Abstract
Ozonation followed by a polishing moving bed biofilm reactor (MBBR) was implemented in pilot and laboratory to remove the residual pharmaceuticals and toxicity from wastewater effluent, which was from a pilot hybrid system of MBBR and activated sludge, receiving municipal wastewater. The delivered ozone dosages achieving 90% pharmaceutical removal were determined both in pilot and laboratory experiments and they were normalised to dissolved organic carbon (DOC), illustrating our findings were comparable with previously published literature. During wastewater ozonation, the intensity of natural fluorescence was found to be greatly associated with the concentrations of the studied pharmaceuticals. In pilot experiments, toxicity, measured by Vibrio fischeri, increased after ozonation at delivered ozone dosages at 0.38–0.47 mg O3/mg DOC and was completely removed by the subsequent polishing MBBR. Laboratory experiments verified that the polishing MBBR was able to remove the toxicity produced by the ozonation.
1. Introduction
Due to recently developed analysis techniques for trace organic contaminants (TrOCs), these compounds are now easily detectable in wastewater. Generally, TrOCs in wastewater discharged into the sewer system eventually end up in municipal wastewater treatment plants (WWTPs) [1,2,3,4]. Despite conventional WWTPs (e.g., activated sludge) removing nutrients and biodegradable TrOCs efficiently [5,6], a considerable amount of non-biodegradable TrOCs, including pharmaceuticals, can be still found in wastewater effluent, thereby worsening the water quality of nearby recipients [7].
The moving bed biofilm reactor (MBBR) has been considered as an alternative biological treatment process in WWTPs, since higher capacity in terms of pharmaceutical removal has been found in MBBRs compared to conventional activated sludge (CAS) [8,9]. Diclofenac, which is a refractory compound, achieved a higher degradation rate per unit of biomass in MBBR than CAS [10,11]. However, persistent and biologically resistant compounds, for example contrast media, are still detected in MBBR effluent [12,13,14].
To strengthen the removal of pharmaceuticals in biologically treated effluents, polishing treatment processes are necessary. Currently, powder-activated carbon, biofiltration and advanced oxidation processes (e.g., electrochemical, ozonation) have been used as tertiary methods to upgrade treatment processes in conventional WWTPs [15,16,17,18]. Among these processes, ozonation appears to be a suitable technology to remove pharmaceuticals sufficiently and at reasonably low operational cost [18,19]. Besides the undeniable benefits of ozone on water quality, higher toxicity in the related by-products than the original compounds can be observed during the reaction process. Therefore, the need for a subsequent polishing treatment used for purifying ozonated wastewater has emerged.
Besides complying with discharge standards in regulations relating to pharmaceuticals in effluent, an overall hazardous assessment for toxicants in effluent is also needed. Currently, a number of well-developed biotest methods are employed for measuring water toxicity, for instance luminescent bacterial (Vibrio fischeri), crustaceans (Daphnia magna) and Umu-Chromotest UmuC for genotoxicity (Salmonella typhimurium) [20,21].
To reduce the time and analysis costs of examining pharmaceuticals with chromatography techniques, fluorescence monitoring seems to be an alternative. Previous studies show that fluorescence with a 254 nm excitation wavelength and UV254 used as an online or offline surrogate parameter is able to predicate solidly the removal of TrOCs by ozonation [22,23,24]. Hence, fluorescence monitoring can be used to indirectly express the removal degree of pharmaceuticals in wastewater by ozonation when LC-MS/MS is not involved.
The aims of this study mainly comprise the following elements. First, in pilot, it set out to study the impact of a delivered ozone dosage on the concentrations of pharmaceuticals in the effluent of a pilot hybrid system of MBBR and activated treating municipal wastewater. To verify the removal efficiency of pharmaceuticals by ozone, the same effluent was ozonated in the laboratory and the same dosages were used as those applied in the pilot. Second, the intensity of the investigated natural fluorescence in ozonated effluent was measured, to study whether fluorescence intensity has any correlation with pharmaceutical concentration or not. Third, wastewater toxicity developed in the pilot hybrid system, followed by ozonation and a subsequent pilot polishing MBBR implemented as previously, was measured with Vibrio fischeri. The effect of ozone dosage on toxicity in the effluent of the pilot hybrid system was also investigated. Finally, to verify the performance of the subsequent polishing MBBR in pilot on reducing toxicity in the ozonated effluent, a polishing MBBR in the laboratory was applied. Additionally, the impact on reaction time of a polishing MBBR and ozonated effluent on toxicity and fluorescence intensity was also examined in the laboratory.
2. Materials and Methods
2.1. Chemicals
All pharmaceuticals used for calibration were obtained from different suppliers, all of which are presented in the Supplementary Information (Table S1). Formic acid and HPLC-gradient-grade methanol were supplied by Merck (Germany). To determine the delivered ozone dosage, potassium indigotrisulfonate was purchased from Sigma-Aldrich (Denmark).
2.2. MBBR Effluent
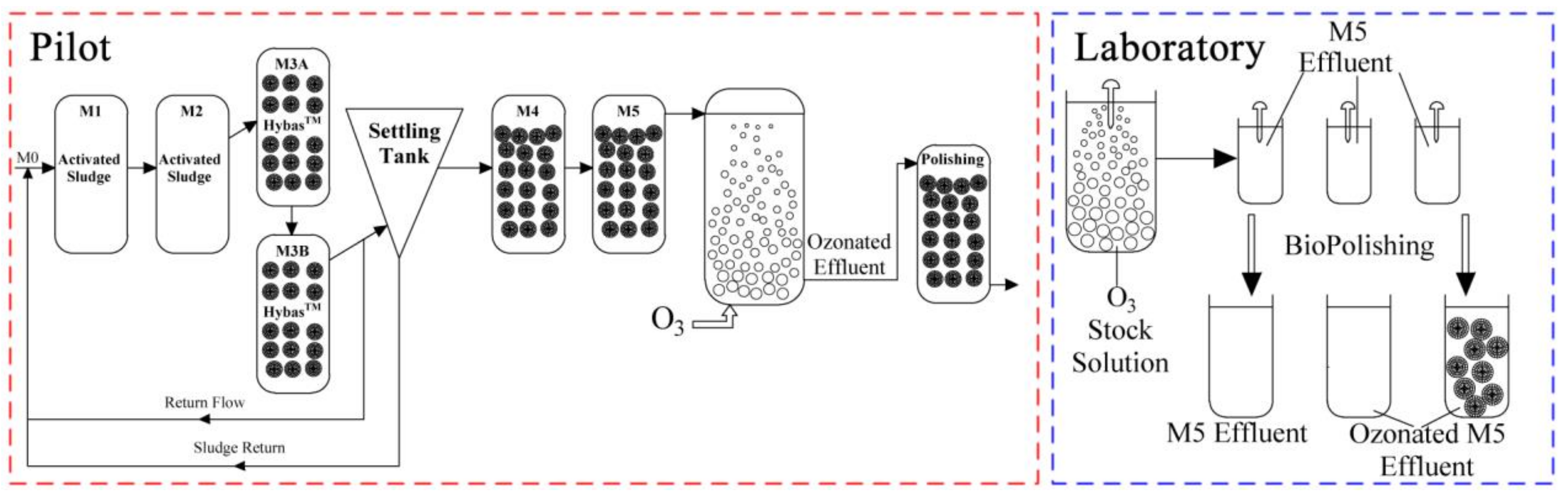
The same wastewater as the influent to the municipal WWTP at Herning, Denmark, which has a total influent of approximately 10,000,000 m3/year, covering 34,000 households, was treated with a pilot hybrid system, which included a non-aerated (mixed) activated sludge reactor (M1, 900 L), an aerated activated sludge reactor (M2, 900 L), two identical reactors (M3A and M3B, 900 L) combined with MBBR and activated sludge, a non-aerated MBBR reactor (M4, 500 L) and an aerated MBBR reactor (M5, 500 L) (Figure 1, left). K5 carriers from AnoxKaldnes (Sweden) were used with a filling ratio of 50%. The inlet flow rate, return flow rate and return sludge flow rate were 250 L/h, 500 L/h and 300 L/h, respectively. The mean values of dissolved oxygen (DO), pH, chemical oxygen demand (COD), NH4-N and NO3-N during the experimental period are shown in Table S2.
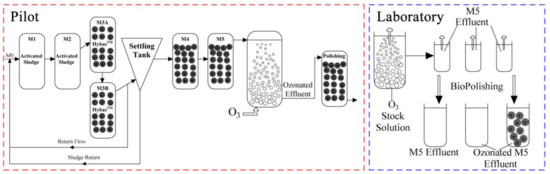
Figure 1.
Schematic diagram of a five-stage pilot-scale MBBR treatment train followed by a pilot-scale ozonation and a polishing MBBR (left). ‘M0’ stands for the municipal wastewater inlet. A laboratory-scale MBBR was used to polish ozonated effluent in the laboratory (right).
2.3. Ozone Setup
The detailed composition of the ozone set-up in both the pilot and the laboratory were described previously [25].
The ozone equipment in the pilot (Figure 1, left) included an ozone generator, a reaction column, an ozone analyser, and an ozone destructor. The water flow and volume of the reaction column were 2 L/min and 18 L, respectively, resulting in 9 min of hydraulic retention time (HRT) in the reaction column. The ozonated effluent was directed to a polishing MBBR tank of 28 L with a HRT of 14 min.
2.4. Fluorescence and MicroTox®
2.4.1. Fluorescence
A fluorimeter (Cary Eclipse, Varian, Santa Clara, US) was used to measure fluorescence intensity. Six transition pairs (Table 1): Peak A (λexcitation/emission: λ249/450), Peak B: (λ231/315, λ275/310), Peak C: (λ335/450), Peak T1: (λ275/340) and Peak T2: (λ231/360), were studied.

Table 1.
Excitation/emission wavelength pair for fluorophores, based on Spiliotopoulou et al. [26].
Samples were transferred to a quartz cuvette and subjected to further analysis. The dilution of fluorescence intensity in ozonated samples caused by adding ozone stock solution was corrected.
2.4.2. MicroTox® Test
The MicroTox® test using Vibrio fischeri is based on an ISO standard method [28]. Vibrio fischeri were purchased from ABOATOX, Finland. Duplicate measurements for toxicity were performed.
2.5. Quantification
2.5.1. Determination of Delivered Ozone Dosage
The details of a modified method based on a former indigo method quantifying the delivered ozone dosage [29] were described in a previous work [19].
2.5.2. Pharmaceuticals Analysis
The detailed sample preparation method, ingredients of the internal standard solution, and the relevant HPLC-MS/MS parameters were described previously [12].
Methanol was added to the samples before centrifugation, the internal standard was spiked into the supernatant and analysed by HPLC-MS/MS, and the limits of quantification (LOQs) are shown in Table S3. The definition used in the following contents with regard to pharmaceutical concentrations above the LOQ was in accordance with the following principles. If there were two or more points of normalised concentrations above the LOQ, then this pharmaceutical was above LOQ (e.g., atenolol in the laboratory experiments). However, if there were fewer than two points, this pharmaceutical was below the LOQ (e.g., atenolol in the pilot experiments).
2.6. Experiments Performed
Five experiments were conducted in different initial conditions (Table 2).

Table 2.
Outline of experiments at the pilot and laboratory scales.
Exp 1: Effluent was treated with six delivered ozone dosages in the pilot (2, 4, 8, 12, 15 and 30 mg O3/L). To investigate the purification capability for the ozonated effluent, a pilot polishing MBBR was applied after the ozonation process. Pharmaceutical analysis and fluorescence and toxicity measurement of ozonated samples were conducted.
Exp 2: Effluent was continuously treated for an entire week with a fixed delivered ozone dosage, during which time three ozone dosages were performed (5, 7 and 20 mg O3/L). Pharmaceutical analysis of ozonated samples was conducted.
Exp 3: Effluent was treated with eight delivered ozone dosages in the laboratory (1.2, 2.4, 4.5, 6.4, 8.9, 12, 16 and 22 mg O3/L). To ensure ozone depletion, the ozonated samples were left for half an hour prior to the subsample being taken for pharmaceutical measurement. Furthermore, fluorescence intensity and MicroTox® in the ozonated samples were measured over time.
Exp 4: Wastewater samples from each reactor in the pilot hybrid system were subjected to the MicroTox® test.
Exp 5: Effluent was treated with four delivered ozone dosages (2, 4, 8 and 12 mg O3/L) in the laboratory. After 30 min of standing, to ensure all ozone had been consumed, the fluorescence intensity as well as the MicroTox® of these ozonated samples were measured. Furthermore, 40 mL of the ozonated samples was transferred to each of two glass bottles (with and without MBBR carriers). A filling ratio of 50% was used in the bottle with carriers (Figure 1, right). Effluent treated by neither ozonation nor carriers was used as a control (Figure 1, right). Fluorescence intensity and MicroTox® were measured over time in the effluent, the ozonated effluent and the polished ozonated effluent by carriers.
2.7. Data Treatment
To determine the delivered ozone dosages that removed 90% of each pharmaceutical in the effluent, the correlation of elimination rate for each pharmaceutical and delivered ozone dosage was fitted by Equation (1).
where C stands for the residue concentration of the pharmaceutical while C0 is related to its initial concentration. DO3 stands for a specific delivered ozone dose (mg O3/L) and DDO3 is with decadic dose of ozone (mg O3/L), which is a compound-related constant explaining the required delivered ozone dosage needed to remove 90% of each pharmaceutical [30,31].
3. Results and Discussion
3.1. Pharmaceuticals Removal
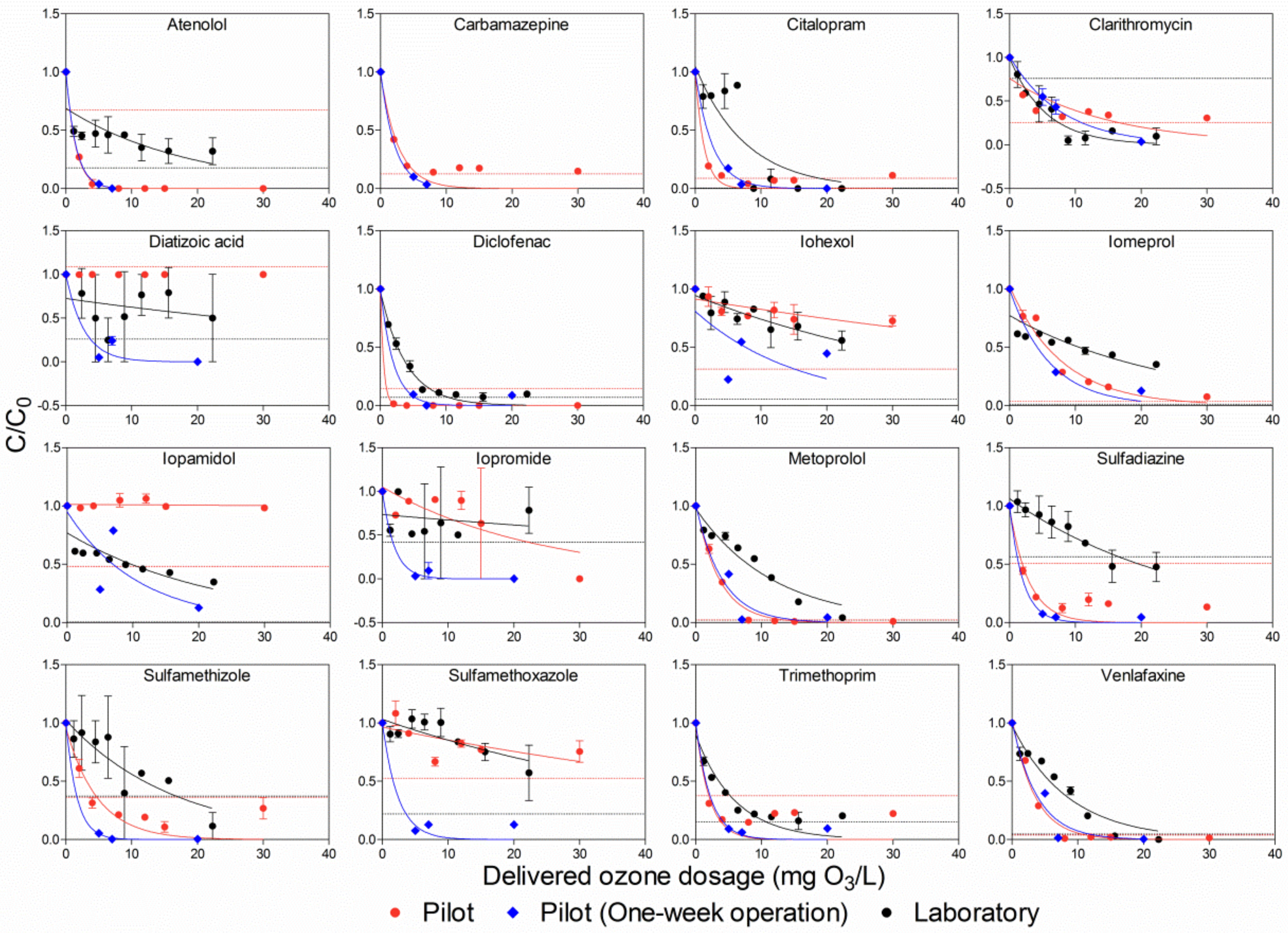
Without spiking, 24 pharmaceuticals were found in the effluent of pre-treated municipal wastewater by the pilot hybrid system. Based on the LOQ definition, early, in Figure 2, 16 pharmaceuticals were above the LOQ, whereas the remaining eight pharmaceuticals belonged to the group below the LOQ (all compounds are present in Figure S1). Although certain points are below the LOQ in the defined pharmaceuticals that are above the LOQ, this factor did not remarkably affect the accuracy of the results of the fitting curve for conversely ensuring the selectively delivered ozone dosage had removed 90% of the pharmaceutical. Pharmaceutical concentrations were negatively correlated with delivered ozone dosages, except for diatrizoic acid, which had no clear removal at all, as well as iopromide, which had no removal in both pilot and laboratory. In Figure 2, it was found that the slopes of the fitting curves in the one-week continuous pilot experiments were generally steeper than those in the pilot and the laboratory experiments, indicating fewer delivered ozone dosages were needed in the one-week continuous pilot experiments in order to remove the same concentrations of pharmaceuticals when compared to the other two experiments. The poor fitting observed for atenolol in the laboratory experiment, as well as sulfamethoxazole in both the pilot and the laboratory experiments, could be attributed to the reaction mechanisms involved between the ozone and pharmaceuticals. The reaction of ozone with pharmaceuticals comprises a non-selective ·OH-based reaction, which happens instantly due to the high reaction rate constants, and a selective molecular ozone-based reaction, which has lower rate constants compared to the former example. The two above reactions can be intuitively expressed with the form of second-order rate constant with ozone (kO3) and hydroxyl radicals (kOH). In a previous study [32], it was shown that kO3 and kOH values ranged from 5 × 10−4 to 105 m−1s−1 and 0.04 × 109 to 18 × 109 m−1s−1, respectively. Consequently, with both atenolol and sulfamethoxazole, because the selective molecular ozone-based reaction was the dominant reaction when the delivered ozone dosages were up to 10 mg/L, it did not have a significant impact on elimination rate of pharmaceutical, resulting in a flat fitting curve. However, by increasing the delivered ozone dosage, the residual ozone, after consumption by reaction with organic matter in wastewater, generates ·OH; thereby, the non-selective ·OH-based reaction becomes the dominant reaction, which results in a fast drop in pharmaceutical concentration.
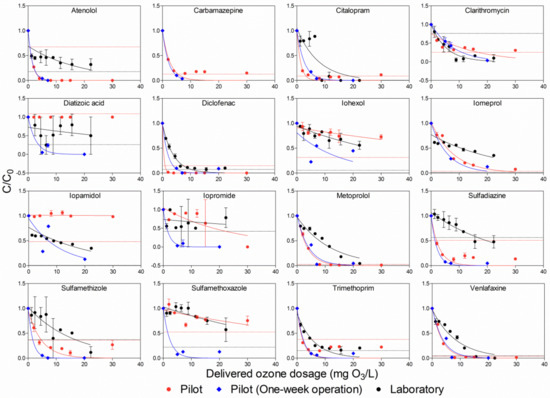
Figure 2.
Comparison of pharmaceutical removal by ozone in the effluent of the pilot hybrid system. The dashed lines in the Figure stand for LOQ of the pharmaceuticals. The same dashed line (red) was applied for both the pilot and the pilot (one-week operation) experiments. Error bars represent standard deviations.
To evaluate pharmaceutical removal efficiency, DDO3 for each pharmaceutical was obtained from a fitting curve based on Equation (1) (Figure 2 and Table 3). It was considered that if the fitting curve had more data points, the obtained DDO3 would be more accurate. As illustrated in Figure 2, there were six and eight delivered ozone dosages performed in both the pilot and the laboratory, respectively, while there were only three delivered ozone dosages conducted in the one-week continuous pilot experiments. Therefore, in the following content, DDO3 is only discussed for the pilot and laboratory experiments.

Table 3.
Delivered ozone dosage for 90% removal of pharmaceuticals in the pilot and the laboratory, and the normalisation of delivered ozone dosage to the relevant DOC condition (Z90, DDO3/DOC, (mg O3/mg DOC)). The DOC for this study was 32 mg/L. Indicated intervals represent the standard deviation.
DOC plays an important role in ozone reactions involved in the degradation of micropollutants in wastewater [33]. The DDO3 of each pharmaceutical was thus normalised to DOC, which was defined as a parameter, Z90 (DDO3/DOC, mg O3/L/mg DOC/L, Table 3), thereby creating a site-unspecific parameter for the sensitivity of each pharmaceutical to ozonation.
Based on the group classification for pharmaceuticals, due to their second-order rate constant with ozone and ·OH [34], diclofenac with aniline moieties, as well as both carbamazepine and trimethoprim with double bonds, reacts highly with ozone [35]. It was observed that these compounds’ concentrations in the present pilot and laboratory experiments significantly decreased, even at a low delivered ozone dosage (around 0.2 of Z90, Table 3). However, substances in the iodinated contrast media (ICM) group (e.g., iohexol, iopamidol and iopromide) did not show any signs of reacting with ozone. Correspondingly, these compounds in the current study were not removed completely, even at high delivered ozone dosages (around 2.0 of Z90). Moreover, metoprolol and venlafaxine, which are considered intermediate ozone reactivity substances, were gradually removed when the delivered ozone dosage increased in this study. Sulfonamide groups, such as sulfadiazine, sulfamethizole and sulfamethoxazole, reacted quickly with ozone, due to their high rate constants of ozone and OH radicals [34]. Conversely, the removal trend for sulfonamide substances from the laboratory experiments was similar to that of ICM in the pilot and the laboratory experiments, thereby indicating that substances were difficult to remove in the laboratory experiments. The lower removal rates of sulfonamide substances in the laboratory experiments than in the pilot experiments could be attributed to a change in the water matrix, due to the transport time for samples before performing the experiments in the laboratory, differences in temperature due to dissimilar experimental circumstances and the different characteristics of ozone devices applied in the ozone experiments. In addition, discrepancies among the above three experiments were caused by the method for delivering ozone. In the laboratory, ozone was added at one time, which led to a relatively high-concentration ozone peak. Meanwhile, in pilot, a bubble column was introduced; as such, ozone and wastewater were added in the countercurrent flow, resulting in a relatively low-concentration ozone peak. This was because ozone was transferred and simultaneously consumed over the height of the column.
Ozonation has also been implemented in removing pharmaceuticals in WWTP effluent or pre-biologically-treated hospital wastewater previously [25,35,36]. Discrepancies regarding normalised delivered ozone dosages to DOC, comparing this pilot experiment with former studies, are due to the following factors. First, different effluent matrixes, such as municipal effluent or hospital wastewater treated by a membrane bioreactor, had impacts on the pharmaceutical removal, as well as further affecting Z90. Second, the DOC value in the literature [35,36] were less than 10 mg/L, while this study had 32 mg/L of DOC. Moreover, the HRT of the reaction in the ozone column could also affect pharmaceutical elimination, in that HRTs in the literature [35,36] run for 12–23 min, while, in this study, the time was 9 min. Furthermore, the transfer efficiency of ozone in the literature was 66%–78%, while it was almost 100% in the current study.
Hansen et al. [19] examined the influence of DOC on the removal of pharmaceuticals in MBBR effluent by ozone in the laboratory, and the resulting Z90 values were close to the Z90 obtained in this study at laboratory for the majority of pharmaceuticals, except for sulfamethizole. Additionally, Antoniou et al. [31] investigated the influence of water matrices on the removal of pharmaceuticals in effluent by zone in the laboratory. Compared to this study, it was found that Z90 of pharmaceuticals such as clarithromycin, iomeprol, iopamidol metoprolol, trimethoprim and venlafaxine in the present study had similar or even better results. As such, Z90 can be considered an important parameter used to evaluate the removal efficiency of pharmaceuticals by ozonation.
3.2. Natural Fluorescence Used for Estimating Delivered Ozone Dosage
3.2.1. Comparison of Fluorescence Results
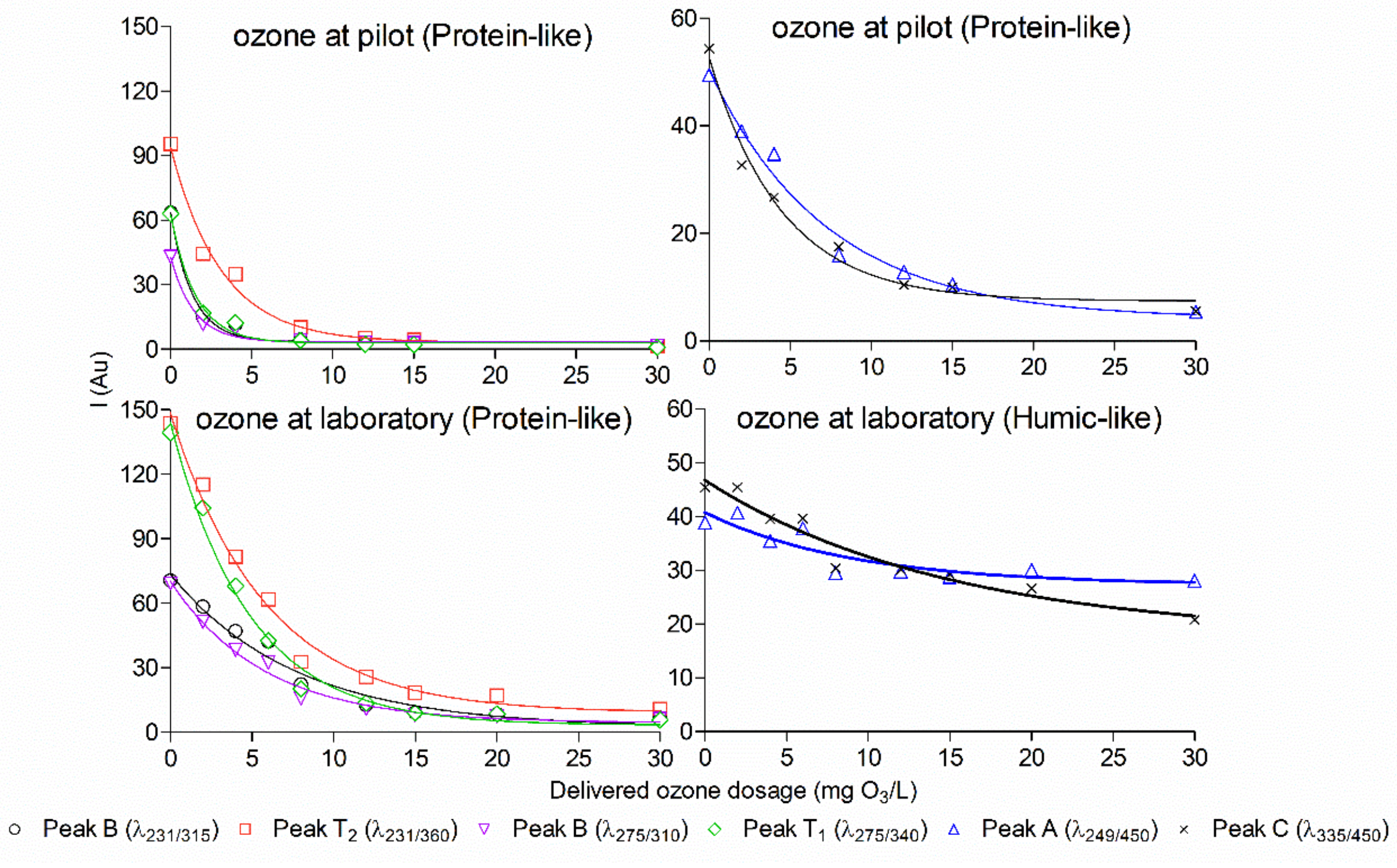
In Figure 3, ozone displays a continuous and significant influence on the intensity of the investigated natural fluorescence of wastewater; namely, ozone dose was negatively correlated with fluorescence intensity. The decreasing of fluorescence intensity was caused by the depletion of aromatic structures and an increase in electron-withdrawing groups, such as –COOH in aromatic compounds [38,39]. For protein-like fluorescence, its intensity instantly dropped to half of the initially detected level when only 5 mg O3/L was delivered. Fluorescence intensity measured from the pilot experiments was removed completely when delivered ozone dosage was approximately 15 mg/L, while fluorescence intensity measured from the laboratory experiments was fully removed when 30 mg/L ozone was dosed. However, for humic-like fluorescence, it was not possible to completely remove intensity measured from the pilot and laboratory experiments, even though the delivered ozone dosage was up to 30 mg/L, where 20%–50% of the initial intensity remained. This could be because humic-like fluorescence was the hardest degradable organic matter in the wastewater [39].
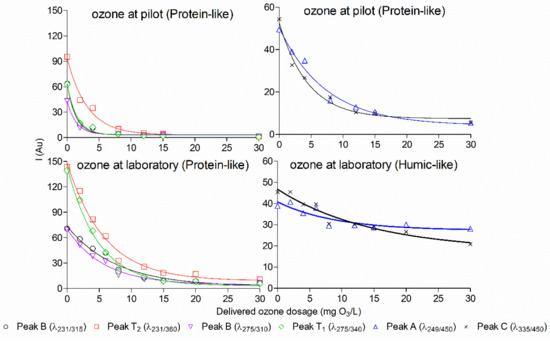
Figure 3.
Comparison of the remaining natural fluorescence of the pilot-scale staged MBBRs effluent treated with ozone, using the pilot or the laboratory method.
Similar results have also been observed in a previous study, where generally humic-like fluorescence intensity was difficult to remove compared with protein-like fluorescence intensity in water from recirculating aquaculture systems when treated with various ozone doses [26].
3.2.2. Correlation of Fluorescence Intensity and Pharmaceutical Concentration
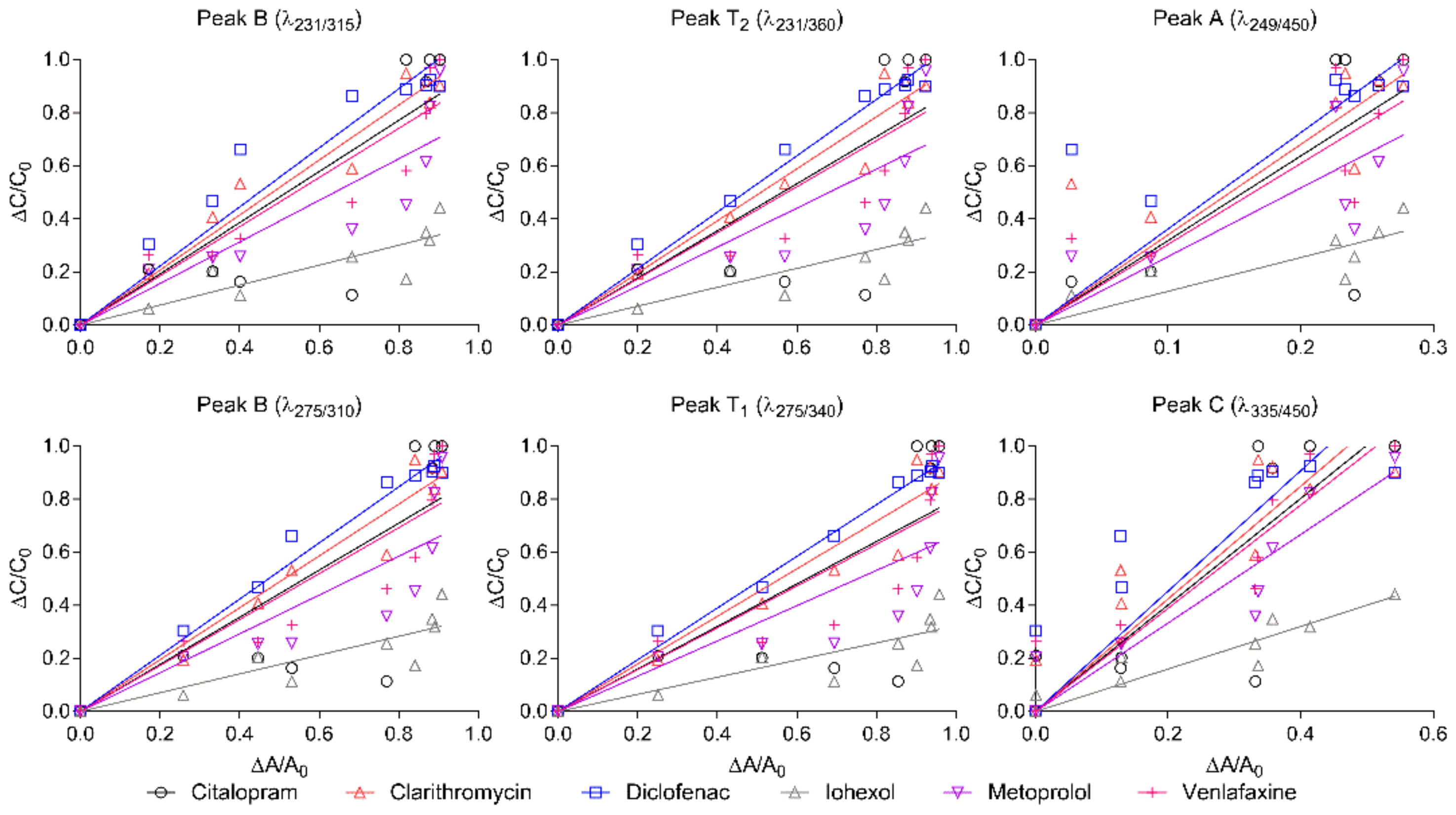
Normalised fluorescence intensity in ozonated wastewater under six investigated wavelengths, as well as the normalised concentrations of selected pharmaceuticals, plotted by the straight line, is shown in Figure 4, as well as the fitting results (see all pharmaceuticals’ fitting results in Figure S2). The fluorescence intensity measured at each wavelength was reduced to a great extent along with a decrease in pharmaceutical concentrations. The correlation between the normalised fluorescence intensity and the normalised pharmaceutical concentration in Figure 4 was not linear for all selected pharmaceuticals under each fluorescence wavelength, and was compound specific. However, for diclofenac, the correlation under Peak T1 can be concluded to be very linear, with an R2 of 0.99. According to the numbers of the highest R2 from the statistically fitting results presented in Table S4, both peak T1 and peak C had the closest correlation, for 6 out of 19 pharmaceuticals. In addition to this, peak B had 3 out of 19 pharmaceuticals correlated the closest. Furthermore, if counting the numbers of the second highest R2 as well, then, 10 out of the 19 pharmaceuticals were observed in peak B.
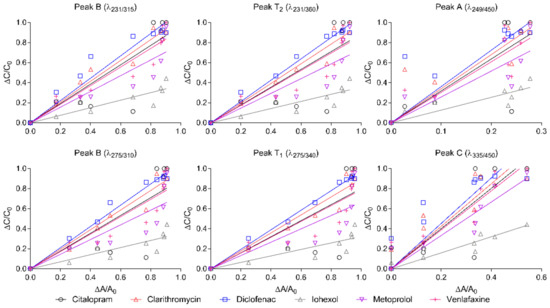
Figure 4.
Correlation between changes in concentrations of selected pharmaceuticals (ΔC/C0) and relative changes in excitation emission matrices fluorescence (ΔA/A0) under different dosages of ozone in the laboratory, fitted by the straight line.
Additionally, if the values of the biggest slope were also counted, all of the pharmaceuticals with the biggest slope were observed in peak A. The biggest slope illustrates that it is more sensitive to pharmaceutical removal compared to the other peaks, thereby indicating that peak A can detect more pharmaceutical removal along with less reduction in fluorescence intensity.
Previous studies have found that total fluorescence (ΔTF) used as an online surrogate during ozonation of wastewater, which is more stable and reliable than UV254, has displayed a concrete ability to predict changes in TrOC concentrations in wastewater (R2 > 0.7) [23,24,40]. Thus, we could conclude that change in fluorescence intensity has potential as a surrogate to represent the elimination trend of pharmaceuticals in wastewater during ozonation.
In addition, if an online sensor of natural fluorescence is developed to apply in monitoring wastewater quality of ozonation, it can automatically control the ozone dosage delivered to wastewater in order to not only eliminate a certain degree of pharmaceutical but to remove humic-like or protein-like fluorescence, corresponding to Section 3.2.1.
3.3. Toxicity Development with Treatment
3.3.1. Development of MicroTox® with Staged Biological Treatment
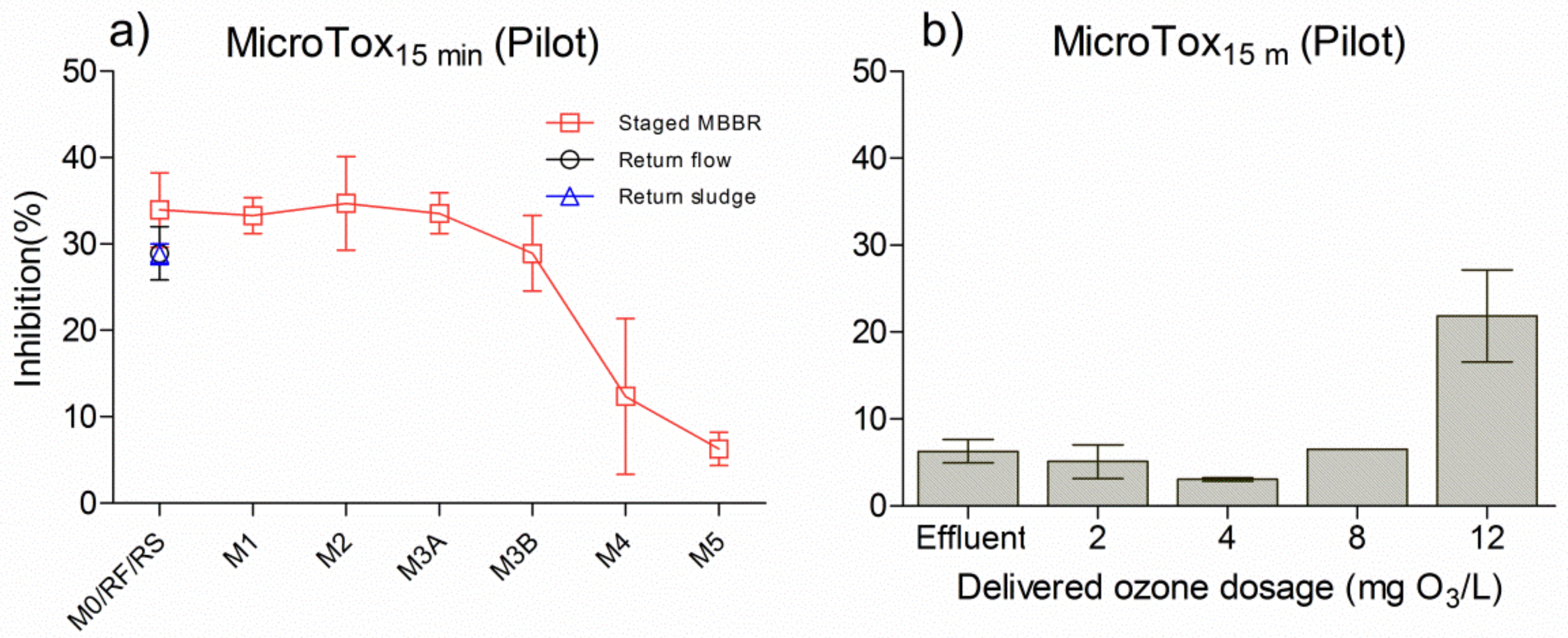
The results of inhibition of Vibrio fischeri at 15 min assay time, in wastewater taken along the pilot system followed by an onsite continuous ozonation, are presented in Figure 5. Both M1 and M4 were under anaerobic conditions (denitrification tank), while M2, M3A, M3B and M5 were under aerobic conditions (nitrification tanks). In the biological configuration, the inhibition of Vibrio fischeri decreased tank by tank (Figure 5a), and especially from M3A to M5, indicating that toxicants were removed during these processes. The relevant removal of toxicity was attributed to the decrease in the biodegradable organic matter in wastewater. It was found that toxicity inhibition in the effluent (M5) was around 10%, which still had a certain level of toxicity, thereby indicating that the influent pollutant was not fully removed by the pilot hybrid system. Moreover, ozonation after the pilot system was able to reduce effluent toxicity to approximately 5% (Figure 5b), thus corresponding to toxicity in M5. However, the Vibrio fischeri inhibition in ozonated effluents first decreased and then increased, from 2 to 12 mg O3/L. It may be assumed that the ozone by-products formed at 12 mg O3/L reached their highest concentration when organic matter from the wastewater interacted with the ozone, thereby increasing the quantity of toxicants.
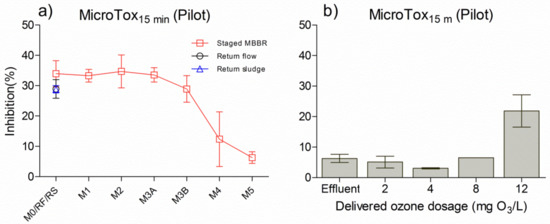
Figure 5.
Toxicity measured with the MicroTox® test with 15 min assay time in a pilot hybrid system (a) and onsite continuous pilot ozonation (b) M0: municipal influent. RF: return flow from M3B marked with a black circle. RS: return sludge flow from a settling tank after M3B marked with blue triangle. M5: effluent from the pilot system, which was also the initial inlet of ozonation (effluent in Figure 5b).
3.3.2. Development of Toxicity Inhibition in Ozonated Effluent Followed with Polishing Biofilm
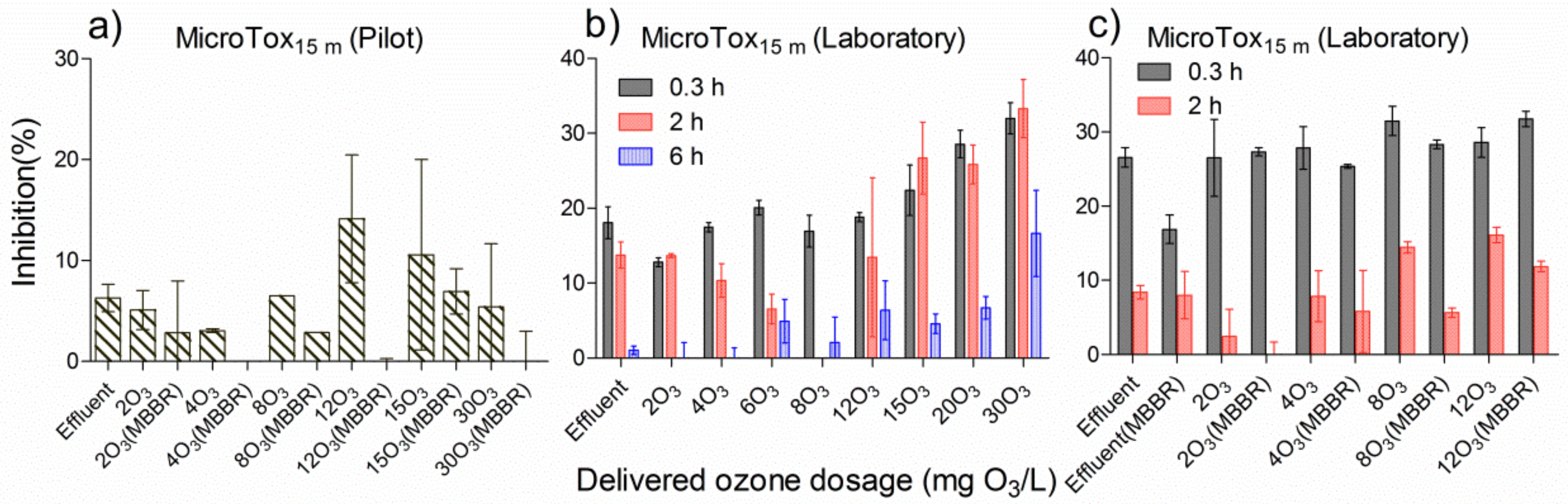
In Figure 6a, the results are shown for the effluent treated with different delivered ozone dosages followed by a pilot polishing MBBR. Vibrio fischeri inhibitions in the ozonated effluent first decreased when increasing the delivered ozone dosages from 2 to 4 mg O3/L, while they increased again when the delivered ozone dosages reached 8 to 12 mg O3/L, and gradually decreased when the ozone dosages ranged from 15 to 30 mg O3/L. In the first stage, toxicity in the ozonated effluent was reduced when increasing the delivered ozone dosage. In the second stage, the DOC was gradually oxidised, and ozonated by-products contributed to the main level of toxicity; thus, the higher the delivered ozone dosages in the effluent, the more toxic ozonated by-products were produced. In the last stage, when decreasing delivered ozone dosage, the inhibitions decreased again due to existing ozone by-products being removed. For each delivered ozone dosage, the subsequent polishing MBBR was able to remove the remaining toxicity from the ozonated effluent to almost null, indicating that the polishing MBBR is an efficient way of enhancing purification ability for ozonated effluent.
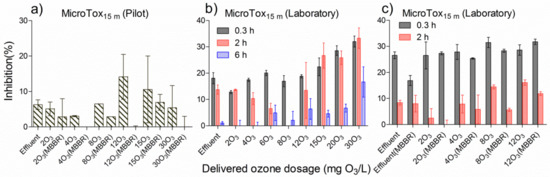
Figure 6.
Comparison of MicroTox® with a 15 min assay time under different ozone dosages in pilot and laboratory, followed by relevant MBBR polishing in pilot and laboratory over time. (a) effluent treated by onsite continuous pilot-scale ozone at various doses and a subsequent pilot MBBR polishing tank, (b) effluent treated by eight doses of ozone in the laboratory, (c) effluent treated by a single polishing MBBR, four doses of ozone in the laboratory, with or without subsequent MBBR polishing.
In Figure 6b, the same effluent was ozonated with various dosages in the laboratory, and inhibitions for the individually ozonated sample decreased over time. At the 2 h ozone reaction time, the toxicity inhibition in ozonated sample initially decreased and then increased when delivered ozone dosage increased, which fitted to the first and second stages mentioned above.
In Figure 6c, the results are shown for the effluent treated with different dosages of ozone followed by a polishing MBBR in the laboratory. At the 2 h contact time, each ozonated sample treated with the polishing MBBR had less inhibition compared to the ozonated samples, which again verified that the polishing MBBR is able to reduce further the toxicity in ozonated wastewater.
3.4. Fluorescence Intensity in the Ozonated Effluent Polished by MBBR
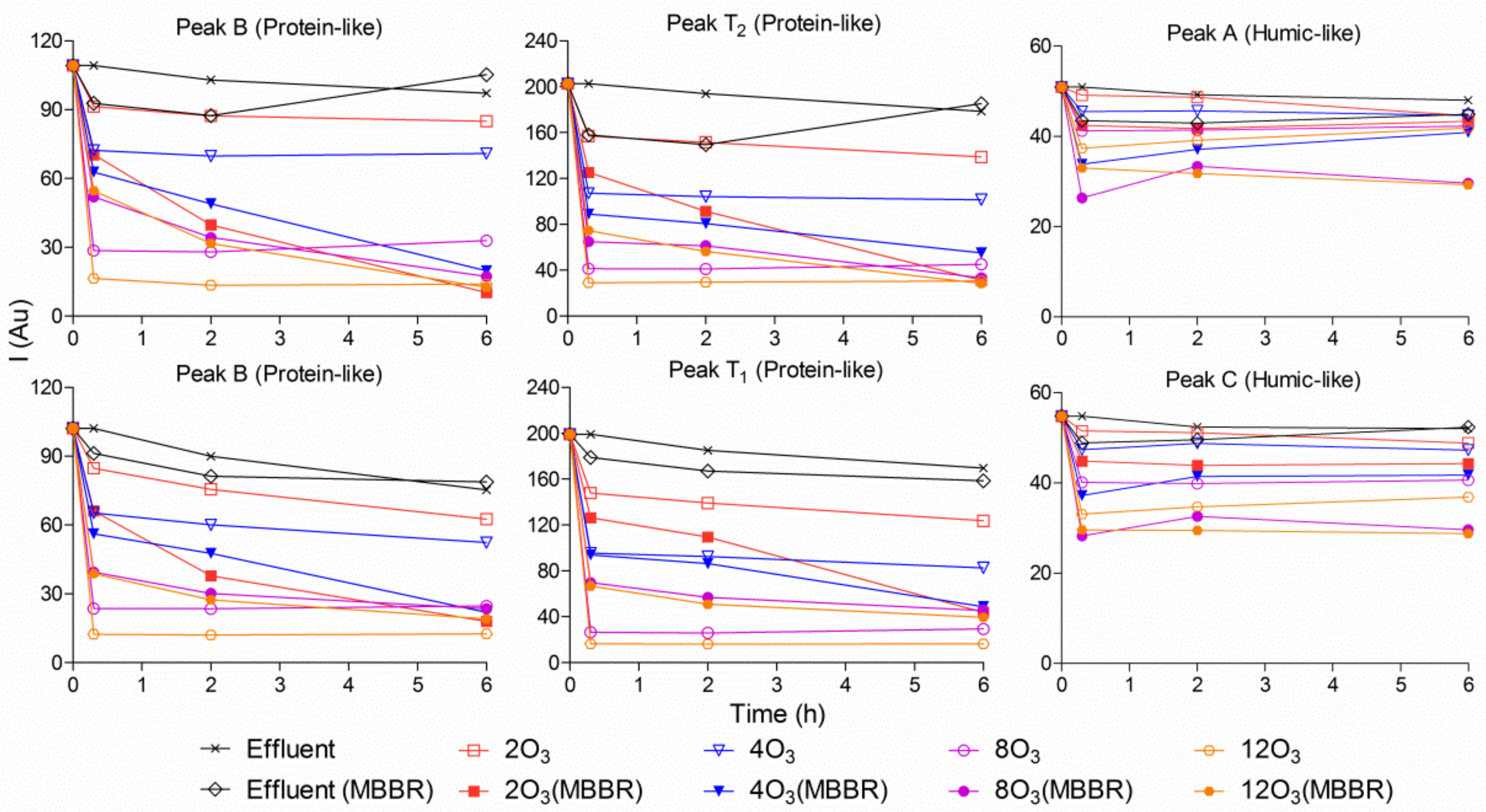
Fluorescence intensities of six wavelength transitions were measured for samples treated with ozone, or ozone followed by a subsequent polishing MBBR, in the pilot and laboratory experiments (Figure 7 and Figure S3). In general, the intensities of protein-like fluorescence were easier to reduce compared to humic-like fluorescence among different treatment processes. In the pilot experiment (Figure S3), there was no difference in fluorescence intensity between samples treated with ozone and those treated with ozone followed by a subsequent polishing MBBR, due to a short contact time between ozonated wastewater and biofilm of MBBR carriers with a HRT of 14 min. Therefore, a longer HRT was investigated in the laboratory experiment as follows.
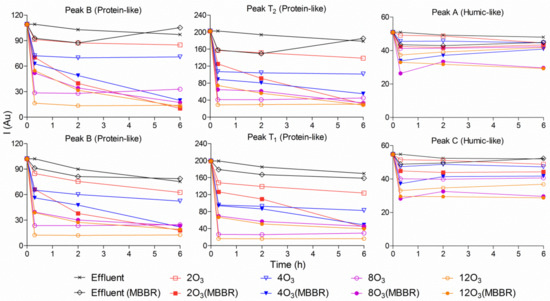
Figure 7.
Development of the natural fluorescence of the effluent treated by a single polishing MBBR, four delivered ozone dosages in the laboratory, with or without subsequent MBBR polishing. Effluent stands for the pilot hybrid system effluent; Effluent (MBBR) stands for effluent treated with only a polishing MBBR; 2O3 stands for effluent treated with 2 mg O3/L; 2O3(MBBR) stands for effluent treated with 2 mg O3/L followed by a polishing MBBR.
The results in Figure 7 show that ozone was able to reduce the investigated fluorescence to some degree compared to the original sample (the staged MBBRs effluent) and the original sample treated with a pure polishing MBBR. Additionally, by increasing the delivered ozone dosages, fluorescence intensities in the related samples were much lower, thereby indicating that less BOD was present in the ozonated samples. On the other hand, after increasing the HRT, ozone followed by a subsequent polishing MBBR had the ability to reduce fluorescence intensities further. At 2 and 4 mg O3/L, there were significant differences in protein-like fluorescence intensities between those samples treated with only ozonation and ozonation followed by a polishing MBBR. Therefore, it could be concluded that a polishing MBBR is necessary after ozonation.
As mentioned above, staged MBBR effluent treated with ozone followed by a subsequent polishing MBBR had the lowest fluorescence intensities; however, at 8 and 12 mg O3/L, ozonated samples treated with the subsequent polishing MBBR had higher intensities of protein-like fluorescence than those exposed only to ozonation for the first five hours. This phenomenon can be explained by the very high BOD reduction in the sample exposed to the high delivered ozone dosage (8 and 12 mg O3/L). After introducing the polishing MBBR, the biofilms were detached from the carriers, which led to the BOD from the biofilm dissolving in the ozonated waster and thus increasing the intensities of protein-like fluorescence.
4. Conclusions
The pharmaceutical concentrations in pre-biologically treated wastewater decreased when the delivered ozone dosage increased. Z90, which was obtained based on delivered ozone dosage achieving 90% removal of pharmaceuticals normalised to DOC in wastewater, was considered an indicator for evaluating the removal efficiency of pharmaceuticals in wastewater by ozonation. Pharmaceutical concentrations in wastewater were positively correlated with fluorescence intensity during ozonation, and less delivered ozone dosage was needed when aiming to reduce the fluorescence intensity of protein-like fluorophores, yet more delivered ozone dosage was required for reducing the fluorescence intensity of humic-like fluorophores. Pilot-scale staged MBBRs were capable of reducing toxicity in wastewater, along the treatment train, and the subsequent ozone further reduced by half the remaining toxicity in the staged MBBR effluent. A polishing MBBR used as post-treatment after ozonation was able to remove almost all remaining toxicity and completely remove the fluorescence intensity of protein-like fluorophores in the ozonated wastewater.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4441/12/4/1059/s1, Table S1: List of Suppliers, Table S2: Common Parameters of Effluent from MBBRs in Herning, Table S3: Initial Concentrations of Pharmaceuticals (C0) in M5 effluent, and the Relevant Limit of Quantification (LOQ) by HPLC-MS/MS from the Pilot and Laboratory Experiments, Table S4: Correlation between the changes of concentrations of selected pharmaceuticals (ΔC/C0) and relative changes of excitation emission matrices fluorescence (ΔA/A0) under different dosages of ozone at laboratory, which was fitted by the straight line, Figure S1: Comparison of pharmaceutical removal by ozone in the effluent of the staged MBBR demonstration plant at Herning municipal wastewater treatment plant using the onsite continuous pilot ozonation system and batch treatment in laboratory. The dash lines in the figure stand for the limit of quantification (LOQ) of pharmaceuticals by HPLC-MS/MS. Error bars represent the standard deviations, Figure S2: Correlation between the changes of concentrations of pharmaceuticals (ΔC/C0) and relative changes of excitation emission matrices fluorescence (ΔA/A0) under different dosages of ozone at laboratory, which was fitted by the straight line, Figure S3: Development of natural fluorescence in M5 effluent treated by six doseages of ozone in pilot with or without a subsequent MBBR polishing.
Author Contributions
K.T.: Conceptualization; Software; Investigation; Writing—review & editing; G.T.H.O.: Conceptualization; Software; Investigation; Writing—original draft; A.S.: Software; Writing—review & editing; K.M.S.K.: Software; K.S.: Resources; Data Curation; B.F.: Resources; Data Curation; C.K.: Resources; Project administration; Funding acquisition; K.B.: Conceptualization; Writing—review & editing; H.R.A.: Conceptualization; Writing—review & editing; Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Danish Environmental Protection Agency [No. 1988, 2018].
Acknowledgments
The authors acknowledge the funding of the MERMISS project (Miljøeffektiv rensning af højpotente lægemiddelstoffer i hospitalsspildevand/Environmentally effective removal of pharmaceuticals from hospital wastewater) as part of the MUDP programme run by the Danish Ministry of the Environment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tran, N.H.; Reinhard, M.; Gin, K.Y.H. Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions-a review. Water Res. 2018, 133, 182–207. [Google Scholar] [CrossRef] [PubMed]
- Sanganyado, E.; Lu, Z.; Fu, Q.; Schlenk, D.; Gan, J. Chiral pharmaceuticals: A review on their environmental occurrence and fate processes. Water Res. 2017, 124, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Alcalá, I.; Guillén-Navarro, J.M.; Fernández-López, C. Pharmaceutical biological degradation, sorption and mass balance determination in a conventional activated-sludge wastewater treatment plant from Murcia, Spain. Chem. Eng. J. 2017, 316, 332–340. [Google Scholar] [CrossRef]
- Yang, Y.; Sik, Y.; Kim, K.; Kwon, E.E.; Fai, Y. Science of the Total Environment Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review. Sci. Total Environ. 2017, 596–597, 303–320. [Google Scholar] [CrossRef]
- Tiwari, B.; Sellamuthu, B.; Ouarda, Y.; Drogui, P.; Tyagi, R.D.; Buelna, G. Bioresource Technology Review on fate and mechanism of removal of pharmaceutical pollutants from wastewater using biological approach. Bioresour. Technol. 2017, 224, 1–12. [Google Scholar] [CrossRef]
- Liu, H.; Lam, J.C.W.; Li, W.; Yu, H.; Lam, P.K.S. Science of the Total Environment Spatial distribution and removal performance of pharmaceuticals in municipal wastewater treatment plants in China. Sci. Total Environ. 2017, 586, 1162–1169. [Google Scholar] [CrossRef]
- Mccallum, E.S.; Krutzelmann, E.; Brodin, T.; Fick, J.; Sundelin, A.; Balshine, S.; Harbour, H. Science of the Total Environment Exposure to wastewater ef fl uent affects fi sh behaviour and tissue-speci fi c uptake of pharmaceuticals. Sci. Total Environ. 2017, 606, 578–588. [Google Scholar] [CrossRef]
- Hapeshi, E.; Lambrianides, A.; Koutsoftas, P.; Kastanos, E.; Michael, C.; Fatta-Kassinos, D. Investigating the fate of iodinated X-ray contrast media iohexol and diatrizoate during microbial degradation in an MBBR system treating urban wastewater. Environ. Sci. Pollut. Res. 2013, 20, 3592–3606. [Google Scholar] [CrossRef]
- Falås, P.; Longrée, P.; la Cour Jansen, J.; Siegrist, H.; Hollender, J.; Joss, A. Micropollutant removal by attached and suspended growth in a hybrid biofilm-activated sludge process. Water Res. 2013, 47, 4498–4506. [Google Scholar] [CrossRef]
- Falås, P.; Baillon-Dhumez, A.; Andersen, H.R.; Ledin, A.; la Cour Jansen, J. Suspended biofilm carrier and activated sludge removal of acidic pharmaceuticals. Water Res. 2012, 46, 1167–1175. [Google Scholar] [CrossRef]
- Tang, K.; Ooi, G.T.H.; Litty, K.; Sundmark, K.; Kaarsholm, K.M.S.; Sund, C.; Kragelund, C.; Christensson, M.; Bester, K.; Andersen, H.R. Bioresource Technology Removal of pharmaceuticals in conventionally treated wastewater by a polishing moving bed biofilm reactor ( MBBR ) with intermittent feeding. Bioresour. Technol. 2017, 236, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Ooi, G.T.H.; Tang, K.; Chhetri, R.K.; Kaarsholm, K.M.S.; Sundmark, K.; Kragelund, C.; Litty, K.; Christensen, A.; Lindholst, S.; Sund, C.; et al. Biological removal of pharmaceuticals from hospital wastewater in a pilot-scale staged moving bed biofilm reactor (MBBR) utilising nitrifying and denitrifying processes. Bioresour. Technol. 2018, 267, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Escolà Casas, M.; Chhetri, R.K.; Ooi, G.; Hansen, K.M.S.; Litty, K.; Christensson, M.; Kragelund, C.; Andersen, H.R.; Bester, K. Biodegradation of pharmaceuticals in hospital wastewater by staged Moving Bed Biofilm Reactors (MBBR). Water Res. 2015, 83, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Escolà Casas, M.; Chhetri, R.K.; Ooi, G.; Hansen, K.M.S.; Litty, K.; Christensson, M.; Kragelund, C.; Andersen, H.R.; Bester, K. Biodegradation of pharmaceuticals in hospital wastewater by a hybrid biofilm and activated sludge system (Hybas). Sci. Total Environ. 2015, 530–531, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Moreira, F.C.; Soler, J.; Alpendurada, M.F.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Tertiary treatment of a municipal wastewater toward pharmaceuticals removal by chemical and electrochemical advanced oxidation processes. Water Res. 2016, 105, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Mailler, R.; Gasperi, J.; Coquet, Y.; Buleté, A.; Vulliet, E.; Deshayes, S.; Zedek, S.; Mirande-bret, C. Science of the Total Environment Removal of a wide range of emerging pollutants from wastewater treatment plant discharges by micro-grain activated carbon in fl uidized bed as tertiary treatment at large pilot scale. Sci. Total Environ. 2016, 542, 983–996. [Google Scholar] [CrossRef]
- Rattier, M.; Reungoat, J.; Keller, J.; Gernjak, W. Removal of micropollutants during tertiary wastewater treatment by biofiltration: Role of nitrifiers and removal mechanisms. Water Res. 2014, 54, 89–99. [Google Scholar] [CrossRef]
- Bui, X.T.; Vo, T.P.T.; Ngo, H.H.; Guo, W.S.; Nguyen, T.T. Science of the Total Environment Multicriteria assessment of advanced treatment technologies for micropollutants removal at large-scale applications. Sci. Total Environ. 2016, 564, 1050–1067. [Google Scholar] [CrossRef]
- Hansen, K.M.S.; Spiliotopoulou, A.; Chhetri, R.K.; Escolà Casas, M.; Bester, K.; Andersen, H.R. Ozonation for source treatment of pharmaceuticals in hospital wastewater—Ozone lifetime and required ozone dose. Chem. Eng. J. 2016, 290, 507–514. [Google Scholar] [CrossRef]
- Baun, A.; Jensen, S.D.; Bjerg, P.L.; Christensen, T.H. Toxicity of Organic Chemical Pollution in Groundwater Downgradient of a Landfill (Grindsted, Denmark ). Environ. Sci. Technol. 2000, 1647–1652. [Google Scholar] [CrossRef]
- Mišík, M.; Knasmueller, S.; Ferk, F.; Cichna-Markl, M.; Grummt, T.; Schaar, H.; Kreuzinger, N. Impact of ozonation on the genotoxic activity of tertiary treated municipal wastewater. Water Res. 2011, 45, 3681–3691. [Google Scholar] [CrossRef] [PubMed]
- Anumol, T.; Sgroi, M.; Park, M.; Roccaro, P.; Snyder, S.A. Predicting trace organic compound breakthrough in granular activated carbon using fluorescence and UV absorbance as surrogates. Water Res. 2015, 76, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Anumol, T.; Daniels, K.D.; Wu, S.; Ziska, A.D.; Snyder, S.A. Predicting trace organic compound attenuation by ozone oxidation: Development of indicator and surrogate models. Water Res. 2017, 119, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Chys, M.; Audenaert, W.T.M.; Vangrinsven, J.; Bauwens, M.; Mortier, S.T.F.C.; Van Langenhove, H.; Nopens, I.; Demeestere, K.; Van Hulle, S.W.H. Dynamic validation of online applied and surrogate-based models for tertiary ozonation on pilot-scale. Chemosphere 2018, 196, 494–501. [Google Scholar] [CrossRef]
- Tang, K.; Spiliotopoulou, A.; Chhetri, R.K.; Ooi, G.T.H.; Kaarsholm, K.M.S.; Sundmark, K.; Florian, B.; Kragelund, C.; Bester, K.; Andersen, H.R. Removal of pharmaceuticals, toxicity and natural fluorescence through the ozonation of biologically-treated hospital wastewater, with further polishing via a suspended biofilm. Chem. Eng. J. 2019, 359, 321–330. [Google Scholar] [CrossRef]
- Spiliotopoulou, A.; Martin, R.; Pedersen, L.; Andersen, H.R. Use of fl uorescence spectroscopy to control ozone dosage in recirculating aquaculture systems. Water Res. 2017, 111, 357–365. [Google Scholar] [CrossRef]
- Coble, P.G. Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy. Mar. Chem. 1996, 51, 325–346. [Google Scholar] [CrossRef]
- Libralato, G.; Ghirardini Annamaria, V.; Francesco, A. How toxic is toxic? A proposal for wastewater toxicity hazard assessment. Ecotoxicol. Environ. Saf. 2010, 73, 1602–1611. [Google Scholar] [CrossRef]
- Bader, H.; Hoigne, J. Determination of ozone in water by the indigo method. Water Res. 1981, 15, 449–456. [Google Scholar] [CrossRef]
- Hansen, K.M.S.; Andersen, H.R.; Ledin, A. Ozonation of estrogenic chemicals in biologically treated sewage. Water Sci. Technol. 2010, 62, 649–657. [Google Scholar] [CrossRef][Green Version]
- Antoniou, M.G.; Hey, G.; Rodríguez Vega, S.; Spiliotopoulou, A.; Fick, J.; Tysklind, M.; la Cour Jansen, J.; Andersen, H.R. Required ozone doses for removing pharmaceuticals from wastewater effluents. Sci. Total Environ. 2013, 456–457, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Sudhakaran, S.; Amy, G.L. QSAR models for oxidation of organic micropollutants in water based on ozone and hydroxyl radical rate constants and their chemical classification. Water Res. 2013, 47, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Blaney, L. Ozone Treatment of Antibiotics in Water. In Water Reclamation and Sustainability; Elsevier: Amsterdam, The Netherlands, 2014; pp. 265–316. ISBN 9780124165762. [Google Scholar]
- Lee, Y.; Kovalova, L.; McArdell, C.S.; von Gunten, U. Prediction of micropollutant elimination during ozonation of a hospital wastewater effluent. Water Res. 2014, 64, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Kovalova, L.; Siegrist, H.; von Gunten, U.; Eugster, J.; Hagenbuch, M.; Wittmer, A.; Moser, R.; McArdell, C.S. Elimination of Micropollutants during Post-Treatment of Hospital Wastewater with Powdered Activated Carbon, Ozone, and UV. Environ. Sci. Technol. 2013, 47, 7899–7908. [Google Scholar] [CrossRef]
- Hollender, J.; Zimmermann, S.G.; Koepke, S.; Krauss, M.; McArdell, C.S.; Ort, C.; Singer, H.; von Gunten, U.; Siegrist, H. Elimination of Organic Micropollutants in a Municipal Wastewater Treatment Plant Upgraded with a Full-Scale Post-Ozonation Followed by Sand Filtration. Environ. Sci. Technol. 2009, 43, 7862–7869. [Google Scholar] [CrossRef]
- Lee, Y.; Gerrity, D.; Lee, M.; Bogeat, A.E.; Salhi, E.; Gamage, S.; Trenholm, R.A.; Wert, E.C.; Snyder, S.A.; von Gunten, U. Prediction of Micropollutant Elimination during Ozonation of Municipal Wastewater Effluents: Use of Kinetic and Water Specific Information. Environ. Sci. Technol. 2013, 47, 5872–5881. [Google Scholar] [CrossRef]
- Świetlik, J.; Sikorska, E. Application of fluorescence spectroscopy in the studies of natural organic matter fractions reactivity with chlorine dioxide and ozone. Water Res. 2004, 38, 3791–3799. [Google Scholar] [CrossRef]
- Uyguner, C.S.; Bekbolet, M. Evaluation of humic acid photocatalytic degradation by UV-vis and fluorescence spectroscopy. Catal. Today 2005, 101, 267–274. [Google Scholar] [CrossRef]
- Wittmer, A.; Heisele, A.; McArdell, C.S.; Böhler, M.; Longree, P.; Siegrist, H. Decreased UV absorbance as an indicator of micropollutant removal efficiency in wastewater treated with ozone. Water Sci. Technol. 2015, 71, 980–985. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).