Mining Activities and the Chemical Composition of R. Modonkul, Transbaikalia
Abstract
1. Introduction
2. Object and Research Methods
3. Results and Discussion
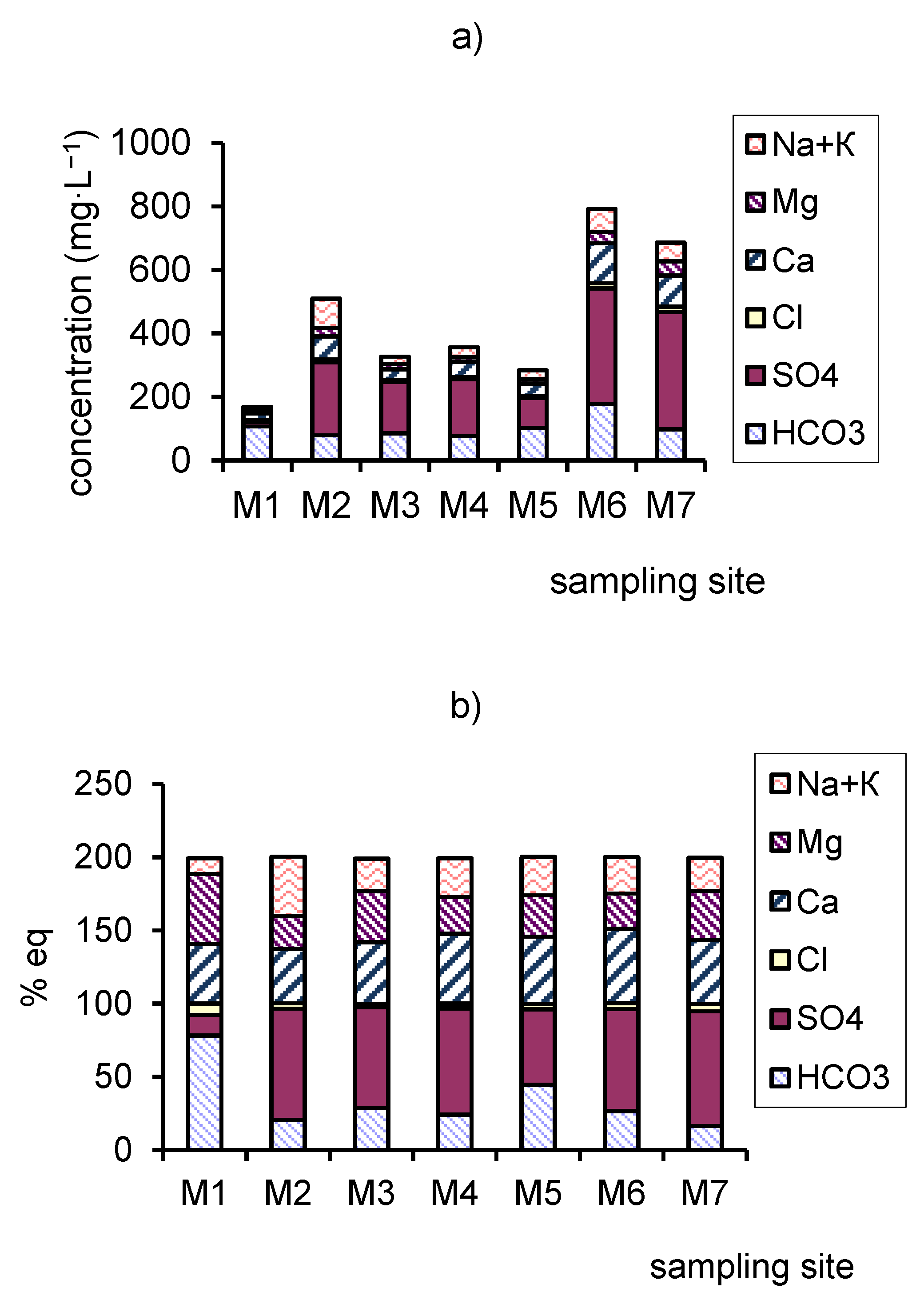
3.1. Main Ions and Total Dissolved Solids (TDS).
3.2. Trace Element
3.3. Distribution of Heavy Metals.
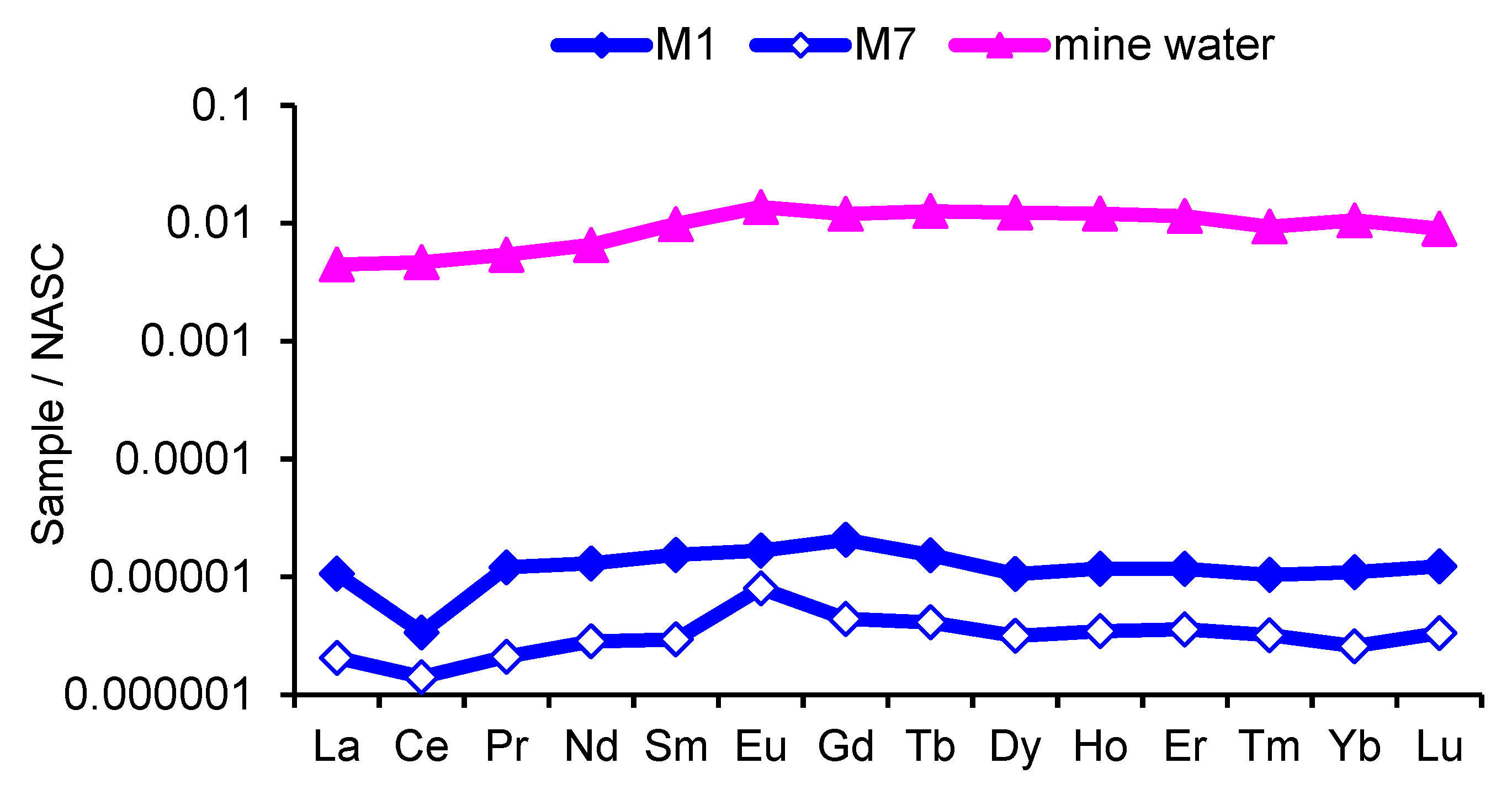
3.4. Rare Earth Elements
4. Conclusions
- (1)
- Along the river, the chemical type of water changes from bicarbonate to sulfate across the sulfate-bicarbonate or bicarbonate-sulfate class and from calcium-magnesium across sodium-calcium-magnesium reverse calcium-magnesium group. TDS increase by a factor of 4.0 to 4.7 between in the upper part and mouth of the river.
- (2)
- Trace-element concentrations in Modonkul river span 6–7 orders of magnitude. A rough classification of trace element mobility in river water can be drawn. The first group comprises the highly mobile elements, with mobility more than 10–100 times greater than that of the world mean values. It consists of Be, Zn, Co, Cd, Cs. The following group of moderately mobile elements includes Li, Cr, Ti, Mn, Co, Ni, Cu, Rb, Sr, Y, Zr, Mo, Sb, Ba, Re, Pb, U. Their mobility close to or is ~2–10 times greater than that of the world mean values. Finally, the last category, the most immobile elements, includes Al, V, Sc, Fe, Ga, As, Hf, Ta, W, Th with mobility less than that of world mean values. The dissolved concentrations of Al and Fe are close to or lower with the world mean values in the river flow. The concentrations of lithophilic elements Ti, Zr, Nb, Y, Hf practically do not change when moving downstream. The dissolved concentrations Zr, Y are 4–9 times greater, and Ti, Nb, Hf are close to that of world mean values.
- (3)
- The mine of the Kholtosonskoe deposit and tributary of the river Inkur are sources of ore technogenic elements into the environment. In the mixing zone, the pH increases, and an alkaline barrier is created. The inflow of mine water and tributary Inkur increases the turbidity of water in the river Modonkul. The river transports material, both in dissolved form and as solid load (suspended matter and bottom sands). Hydrous oxides of iron, aluminum, manganese, and silicon are dominant sorbents in rivers. The pH values and dissolved oxygen cycles could change the uptake of heavy metals on suspended iron and manganese oxides and the mechanism of removing it from the water. River particulates have the potential of regulating heavy metal input to aquatic systems from pollution. The ore elements or heavy metals are removed from water by two ways: by the runoff of Modonkul and sedimentation of suspended material at the bottom.
- (4)
- Sources of REEs in the river Modonkul are lanthanides from basin rocks and the mine waters of the Kholtosonskoe tungsten deposit. The inflow of mine water into the Modonkul river leads to the REEs composition with negative cerium and positive europium anomalies.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gordienko, I.V.; Gorokhovsky, D.V.; Smirnova, O.K.; Lantseva, V.S.; Badmatsyrenova, R.A.; Orsoev, D.A. Dzhida ore district: geology, structural and metallogenic regionalization, genetic types of ore deposits, geodynamic conditions of their formation, forecast, and outlook for development. Geol. Ore Depos. 2018, 60, 3–37. [Google Scholar] [CrossRef]
- Damdinova, L.B.; Damdinov, B.B.; Huang, X.-W.; Bryansky, N.V.; Khybanov, V.B.; Yudin, D.S. Age, conditions of formation and fluid composition of the pervomaiskoe molybdenum deposit (Dzhidinskoe ore field, southwestern Transbaikalia, Russia). Minerals 2019, 9, 572. [Google Scholar] [CrossRef]
- Khodanovich, P.Y.; Smirnova, O.K. Tungsten-Bearing Berezites And A Local Forecast of Mineralization; Nauka: Novosibirsk, Russia, 1991; p. 208. [Google Scholar]
- Smirnova, O.K.; Plyusnin, A.M. Dzhidinsky Ore District (Environmental Problems); Buryat Science Center SB RAS: Ulan-Ude, Russia, 2013; p. 181. [Google Scholar]
- Gordienko, I.V.; Filimonov, A.V.; Minina, O.R.; Klimuk, V.S.; Elbaev, A.L.; Gornova, M.A.; Medvedev, A.Y.; Tomurtogoo, O. Dzhida island-arc system in the paleoasian ocean: Structure and main stages of vendian-paleozoic geodynamic evolution. Russ. Geol. Geophys. 2007, 48, 91–106. [Google Scholar] [CrossRef]
- Reyf, F.G. Ore-Forming Potential of Granites and Conditions for Its Realization; Nauka: Moscow, Russia, 1990; p. 180. (In Russian) [Google Scholar]
- Ontoev, D.O. Mineralization Staging And Zoning of Transbaikalian Deposits; Nauka: Moscow, Russia, 1974; p. 241. (In Russian) [Google Scholar]
- Integrated Water Management. In The Selenge Basin River. Report of Project “Selenga Basin River”; Sovintervod: Moscow, Russia, 2004; p. 556.
- State Control of Water Quality. Handbook of The Technical Committee For Standardization, 2nd ed.; IPK Standards Publishing House: Moscow, Russia, 2003; p. 776. (In Russian) [Google Scholar]
- Alekin, O.A. Fundamental of Hidrochemistry; Gidrometeoizdat: Leningrad, Russia, 1970; p. 444. [Google Scholar]
- Gibbs, R. Mechanisms controlling world water chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef] [PubMed]
- Edmond, Y.M.; Spivack, A.; Grant, B.C.; Hum, H.; Chen, Z.X.; Chen, S.; Zong, X.S. Chemical dynamics of the Changjiang estuary. Cont. Shelf Res. 1985, 4, 17–36. [Google Scholar] [CrossRef]
- Gaillardet, J.; Viers, J.; Dupre, B. Trace elements in River Waters. In Surface And Ground Water: Treatise on Geochemistry Weathering And Soils; Drever, J.I., Turikan, K.K., Eds.; Elsevier: Berlin, Germany, 2003; Volume 5, pp. 225–272. [Google Scholar]
- Pokrovsky, O.S.; Shott, J. Iron colloids/organic matter associated transport of major and trace elements in small boreal rivers and their estuarias (NW Russia). Chem. Geol. 2002, 190, 141–179. [Google Scholar] [CrossRef]
- Shibata, H. Process Of Iron Transport From Terrestrial Ecosystem. In To River: Preliminary Analysis Of Spatial And Temporal Patterns Of Iron Concentrations In Amur River, Proceedings of the International Kyoto Symposium Representative Amur-Okhotsk Project, RIHN, Kyoto, Japan, 26 October 2011; Springer: Berlin, Germany, 2005; Volume 3, pp. 3273–3277. [Google Scholar]
- Elbaz-Poulichet, F.; Seyler, P.; Maurice-Bourgoin, L. Trace element geochemistry in the upper Amazon drainage basin (Bolivia). Chem. Geol. 1999, 157, 319–334. [Google Scholar] [CrossRef]
- Abrosimova, N.; Gaskova, O.; Loshkareva, A.; Edelev, A.; Bortnikova, S. Assessment of the acid mine drainage potential of waste rocks at the Ak-Sug porphyry Cu-Mo deposit. J. Geochem. Explor. 2015, 157, 1–14. [Google Scholar] [CrossRef]
- Wei, X.; Wolfe, F.A.; Han, Y. Mine drainage characterization, treatment, modeling, and environmental aspect. Water Env. Res. 2014, 86, 1515–1534. [Google Scholar] [CrossRef]
- Moncur, M.C.; Ptacek, C.J.; Blowes, D.W.; Jambor, J.L. Release, transport and attenuation of metals from an old tailings impoundment. Appl. Geochem. 2005, 215, 639–659. [Google Scholar] [CrossRef]
- Lengke, M.F.; Davis, A.; Buckman, C. Improving management of potentially acid generating waste rock. Mine. Water Env. 2010, 29, 29–44. [Google Scholar] [CrossRef]
- Flores, R.G.; Andersen, S.L.F.; Maia, L.K.K.; Jose, H.J.; Moreira, R.F.P.M. Recovery of iron oxides from acid mine drainage and their application as adsorbent or catalyst. J. Env. Manag. 2012, 111, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Wang, H.; Liu, H.; Yang, C.; Wei, Y.; Hou, D. Evaluation of the ability of ferrihydrite to bind heavy metal ions: Based on formation environment, adsorption reversibility and ageing. Appl. Geochem. 2014, 45, 114–119. [Google Scholar] [CrossRef]
- Komarek, M.; Vanek, A.; Ettler, V. Chemical stabilization of metals and arsenic in contaminated soils using oxides–a review. Env. Pollut. 2013, 172, 9–22. [Google Scholar] [CrossRef]
- Servida, D.; Grieco, G.; De Capitani, L. Geochemical hazard evalution of sulfide-rich iron mines: The Rio Marina district (Elba Island, Italy). J. Geochem. Explor. 2009, 100, 75–89. [Google Scholar] [CrossRef]
- Negrel, P.; Grosbois, C.; Klopppman, W. The labile fraction of suspended matter in the Loire River (France): Multi-element chemistry and isotopic (Rb-Sr and C-O) systematics. Chem. Geol. 2000, 166, 271–285. [Google Scholar] [CrossRef]
- Schafer, J.; Blan, G. Relationship between ore deposits in river catchments and geochemistry of suspended matter from six rivers in southwest France. Sci. Total Environ. 2002, 298, 103–118. [Google Scholar] [CrossRef]
- Kraus, U.; Wiegand, J. Long-term effects of the Aznalcollar mine spill-heavy metal content and mobility in soils and sediments of the Guadiamar River valley (SW Spain). Sci. Total Environ. 2006, 367, 855–871. [Google Scholar] [CrossRef]
- Li, Y.L.; Hall, K.; Yuan, Y.; Mattu, G.; McCallun, D.; Chen, M. Mobility and bioavailibility of trace metals in the water-sediment system of the highly urbanizid brunette watershed. Water Air Soil Pollution. 2009, 197, 249–266. [Google Scholar] [CrossRef]
- Riba, I.; DelValls, T.A.; Reynoldson, T.B.; Milani, D. Sediment quality in Rio Guadiamar (SW, Spain) after a tailing dam collapse: Contamination, toxicity and bioavailability. Environ. Int. 2006, 32, 891–900. [Google Scholar] [CrossRef]
- Blowes, D.W.; Ptacek, C.J.; Jambor, J.L.; Weisener, C.C. The geochemistry of acid mine drainage. Treatise Geochem. 2003, 9, 149–204. [Google Scholar]
- Kosoff, D.; Hudson-Edvards, K.A.; Dubbin, W.E.; Alfredsson, M.A. Incongruent weathering of Cd and Zn from mine tailings: A column leaching study. Chem. Geol. 2011, 281, 52–71. [Google Scholar] [CrossRef]
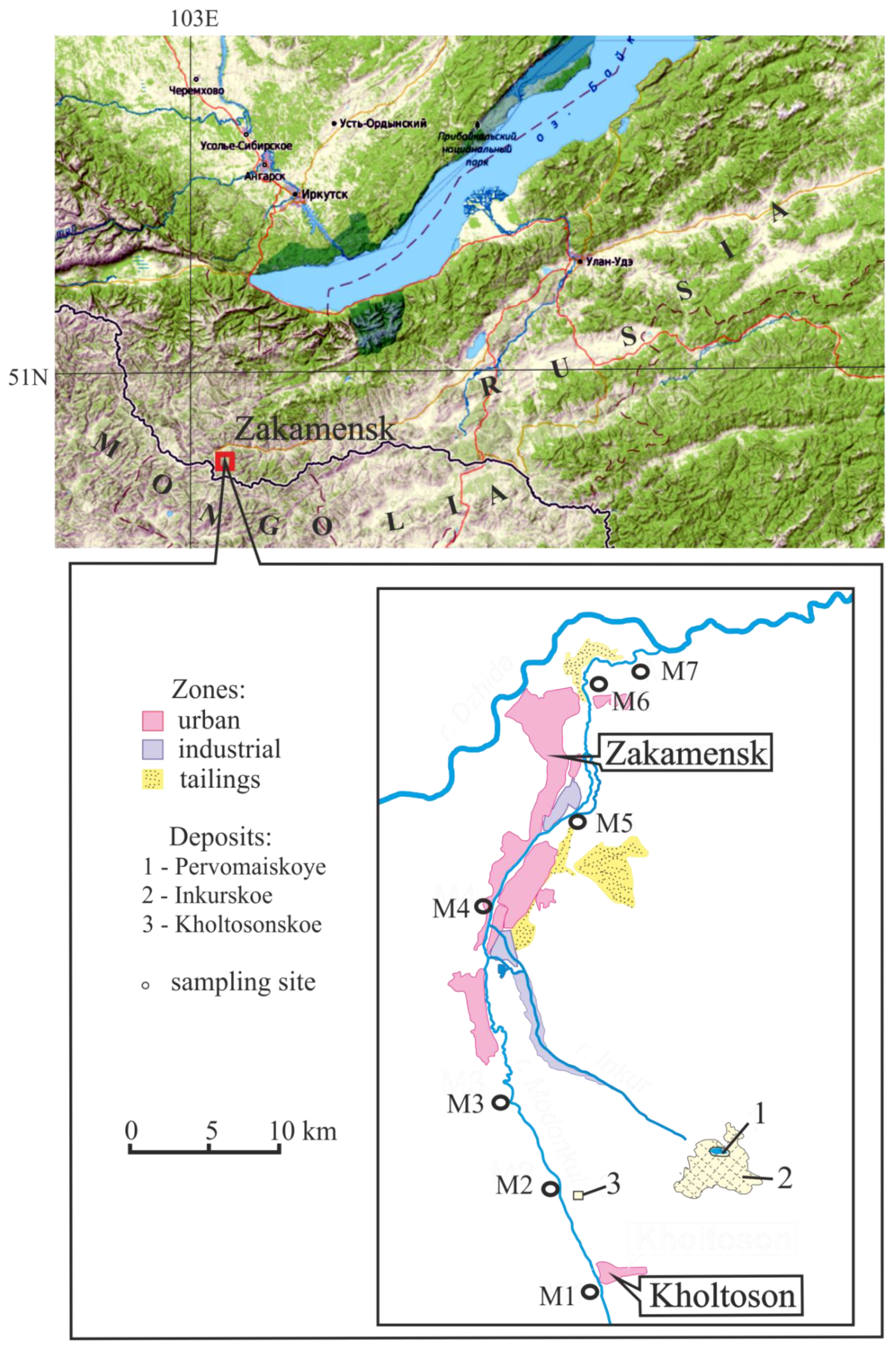





| Sample Site | Location |
|---|---|
| M1 | The upper river, village Kholtoson |
| M2 | Below inflow mine water from Kholtosonskoe |
| M3 | Downstream to the city Zakamensk |
| M4 | After inflow tributary Inkur |
| M5 | In the city Zakamensk |
| M6 | After the city Zakamensk |
| M7 | The mouth of river |
| Component | Data | Site | ||||||
|---|---|---|---|---|---|---|---|---|
| M1 | M2 | M3 | M4 | M5 | M6 | M7 | ||
| pH | 7.1–7.6 7.3 | 6.6–7.2 6.9 | 7.0–7.15 7.1 | 7.1–7.32 7.1 | 7.0–7.6 7.4 | 6.85–7.4 7.3 | 7.2–7.8 7.6 | |
| HCO3− | mg∙L−1 | 100.3–109.6 106.7 | 76.2–81.8 79.3 | 78.3–87.2 85.4 | 70.5–98.3 76.2 | 95.7–106.4 103.7 | 151.7–185.2 177 | 91.8–101.3 97.6 |
| SO42− | -//- | 10.5–16.8 15.2 | 210–252 230.5 | 128.6–197.4 162.3 | 110.4–245.8 180 | 70.2–135.6 94 | 340.5–381.2 365.4 | 345.7–380.1 369.6 |
| Cl− | -//- | 5.1–6.9 6.1 | 7.8–8.2 8.1 | 4.0–5.6 4.2 | 5.5–6.9 6.7 | 5.0–5.6 5.2 | 14.0–16.9 15.6 | 16.8–18.9 17.4 |
| Ca2+ | -//- | 19.6–22.5 20.1 | 70.3–76.8 73.4 | 33.8–39.4 35.1 | 44.7–54.6 47.5 | 38.6–46.1 40.2 | 121.6–133.7 126.2 | 95.8–109.4 98.6 |
| Mg2+ | -//- | 13.6–16.1 14.1 | 24.9–29.5 26.2 | 17.2–18.1 17.5 | 14.5–17.2 15.2 | 14.3–18.6 14.6 | 34.6–40.1 36.1 | 44.4–46.3 45.6 |
| Na+ + K+ | -//- | 5.8–8.4 6.3 | 80.2–115.0 92.4 | 20.1–26.3 22.2 | 29.8–34.5 31.4 | 24.3–29.6 27.1 | 63.0–94.5 72.8 | 53.6–63.4 59.1 |
| F− | -//- | 2.3–2.9 2.7 | 64.2–66.7 65.2 | 6.6–8.7 7.1 | 10.3–16.2 14.5 | 3.2–8.4 5.3 | 23.8–28.6 25.7 | 24.6–29.8 26.2 |
| TDS | -//- | 162–180 172 | 529–618 578 | 292–374 337 | 282–468 356 | 268–335 318 | 758–867 821 | 672–727 703 |
| Si | -//- | 2.8–3.5 3.2 | 4.9–5.5 5.2 | 4.2–4.6 4.4 | 3.9–5.1 4.9 | 4.1–5.1 4.8 | 4.3–4.9 4.6 | 4.7–5.4 5.1 |
| NO3− | -//- | 2.9–3.8 3.3 | 5.1–6.1 5.6 | 3.1–4.5 3.9 | 3.9–4.5 4.3 | 9.5–11.2 10.9 | 7.5–9.3 8.1 | 6.6–7.5 7.0 |
| Element | Site | World Mean Value [13] | K1 | K2 | K3 | Detection Limit | |
|---|---|---|---|---|---|---|---|
| M1 | M7 | ||||||
| Li | 2.11 | 15.5 | 1.84 | 1.15 | 8.42 | 7.3 | 0.057 |
| Be | 0.05 | 0.99 | 0.0089 | 5.62 | 111.24 | 19.8 | 0.0027 |
| Al | 5.6 | 14.6 | 32 | 0.18 | 0.46 | 2.6 | 2.62 |
| Sc | 0.0390 | 0.0136 | 1.2 | 0.03 | 0.01 | 0.3 | 0.0031 |
| Ti | 0.32 | 0.56 | 0.48 | 0.67 | 1.17 | 1.8 | 0.41 |
| V | 0.3514 | 0.3252 | 0.71 | 0.49 | 0.46 | 0.9 | 0.0035 |
| Cr | 1.74 | 1.46 | 0.7 | 2.49 | 2.09 | 0.8 | 0.017 |
| Mn | 6.7 | 483 | 34 | 0.20 | 14.2 | 72 | 0.024 |
| Fe | 30.4 | 52.7 | 66 | 0.46 | 0.8 | 1.73 | 0.56 |
| Co | 0.1271 | 4.8 | 0.14 | 0.91 | 34.29 | 37.8 | 0.0014 |
| Ni | 2.34 | 3.53 | 0.801 | 2.92 | 4.41 | 1.5 | 0.024 |
| Cu | 7.6 | 22.48 | 1.48 | 5.14 | 15.19 | 3.0 | 0.052 |
| Zn | 11.06 | 216.32 | 0.6 | 18.44 | 360.53 | 19.6 | 0.12 |
| Ga | 0.0182 | 0.0143 | 0.03 | 0.61 | 0.48 | 0.8 | 0.0135 |
| As | 0.19 | 0.49 | 0.62 | 0.31 | 0.79 | 2.6 | 0.011 |
| Rb | 1.2 | 4.4 | 1.63 | 0.74 | 2.70 | 3.7 | 0.0087 |
| Sr | 211.54 | 409.44 | 60 | 3.53 | 6.82 | 1.9 | 0.14 |
| Y | 0.3596 | 0.0958 | 0.04 | 8.99 | 2.40 | 0.3 | 0.0006 |
| Zr | 0.1857 | 0.0580 | 0.039 | 4.76 | 1.49 | 0.3 | 0.0052 |
| Nb | 0.0038 | 0.0032 | 0.0017 | 2.24 | 1.88 | 0.8 | 0.0032 |
| Mo | 2.38 | 6.1500 | 0.42 | 5.67 | 14.64 | 2.6 | 0.057 |
| Cd | 0.0750 | 4.4 | 0.08 | 0.94 | 55.00 | 58.7 | 0.0016 |
| Sb | 0.1238 | 0.7829 | 0.07 | 1.77 | 11.18 | 6.3 | 0.0091 |
| Cs | 0.5639 | 0.5250 | 0.011 | 51.26 | 47.73 | 0.9 | 0.0007 |
| Ba | 69.5014 | 109.09 | 23 | 3.02 | 4.74 | 1.6 | 0.040 |
| Hf | 0.0052 | 0.0056 | 0.0059 | 0.88 | 0.95 | 1.1 | 0.0007 |
| Ta | 0.0005 | 0.0005 | 0.0011 | 0.45 | 0.45 | 1.0 | 0.0003 |
| W | 0.018 | 0.056 | 0.1 | 0.18 | 0.56 | 3.1 | 0.012 |
| Re | 0.0031 | 0.0103 | 0.0004 | 7.75 | 25.75 | 3.3 | 0.002 |
| Pb | 1.19 | 0.9938 | 0.079 | 15.06 | 12.58 | 0.8 | 0.014 |
| Th | 0.0663 | 0.0121 | 0.041 | 1.62 | 0.30 | 0.2 | 0.0023 |
| U | 2.3 | 3.5 | 0.372 | 6.18 | 9.41 | 1.5 | 0.0011 |
| Site | Fe | Mn | Ni | Cu | Zn | Cd | Pb |
|---|---|---|---|---|---|---|---|
| M1 | 15.9–56.8 30.4 | 4.7–9.4 7.7 | 1.7–3.2 2.3 | 1.9–9.2 7.6 | 7.3–29.5 11 | 0.8–1.2 1.1 | 0.7–1.5 1.1 |
| M2 | 5.1–36.2 26.4 | 82.0–165 126 | 1.2–8.9 5.8 | 7.6–21.3 15.2 | 120–273 186 | 1.1–3.4 2.8 | 1.1–2.9 0.9 |
| M3 | 32–63.4 48 | 8.2–24.8 16.4 | 1.7–5.6 2.5 | 4.3–12.6 9.4 | 15.8–45 32 | 0.8–1.2 1.0 | 0.8–1.5 1.1 |
| M4 | 14–38.7 27.6 | 141.8–352 248 | 2.1–3.9 2.6 | 9.6–28.1 20.5 | 256–483 367 | 2.1–5.7 3.8 | 1.8–4.4 3.2 |
| M5 | 16.8–46.3 38.6 | 12.8–53.2 40.9 | 1.2–2.9 1.8 | 3.4–12.8 8.6 | 30.3–123 72 | 1.2–3.2 2.4 | 1.1–2.6 1.8 |
| M6 | 15.3–28.9 22.5 | 87.3–254 198 | 1.8–5.8 3.2 | 8.7–21.9 16.6 | 115–192 153 | 1.1–5.2 3.2 | 1.0–3.2 2.4 |
| M7 | 21.4–62.7 52.7 | 315–572 483 | 1.7–6.1 3.5 | 12.5–32.8 22.5 | 148–321 216 | 2.8–6.1 4.4 | 1.2–3.5 2.8 |
| Site | Fe | Mn | Ni | Cu | Zn | Cd | Pb |
|---|---|---|---|---|---|---|---|
| M1 | 156–347 259 | 23.2–41.3 38.3 | 1.1–3.6 1.9 | 3.6–13.2 9.8 | 45–82 67 | 0.6–1.4 0.8 | 1.2–2.1 1.6 |
| M2 | 124–248 185 | 97–151 126 | 3.6–8.2 5.3 | 9.2–34 15 | 134–186 152 | 16.8–32.8 24 | 3.5–10.7 6.2 |
| M3 | 476–812 684 | 272–506 386 | 2.3–6.4 4.2 | 15–29 21 | 183–262 214 | 19.2–42.7 32 | 5.8–12.6 7.2 |
| M4 | 342–722 575 | 265–431 353 | 2.8–7.2 5.4 | 11–26 18 | 98–167 129 | 12.1–23.3 17 | 4.2–9.2 6.4 |
| M5 | 491–832 692 | 177–348 215 | 2.2–6.7 4.8 | 12–29 21 | 116–173 146 | 11.5–24.2 18 | 3.6–8.9 5.7 |
| M6 | 923–1280 1012 | 198–314 268 | 2.8–7.9 5.1 | 8.6–21.4 14 | 102–172 137 | 2.7–10.5 6.2 | 6.7–14.5 9.8 |
| M7 | 1006–1365 1238 | 243–376 315 | 2.3–6.8 4.5 | 11–20.4 17 | 128–217 186 | 3.6–12.7 8.4 | 4.6–13.8 8.6 |
| Element | Site | Mine Water Kholtosonskoe Deposit | Detection Limit | |
|---|---|---|---|---|
| M1 | M7 | |||
| La | 0.3399 | 0.0659 | 143.08 | 0.011 |
| Ce | 0.2466 | 0.1029 | 340.8 | 0.0022 |
| Pr | 0.0952 | 0.0166 | 42.71 | 0.0003 |
| Nd | 0.4289 | 0.0936 | 215.48 | 0.0020 |
| Sm | 0.0875 | 0.0168 | 56.09 | 0.0003 |
| Eu | 0.0207 | 0.0099 | 16.94 | 0.0004 |
| Gd | 0.1071 | 0.0229 | 62.33 | 0.0003 |
| Tb | 0.013 | 0.0035 | 10.75 | 0.0009 |
| Dy | 0.0612 | 0.0183 | 71.34 | 0.0002 |
| Ho | 0.0122 | 0.0036 | 12.52 | 0.0003 |
| Er | 0.0398 | 0.0122 | 38.98 | 0.0006 |
| Tm | 0.0052 | 0.0016 | 4.67 | 0.0001 |
| Lu | 0.0059 | 0.0016 | 4.31 | 0.0005 |
| Σ LREE | 1.22 | 0.31 | 815.10 | - |
| Σ HREE | 0.24 | 0.06 | 204.90 | - |
| Σ REE | 1.46 | 0.37 | 1020.00 | - |
| Ce/Ce* | 0.30 | 0.68 | 0.95 | - |
| Eu/Eu* | 0.93 | 2.17 | 1.25 | - |
| (La/Yb)N | 0.97 | 0.80 | 0.42 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khazheeva, Z.I.; Plyusnin, A.M.; Smirnova, O.K.; Peryazeva, E.G.; Zhambalova, D.I.; Doroshkevich, S.G.; Dabaeva, V.V. Mining Activities and the Chemical Composition of R. Modonkul, Transbaikalia. Water 2020, 12, 979. https://doi.org/10.3390/w12040979
Khazheeva ZI, Plyusnin AM, Smirnova OK, Peryazeva EG, Zhambalova DI, Doroshkevich SG, Dabaeva VV. Mining Activities and the Chemical Composition of R. Modonkul, Transbaikalia. Water. 2020; 12(4):979. https://doi.org/10.3390/w12040979
Chicago/Turabian StyleKhazheeva, Zinaida Ivanovna, Aleksey Maksimovich Plyusnin, Olga Konstantinovna Smirnova, Elena Georgievna Peryazeva, Dashima Ivanovna Zhambalova, Svetlana Gennadievna Doroshkevich, and Viktoriya Valerievna Dabaeva. 2020. "Mining Activities and the Chemical Composition of R. Modonkul, Transbaikalia" Water 12, no. 4: 979. https://doi.org/10.3390/w12040979
APA StyleKhazheeva, Z. I., Plyusnin, A. M., Smirnova, O. K., Peryazeva, E. G., Zhambalova, D. I., Doroshkevich, S. G., & Dabaeva, V. V. (2020). Mining Activities and the Chemical Composition of R. Modonkul, Transbaikalia. Water, 12(4), 979. https://doi.org/10.3390/w12040979




