scholar] [CrossRef]
- Da Silva, F.F.; Wallach, R.; Chen, Y. Hydraulic properties of Sphagnum peat moss and tuff (scoria) and their potential effects on water availability. Plant. Soil 1993, 154, 119–126. [Google Scholar] [CrossRef]
- Talsma, T. Prediction of hydraulic conductivity from soil water retention data. Soil Sci. 1985, 140, 184–188. [Google Scholar] [CrossRef]
- Poulovassilis, A.; Polychronides, M.; Kerkides, P. Evaluation of various computational schemes in calculating unsaturated hydraulic conductivity. Agric. Water Manag. 1988, 13, 317–327. [Google Scholar] [CrossRef]
- Valiantzas, J.D.; Sassalou, A. Laboratory determination of unsaturated hydraulic conductivity using a generalized-form hydraulic model. J. Hydrol. 1991, 128, 293–304. [Google Scholar] [CrossRef]
- Kargas, G.; Londra, P.A. Effect of tillage practices on the hydraulic properties of a loamy soil. Desalin. Water Treat. 2015, 54, 2138–2146. [Google Scholar] [CrossRef]
- Londra, P.; Kargas, G. Evaluation of hydrodynamic characteristics of porous media from one-step outflow experiments using RETC code. J. Hydroinform. 2018, 20, 699–707. [Google Scholar] [CrossRef]
- Schroll, E.; Lambrinos, J.G.; Sandrock, D. An Evaluation of Plant Selections and Irrigation Requirements for Extensive Green Roofs in the Pacific Northwestern United States. HortTechnology 2011, 21, 314–322. [Google Scholar] [CrossRef]
- Vahdati, N.; Tehranifar, A.; Kazemi, F. Assessing chilling and drought tolerance of different plant genea on extensive green roofs in an arid climate region in Iran. J. Environ. Manage. 2017, 192, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Nazemi Rafi, Z.; Kazemi, F.; Tehranifar, A. Effects of various irrigation regimes on water use efficiency and visual quality of some ornamental herbaceous plants in the field. Agr. Water Manage. 2019, 212, 78–87. [Google Scholar] [CrossRef]
- Savi, T.; Dal Borgo, A.; Love, V.L.; Andri, S.; Tretiach, M.; Nardini, A. Drought versus heat: What’s the major constraint on Mediterranean green roof plants? Sci. Total Environ. 2016, 566–567, 753–760. [Google Scholar] [CrossRef]
- Kazemi, F.; Mohorko, R. Review on the roles and effects of growing media on plant performance in green roofs in world climates. Urban. Forestry and Urban. Greening 2017, 23, 13–26. [Google Scholar] [CrossRef]
- Huang, B.; Rachmilevitch, S.; Xu, J. Root carbon and protein metabolism associated with heat tolerance. J. Experimental Botany 2012, 63, 3455–3465. [Google Scholar] [CrossRef]
- Olivieri, F.; Di Perna, C.; D’Orazio, M.; Olivieri, L.; Neila, J. Experimental measurements and numerical model for the summer performance assessment of extensive green roofs in a Mediterranean coastal climate. Energ. Buildings 2013, 63, 1–14. [Google Scholar] [CrossRef]
- Simmons, M.T.; Gardiner, B.; Windhager, S.; Tinsley, J. Green roofs are not created equal: The hydrologic and thermal performance of six different extensive green roofs and reflective and non-reflective roofs in a sub-tropical climate. Urban. Ecosyst. 2008, 11, 339–348. [Google Scholar] [CrossRef]
- Theodosiou, T.G. Summer period analysis of the performance of a planted roof as a passive cooling technique. Energ. Buildings 2003, 35, 909–917. [Google Scholar] [CrossRef]
- Chapin, F.S., III. The mineral nutrition of wild plants. Ann. Rev. Ecol. Syst. 1983, 11, 233–260. [Google Scholar] [CrossRef]
- Schuppler, U.; He, P.H.; John, P.C.L.; Munns, R. Effects of water stress on cell division and cell-division-cycle2-like cell-cycle kinase activity in wheat leaves. Plant. Physiol. 1998, 117, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Sendo, T.; Kanechi, M.; Uno, Y.; Inagaki, N. Evaluation of Growth and Green Coverage of Ten Ornamental Species for Planting as Urban Rooftop Greening. J. Japan. Soc. Hort. Sci. 2010, 79, 69–76. [Google Scholar] [CrossRef]
- Savi, T.; Andri, S.; Nardini, A. Impact of different green roof layering on plant water status and drought survival. Ecol. Eng. 2013, 57, 188–196. [Google Scholar] [CrossRef]
- Mirás-Avalos, J.M.; Pérez-Sarmiento, F.; Alcobendas, R.; Alarcón, J.J.; Mounzer, O.; Nicolás, E. Using midday stem water potential for scheduling deficit irrigation in mid–late maturing peach trees under Mediterranean conditions. Irr. Sci. 2016, 32, 161–173. [Google Scholar] [CrossRef]
- Molineux, C.J.; Fentiman, C.H.; Gange, A.C. Characterising alternative recycled waste materials for use as green roof growing media in the U.K. Ecol. Eng. 2009, 35, 1507–1513. [Google Scholar] [CrossRef]






| Particle Size (mm) | Particle Size Distribution (% by wt) |
|---|---|
| >10 | 0.00 |
| 10–8 | 0.34 |
| 8–4 | 13.55 |
| 4–2 | 23.16 |
| 2–1 | 12.49 |
| 1–0.5 | 8.98 |
| 0.5–0.25 | 12.25 |
| 0.25–0.106 | 23.34 |
| 0.106–0.053 | 4.54 |
| <0.053 | 1.35 |
| Total Porosity 1 (cm3 cm−3) | Water Content at −50 cm (cm3 cm−3) | Water Content at −100 cm (cm3 cm−3) | Easily Available Water (EAW) 2 (cm3 cm−3) | Air-Filled Porosity at −50 cm (cm3 cm−3) | Ks 3 (cm min−1) |
|---|---|---|---|---|---|
| 0.513 | 0.3120 | 0.2920 | 0.081 | 0.201 | 0.547 |
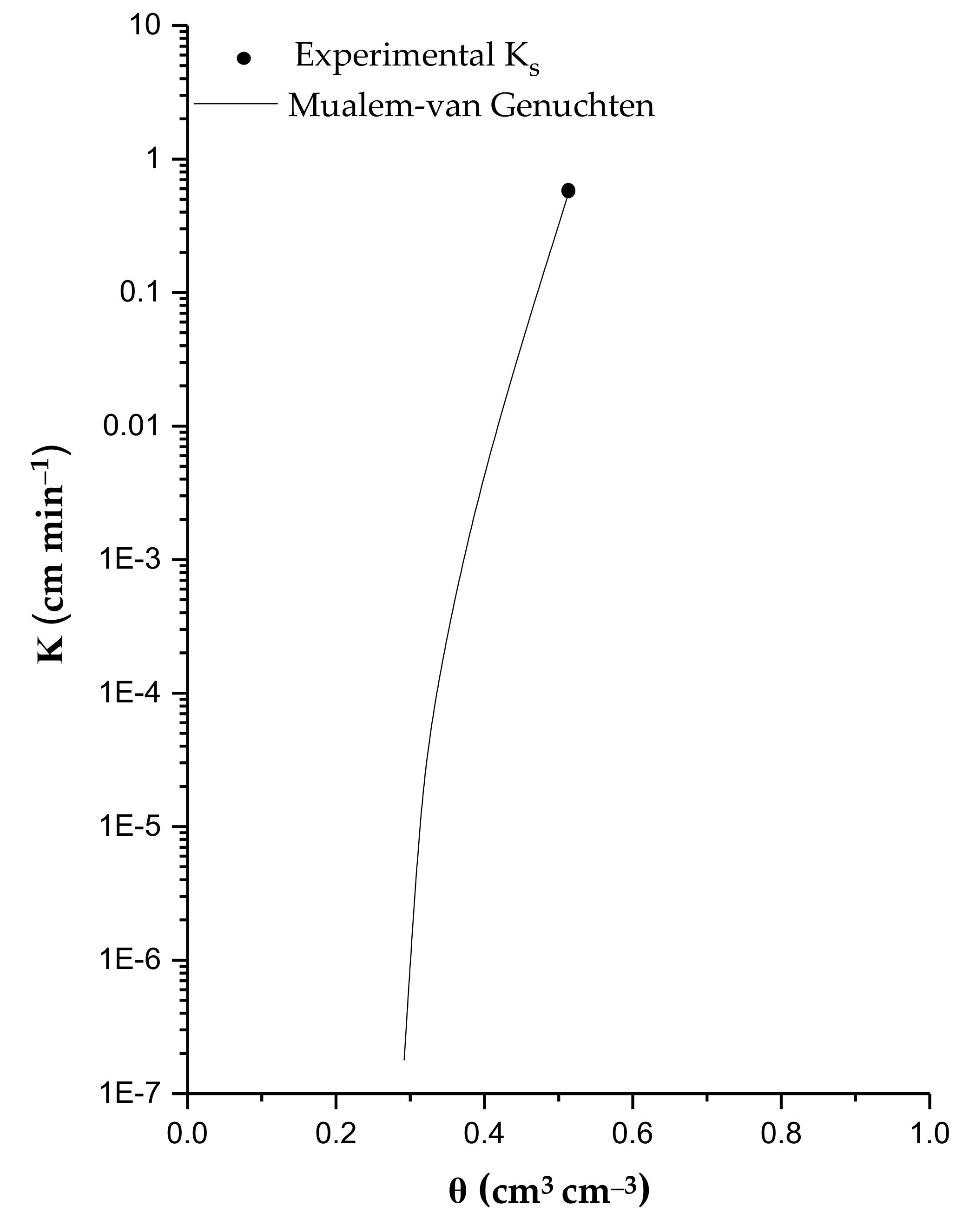
| H (cm) | θ (cm3 cm−3) | Κ (cm min−1) |
|---|---|---|
| 0 | 0.513 | 0.547 |
| −10 | 0.393 | 4.06 × 10−3 |
| −30 | 0.330 | 8.16 × 10−5 |
| −50 | 0.312 | 1.07 × 10−5 |
| −100 | 0.292 | 1.78 × 10−7 |
| Species | Irrigation Treatment | |
|---|---|---|
| High | Low | |
| L. angustifolia | 5 a | 5 a |
| L. stoechas | 3 b | 2 c |
| L. dentata var. candicans | 5 a | 5 a |
| L. dentata var. dentata | 5 a | 5 a |
| Species | Percentage Increase (%) | |||
|---|---|---|---|---|
| H | D | GI | ||
| L. dentata var. candicans | 157 ± 7.305 a | 335 ± 3.554 a | 235 ± 3.669 a | |
| L. dentata var. dentata | 79 ± 7.305 b | 277 ± 3.554 b | 173 ± 3.669 b | |
| L. angustifolia | 48 ± 7.305 c | 178 ± 3.554 c | 107 ± 3.669 c | |
| L. stoechas | 42 ± 7.305 c | 53 ± 3.554 d | 38 ± 3.669 d | |
| Irrigation treatment | ||||
| high | 96 ± 5.165 a | 237 ± 2.513 a | 158 ± 2.595 a | |
| low | 67 ± 5.165 b | 184 ± 2.513 b | 118 ± 2.595 b | |
| Interaction (species × irrigation treatment) | ||||
| L. dentata var. candicans | × high | 179 ± 10.331 | 358 ± 5.025 | 256 ± 5.189 |
| × low | 135 ± 10.331 | 313 ± 5.025 | 214 ± 5.189 | |
| L. dentata var. dentata | × high | 94 ± 10.331 | 299 ± 5.025 | 191 ± 5.189 |
| × low | 64 ± 10.331 | 254 ± 5.025 | 154 ± 5.189 | |
| L. angustifolia | × high | 60 ± 10.331 | 213 ± 5.025 | 131 ± 5.189 |
| × low | 36 ± 10.331 | 143 ± 5.025 | 84 ± 5.189 | |
| L. stoechas | × high | 51 ± 10.331 | 78 ± 5.025 | 56 ± 5.189 |
| × low | 32 ± 10.331 | 27 ± 5.025 | 21 ± 5.189 | |
| Fspecies/sig. | * | * | * | |
| Firrigation/sig. | * | * | * | |
| Finteraction/sig. | ns | ns | ns | |
| Species | Shoot Dry Weight (g) | Stomatal Conductance (mmol m−2 s−1) | |
|---|---|---|---|
| L. dentata var. candicans | 188 ± 3.165 a | 44 ± 1.123 c | |
| L. dentata var. dentata | 126 ± 3.165 b | 47 ± 1.123 bc | |
| L. angustifolia | 76 ± 3.165 c | 64 ± 1.123 b | |
| L. stoechas | 35 ± 3.165 d | 50 ± 1.123 a | |
| Irrigation Treatment | |||
| high | 120 ± 2.238 a | 54 ± 0.794 a | |
| low | 92 ± 2.238 b | 49 ± 0.794 b | |
| Interaction (species × irrigation treatment) | |||
| L. dentata var. candicans | × high | 205 ± 4.477 | 49 ± 1.589 |
| × low | 171 ± 4.477 | 45 ± 1.589 | |
| L. dentata var. dentata | × high | 141 ± 4.477 | 45 ± 1.589 |
| × low | 111 ± 4.477 | 43 ± 1.589 | |
| L. angustifolia | × high | 86 ± 4.477 | 68 ± 1.589 |
| × low | 65 ± 4.477 | 60 ± 1.589 | |
| L. stoechas | × high | 49 ± 4.477 | 53 ± 1.589 |
| × low | 21 ± 4.477 | 48 ± 1.589 | |
| Fspecies/sig. | * | * | |
| Firrigation/sig. | * | * | |
| Finteraction/sig. | ns | ns | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paraskevopoulou, A.T.; Tsarouchas, P.; Londra, P.A.; Kamoutsis, A.P. The Effect of Irrigation Treatment on the Growth of Lavender Species in an Extensive Green Roof System. Water 2020, 12, 863. https://doi.org/10.3390/w12030863
Paraskevopoulou AT, Tsarouchas P, Londra PA, Kamoutsis AP. The Effect of Irrigation Treatment on the Growth of Lavender Species in an Extensive Green Roof System. Water. 2020; 12(3):863. https://doi.org/10.3390/w12030863
Chicago/Turabian StyleParaskevopoulou, Angeliki T., Panagiotis Tsarouchas, Paraskevi A. Londra, and Athanasios P. Kamoutsis. 2020. "The Effect of Irrigation Treatment on the Growth of Lavender Species in an Extensive Green Roof System" Water 12, no. 3: 863. https://doi.org/10.3390/w12030863
APA StyleParaskevopoulou, A. T., Tsarouchas, P., Londra, P. A., & Kamoutsis, A. P. (2020). The Effect of Irrigation Treatment on the Growth of Lavender Species in an Extensive Green Roof System. Water, 12(3), 863. https://doi.org/10.3390/w12030863







