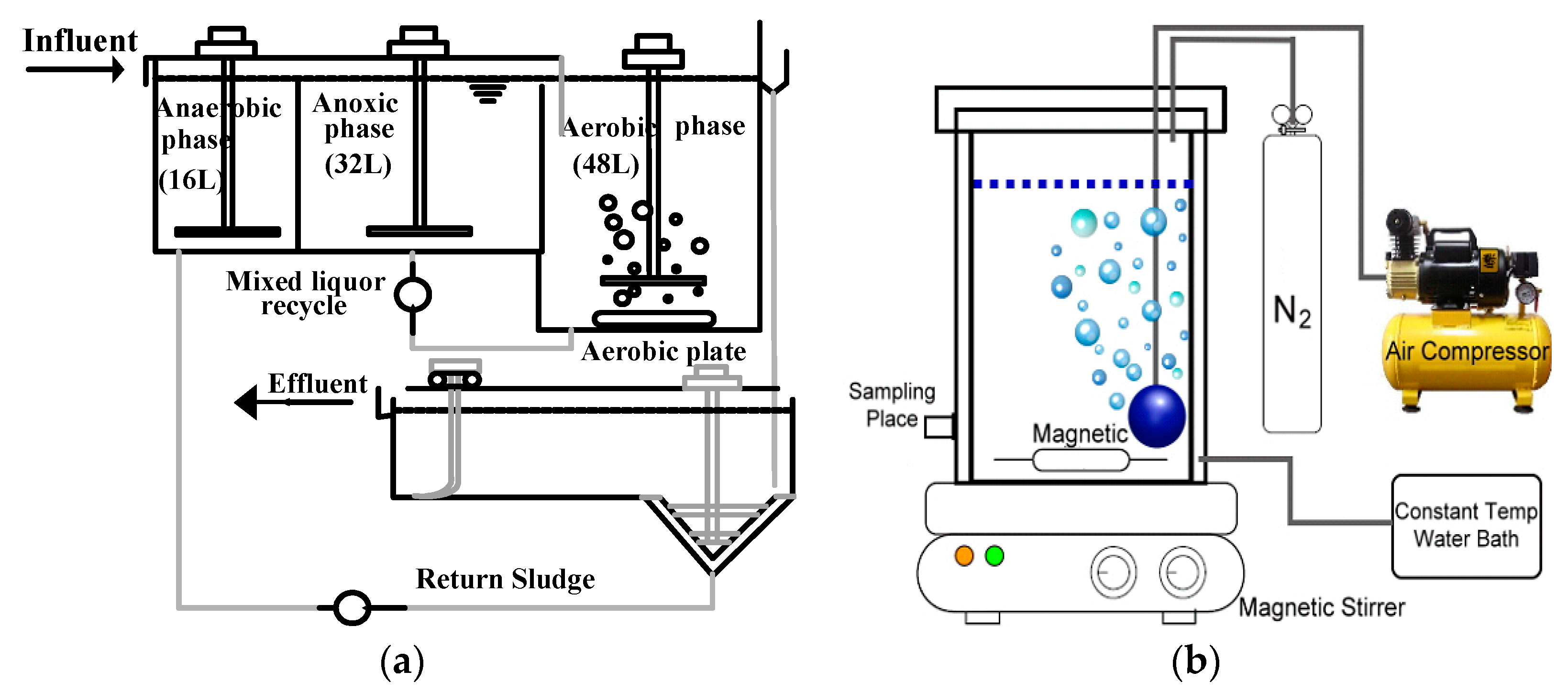
3.1. Performance of Acetic Acid and Glucose as Carbon Sources in the Absence Cd2+
To investigate the effect of carbon source and its concentration on the EBPR process, a series of batch experiments were performed in this study. Two carbon sources such as acetic acid (A) and glucose (G) were added with high (H) and low (L) concentrations.
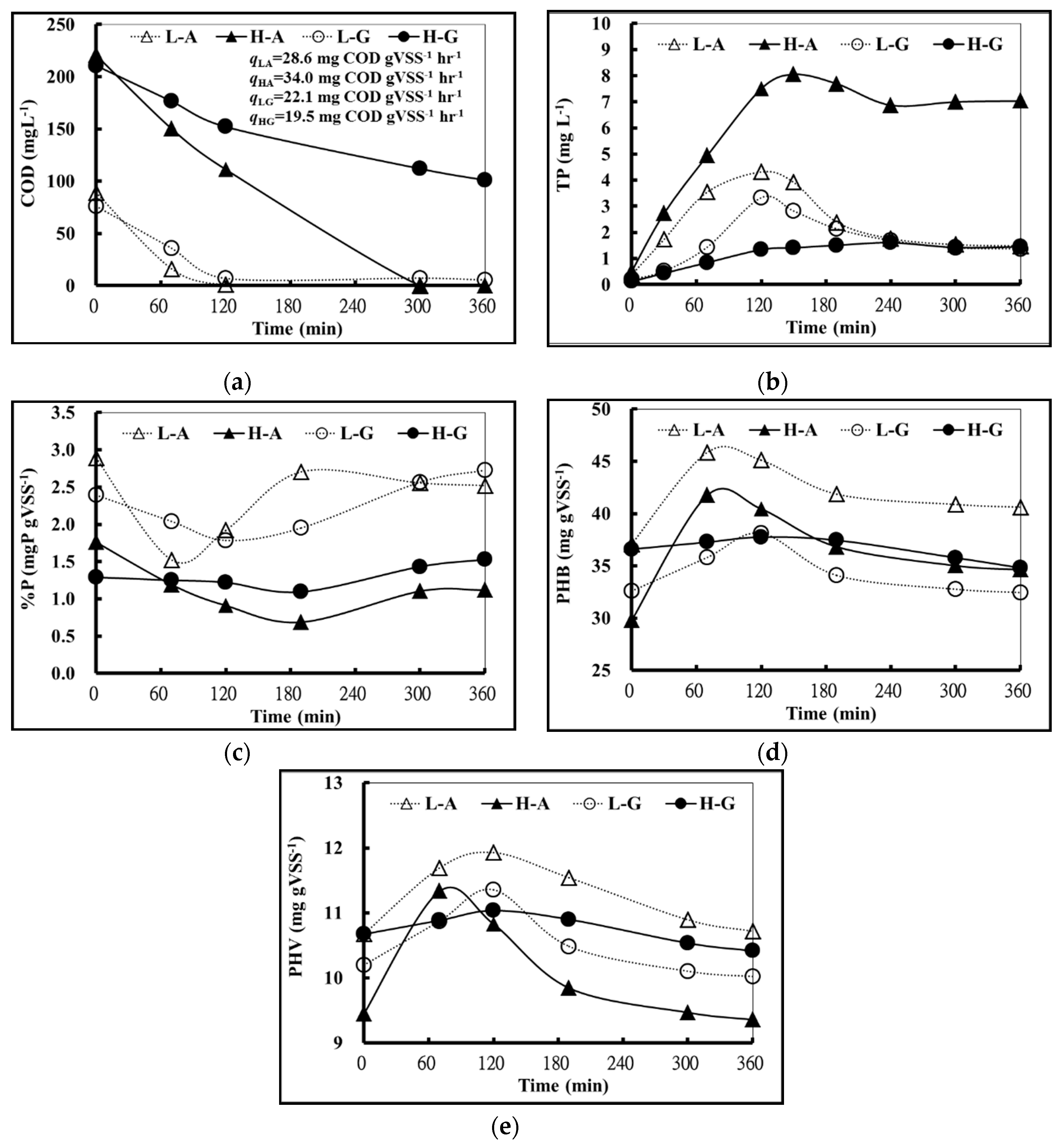
Figure 1 shows the changes in COD, TP, P%, PHB, and PHV of high concentration acetic acid (HA), low concentration acetic acid (LA), high concentration glucose (HG), and low concentration glucose (LG) in 360 min.
Under anaerobic conditions (0–120 min), it was observed that the specific acetic acid utilization rate (qLA and qHA) was higher than that of specific glucose utilization rate (qLG and qHG), in either high or low substrate concentration as shown in
Figure 2a. This vividly indicated that the A
2O process preferred acetic acid as a carbon source rather than glucose. Additionally, the specific substrate utilization rate of the high concentration acetic acid trials (qHA = 34.0 mg COD g
−1 VSS h
−1) was higher than that of low concentration acetic acid trials (qLA = 28.6 mg COD g
−1 VSS h
−1). Contrarily, the specific utilization rate of the high concentration glucose trials (qHG = 19.5 mg COD g
−1 VSS h
−1) was lower than that of low concentration glucose trials (qLG = 22.1 mg COD g
−1 VSS h
−1). This indicated that the activated sludge of A
2O did not only dislike the glucose as a carbon source but also decreased its substrate utilization rate when the glucose concentration was increased. This result varied from the study of Hollender et al. [
2], which reported that the degradation of glucose was fast, whereas the degradation of acetate was slow and incomplete.
It was also observed that the anaerobic phosphate release rates of glucose trials were all lower than that of acetic acid trials, in either high or low substrate concentration as shown in
Figure 1b. Additionally, the anaerobic phosphate release rates of high acetic acid concentration trials were higher than that of the low acetic acid concentration trials. However, the anaerobic phosphate release rates of high glucose concentration trials were lower than that of low glucose concentration trials. This suggested that the amount of phosphate released was relatively low when the activated sludge of the A
2O process utilized glucose as a substrate.
Table 6 shows the required amount of COD that was consumed to release 1 mg phosphorous (γ), which is calculated from
Figure 1a,b. It was observed that 15.6 mg of COD was consumed to release 1 mg of phosphate when a high concentration of acetic acid was used as a carbon source. Meanwhile, almost three times more COD (47.9 mg) was consumed to release 1 mg of phosphate by using a high concentration of glucose. This might be due to the existence of GAOs in the activated sludge. Some bacteria could be observed in failed EBPR systems that had no anaerobic phosphate release with carbon uptake and these bacteria were easily observed in glucose reactors. Cech and Hartman [
24] called the bacteria “G-bacterium”, which consumed intracellular glycogen under anaerobic conditions and accumulated glycogen under aerobic conditions. Trials using a low concentration of glucose indicated that there was no anaerobic phosphate release. In this study, a high concentration of glucose possessed a remarkably high γ value (47.9 mg COD mg
−1 P). It showed that the phosphate release in glucose trial was much more difficult than that of acetic acid trial. This might be ascribed to the predominance of GAOs, which could utilize a lot of carbon but without any phosphate release.
Figure 1b and
Figure 2a show that the residual COD at the beginning of the aerobic phase (t = 120 min) was 111 mg/L for high concentration acetic acid trial, with a phosphate uptake rate of 0.11 mgP gVSS
−1 h
−1 (120–360 min). Additionally, the residual COD at t = 120 min was 152 mg/L of high concentration glucose trial, with a phosphate uptake rate of −0.01 mg P g VSS
−1 h
−1. These data suggested that regardless of the type of carbon source, the phosphate uptake rate decreased when residual COD at the beginning of the aerobic phase increased. The results indicated that the phosphate release possibly would occur during aerobic conditions if there was too much residual COD in the aerobic phase. A similar observation was reported by You et al. [
25], stating that the anaerobic phosphate uptake rate decreased as the residual COD concentration in the aerobic phase increased. In their study, it was deemed that this phenomenon might be due to the denitrifying phosphate accumulating organisms (DNPAOs), which could further release phosphate under aerobic conditions in the occurrence of a high amount of residual COD.
Figure 3a shows the phosphate release rate with substrate utilization rate under different concentrations of carbon sources. It was observed when the phosphate release rate increased, the substrate utilization rate also increased. This denoted that the amount of released phosphate increased with the increase of substrate utilization rate under any type of carbon source in A
2O. Kuba et al. [
26] showed that the amount of anaerobic phosphate release was proportional to acetate uptake. Hence, when the amount of acetate was higher than the minimum carbon requirement, extra carbon source contributed to increasing the amount of anaerobic phosphate release but had no impact on total phosphate removal performance. However, they did not investigate the impact of carbon sources on the contribution of phosphorus release. In this study, it was found that the phosphate release rate was proportional to the substrate utilization rate in any type or concentration of carbon source. The y-intercept of the trend line in
Figure 3a shows the minimum carbon source for phosphate release (15.7 mg COD g
−1 VSS h
−1). Thus, when a high concentration of acetic acid was used as a carbon source, more phosphate was released under anaerobic conditions. However, the phosphorus removal performance was also inhibited due to the absence of phosphate uptake under aerobic conditions, indicating a high concentration of acetic acid resulted in the collapse of the EBPR process [
27]. Besides, acetic acid caused a serious bulking problem of activated sludge in this study. Similar observations were also reported by the previous studies.
However, a previous study showed that the necessary conditions for stable EBPR processes were long anaerobic retention time, short aerobic retention time with low dissolved oxygen (DO), and high concentration of glucose [
3]. The result of this study was inconsistent with previous reports when the EBPR process was inefficient under a high concentration of glucose, due to the release of phosphate instead of uptake under aerobic conditions. Thus, this study demonstrated that phosphate removal potential of the A
2O process would increase with the increase of initial anaerobic COD concentration and decrease of residual aerobic COD. Moreover, higher phosphate removal performance was observed when acetic acid was used as a carbon source.
It was also noticeable that intracellular phosphorous content (P%) and phosphate concentration in bulk solution (TP) showed a highly negative correlation when acetic acid was used as a carbon source. The correlation was not as nearly as high when glucose was the carbon source (
Figure 2b,c and
Figure 3b). This revealed that in acetic acid, the activated sludge released phosphate by degrading the long-chain polyphosphate under anaerobic conditions, resulting in the reduction of intracellular polyphosphate content. Besides, the intracellular PHAs were oxidized to obtain the energy for phosphate uptake under aerobic conditions to enrich intracellular phosphorous content. On the contrary, when high concentration glucose was used as a carbon source, the anaerobic intracellular phosphorous content decreased and the rate was 0.02 mgP g VSS
−1 h
−1, with the phosphate release rate of 0.39 mgP g VSS
−1 h
−1. It was also observed that the aerobic intracellular phosphorous increase rate was found to be 0.04 mgP g VSS
−1 h
−1, with a phosphate uptake rate of −0.01 mgP g VSS
−1 h
−1. It was observed that the phosphate release rate did not reflect the phosphate content in the bulk solution. This might be due to the adsorption of phosphate onto extracellular polymeric substances (EPS).
The constituents of EPS were similar to microbial polymers. It included different types of macromolecules, such as protein, polysaccharide, nucleic acid, phosphoric acid, and other polymers. They could produce sticky material to flocculate the biofilm and activated sludge [
28]. By X-ray observation, Cloete and Oosthuizen [
29] observed that 80% of phosphate removal was due to the biochemical action of PAO, and 20% was due to the physical adsorption of EPS. Because of this, EPS was considered as the storage tank of phosphate. Additionally, the presence of glucose promoted the formation of EPS and enhanced adsorption ability [
30,
31]. It was also observed that EPS concentration decreased under anaerobic conditions, and increased under aerobic conditions. As a consequence, this resulted in the reduction of phosphate removal performance under anaerobic conditions in comparison with aerobic conditions. The glucose trials contained higher EPS under aerobic conditions, which caused the enhancement of the phosphate adsorption ability of EPS, increasing the phosphate content of activated sludge. The results of this study suggested that a similar phenomenon has occurred in the A
2O process.
Figure 2d,e shows that the PHB and PHV anaerobic synthesis rate of acetic acid trials were higher than that of the glucose trials, regardless of the concentration of the substrate. This indicated that the activated sludge of A
2O preferred to uptake acetic acid as a carbon source, and transformed it to PHA for energy storage. However, activated sludge did not transform glucose as easily. It could be seen from
Figure 4a that the anaerobic PHA synthesis rate of acetic acid trials was higher than that of glucose trials, regardless of concentration. The specific PHA synthesis (PHA/q) of the trial that used low concentration acetic acid was 0.1 mg g
−1 VSS h
−1, while the trial that used high concentration was 0.11 mg g
−1VSS h
−1. Meanwhile, a low concentration glucose trial was 0.09 mg g
−1 VSS h
−1, and a high concentration glucose trial was 0.02 mg g
−1 VSS h
−1. These results suggested that it was easy for activated sludge to convert acetic acid to PHA, mostly in the form of PHB, via the ED pathway. On the contrary, the PHA synthesis rate was lower for glucose trials. This perhaps was because of the reason that the activated sludge could not utilize glucose well under anaerobic conditions.
The substrate utilization rate and PHB synthesis rate showed a high positive correlation, regardless of the type and concentration of carbon source as shown in
Figure 3b. This conveyed that the activated sludge readily converted the substrate to PHB by ED pathway, but not as easily to PHV by EMP pathways. From
Figure 4, it was observed that the ratio of PHB:PHV was about 80:20. The results thus implied that under aerobic conditions, the activated sludge could degrade the intracellular PHA to produce energy for the uptake of extracellular phosphate as shown in
Figure 4b. However, the activated sludge of the high concentration glucose trial could not degrade PHA as easily. Under anaerobic conditions, activated sludge consumed more PHA for metabolic maintenance. Notably, the ratio of the degradation rates (PHV: PHB) was also found to be 80:20.
3.2. Effect of Cd2+ Shock Loading on the EBPR Process
To investigate the effect of Cd2+ shock loading on the EBPR process under different types and concentrations of carbon source, different concentrations such 0, 1, 3, 6, 10, and 15 mg L−1 were used. Substrate utilization rate, residual substrate amount, phosphate release rate, phosphate uptake rate, PHA synthesis rate, and PHA degradation rate were compared under different concentrations of Cd2+ to investigate the effect of the heavy metal on the A2O system.
The activated sludge that originally possessed a higher substrate utilization rate was more affected by the addition of Cd
2+ than that of activated sludge that was originally associated with lower substrate utilization rate, regardless of the concentration as shown in
Figure 5a. It might be ascribed to the competition of cadmium ions with organic substrates on cellular adsorption sites, and decreased the interaction of the substrate with the floc [
10] because of the reason that the heavy metals were generally bound on microbial surfaces instead of accumulating within the cells [
32].
In this study, it was observed that the activated sludge fed with acetic acid was more vulnerable to the shock loading of Cd
2+ than that of glucose for the inhibition on substrate utilization rate as shown in
Figure 5a. The inhibition rate increased when Cd
2+ concentration increased. This suggested that in the presence of toxic heavy metal such as Cd
2+, the activated sludge could adapt to the toxic environment and lower its consumption of carbon source to avoid the inhibition of regular cell metabolisms. The average of the slope of HA or LA obtained from
Figure 4a is −0.75. As such, it could be suggested that Cd
2+ significantly inhibited the substrate utilization rate of activated sludge that used acetic acid as a carbon source i.e., the addition of 1 mg L
−1 of Cd
2+ could decrease the substrate utilization rate by 0.75 mg g VSS
−1 h
−1. From
Figure 6a, it was also observed that the aerobic residual COD increased when Cd
2+ concentration increased, with an average slope of HA or LA equal to 2.38.
Figure 5b shows that for the acetic acid trials, phosphate release rate (PRR) significantly decreased when Cd
2+ concentration increased, and the activated sludge fed with a high concentration of acetic acids was more vulnerable to the shock loading of Cd
2+ than that fed with a low concentration of acetic acid. A similar trend was observed for the glucose trials, but the slope of PRR was comparatively lower. This indicated that the inhibition effect of Cd
2+ on the glucose trials was not as great as the acetic acid trials. The average phosphate release rate was about 33% when Cd
2+ concentration was 6 mg L
−1 in the high concentration of acetic acid trials. Meanwhile, the average phosphate release rate reached 50% when Cd
2+ concentration was 10 mg L
−1. The average phosphate release rate was 66% when Cd
2+ concentration was 15 mg L
−1. It was evident when acetic acid was used as a substrate, the sudden introduction of Cd
2+ in the anaerobic phase caused a great reduction of phosphate release rate. The phosphate release rate was half when the concentration of Cd
2+ was 10 mg L
−1. On the other hand, the inhibition effect of Cd
2+ on the phosphate release of glucose trials was much less. Thus, it could be said that the A
2O process that utilized the glucose as a substrate showed more tolerance to the sudden intrusion of Cd
2+. In general, GAOs were dominant in glucose trials and PAOs were dominant in acetic acid trials. Therefore, it could be said that PAOs were more sensitive to Cd
2+ toxicity than GAOs. That is why the phosphate removal was easily deteriorated by the presence of heavy metals in an EBPR system, as proposed by Tsai et al. [
18].
Figure 6b shows the relationship between Cd
2+ and phosphate uptake. Regardless of the high or low concentration of carbon source, the phosphate uptake rate of the acetic acid trials was inhibited more by the introduction of Cd
2+ than that of the glucose trials. This was because the acetic acid trials were more vulnerable to the toxicity of heavy metal. Another reason was that the increased residual COD (
Figure 6a) suppressed the phosphate uptake rate. It was observed for the trial that used a high concentration of acetic acid or glucose that the value of phosphate uptake rate became negative, even in the absence of Cd
2+, implying that the activated sludge did not uptake any orthophosphate from water during the aerobic phase. This was because the level of COD was high. The negative phosphate uptake rate became more obvious when Cd
2+ was introduced and further increased. Hence, the activated sludge did not uptake orthophosphate in the aerobic phase, but rather, it released cellular polyphosphate.
The main reason for the aforementioned phenomenon was the toxicity of Cd
2+, which caused the inhibition of cell metabolism causing cell death and subsequent lysis. The disturbance due to the aeration in the succeeding aerobic phase further disintegrated the activated sludge, until all cells were lysed. Thus, intracellular phosphate was released to the water, causing the phosphate uptake rate to be negative. The second reason was that Cd
2+ did harm the activated sludge without the floc lysis. However, because of the toxicity, the activated sludge did not have enough energy to perform aerobic phosphate uptake. The cells utilized intracellular phosphate to continue metabolic activities. From
Figure 6d, it was noticed that the amount of intracellular phosphate in the initial stage of the aerobic phase slightly decreased upon the introduction of Cd
2+ in the trial that used a high concentration of acetic acid.
Figure 6b indicates the apparent inhibition of PHB degradation. This implied that the activated sludge that has been poisoned by Cd
2+ in the aerobic phase could decompose its intracellular polyphosphate and PHB to produce sufficient energy for continued metabolic activities. This was the reason for the phosphate uptake rate to be negative. It has been proven that shock loading of Cd
2+ on the trial that used a high concentration of acetic acid caused its phosphate uptake rate to become negative.
It was observed that the PHB synthesis rate of the acetic acid trials was more suppressed by the increase of Cd
2+ concentration than that of the glucose trials as shown in
Figure 5c. The inhibition was more apparent for low concentration of acetic acid trials (slope = −0.08) and also the PHB degradation of the acetic acid trials was more suppressed by the increase of Cd
2+ concentration than that of the glucose trials as shown in
Figure 6c. Again, the inhibition was more apparent in the low concentration of acetic acid trials (slope = −0.11).
The metabolism of PHV was similarly influenced by Cd
2+. From
Figure 5d, it was observed that the PHV synthesis rate of the acetic acid trials was more suppressed by the increase of Cd
2+ concentration than that of the glucose trials. The inhibition could be seen more clearly in the low concentration of acetic acid trials (slope = −0.03) and also from
Figure 6d, it was observed that the PHV degradation rate of the acetic acid trials was more suppressed by the increase of Cd
2+ concentration than that of the glucose trials. The inhibition was more apparent for the low concentration of acetic acid trials (slope = −0.02). Furthermore, by comparing the slopes of
Figure 5c and
Figure 6c with slopes of
Figure 5d and
Figure 6d, it was also apparent that the PHB synthesis/degradation rates were more suppressed by the increase of Cd
2+ concentration than PHV synthesis/degradation rates, regardless of the type and concentration of carbon source. It indicated that the ED pathway, which related to PHB metabolism, was suppressed more severely by lead shock loading than that of the EMP pathway, which related to PHV metabolism.
In conclusion, it was stated that from all the aforementioned findings, the increase of concentration of Cd
2+ inhibited the synthesis and decomposition of both PHB and PHV. Comparing the slopes of the trend lines of
Figure 5c,d with
Figure 6c,d, it could be seen that the shock loading of Cd
2+ suppressed the synthesis and decomposition of PHAs more easily in acetic acid trials than that of glucose trials. This implied that if glucose was used as the main carbon source in the A
2O process, synthesis and decomposition of intracellular PHAs would not be easily influenced by Cd
2+. In other words, an EBPR process that used glucose as a carbon source has more tolerance to the sudden introduction of toxic Cd
2+.