Cyanobacterial Blooms and Zooplankton Structure in Lake Ecosystem under Limited Human Impact
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Sampling and Determination
2.3. Data Analysis and Statistics
- (1)
- TZB:TPB
- (2)
- BCil:TPB
- (3)
- BRot:TPB
- (4)
- BClad:TPB
- (5)
- BCop:TPB
3. Results
3.1. Environmental Parameters
3.2. Total Phytoplankton and Cyanobacterial Biomass and Composition
3.3. Heterotrophic Nanoflagellate (HNF) Biomass
3.4. Ciliate Biomass and Composition
3.5. Rotifer and Crustacean Biomass and Composition
3.6. Relationships between Biotic and Abiotic Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nature 2018, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Monchamp, M.E.; Spaak, P.; Domaizon, I.; Dubois, N.; Bouffard, D.; Pomati, F. Homogenization of lake cyanobacterial communities over a century of climate change and eutrophication. Nat. Ecol. Evol. 2018, 2, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Wilk-Woźniak, E. An introduction to the ‘micronet’ of cyanobacterial harmful algal blooms (CyanoHABs): Cyanobacteria, zooplankton and microorganisms: A review. Mar. Freshw. Res. 2019. [Google Scholar] [CrossRef]
- Yan, D.; Xu, H.; Yang, M.; Lan, J.; Hou, W.; Wang, F.; Zhang, J.; Zhou, K.; An, Z.; Goldsmith, Y. Responses of cyanobacteria to climate and human activities at Lake Chenghai over the past 100 years. Ecol. Ind. 2019, 104, 755–763. [Google Scholar] [CrossRef]
- Jeppesen, E.; Canfield, D.E.; Bachmann, R.W.; Søndergaard, M.; Havens, K.E.; Johansson, L.S.; Lauridsen, T.L.; Sh, T.; Rutter, R.P.; Warren, G.; et al. Toward predicting climate change effects on lakes: A comparison of 1656 shallow lakes from Florida and Denmark reveals substantial differences in nutrient dynamics, metabolism, trophic structure, and top-down control. Inland Waters 2020. [Google Scholar] [CrossRef]
- Godlewska, M.; Izydorczyk, K.; Kaczkowski, Z.; Jóźwik, A.; Długoszewski, B.; Ye, S.; Lian, Y.; Guillard, J. Do Fish and blue-green algae blooms coexist in space and time? Fish. Res. 2016, 173, 93–100. [Google Scholar] [CrossRef]
- Godlewska, M.; Balk, H.; Kaczkowski, Z.; Jurczak, T.; Izydorczyk, K.; Długoszewski, B.; Jaskulska, A.; Gągała-Borowska, I.; Mankiewicz-Boczek, J. Night fish avoidance of Microcystis bloom revealed by simultaneous hydroacoustic measurements of both organisms. Fish. Res. 2018, 207, 74–84. [Google Scholar] [CrossRef]
- Krztoń, W.; Kosiba, J.; Pociecha, A.; Wilk-Woźniak, E. The effect of cyanobacterial blooms on bio- and functional diversity of zooplankton communities. Biodivers. Conserv. 2019, 28, 1815–1835. [Google Scholar] [CrossRef]
- Budzyńska, A.; Rosińska, J.; Pełechata, A.; Toporowska, M.; Napiórkowska-Krzebietke, A.; Kozak, A.; Messyasz, B.; Pęczuła, W.; Kokociński, M.; Szeląg-Wasielewska, E.; et al. Environmental factors driving the occurrence of the invasive cyanobacterium Sphaerospermopsis aphanizomenoides (Nostocales) in temperate lakes. Sci. Total Environ. 2019, 650, 1338–1347. [Google Scholar] [CrossRef]
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef]
- Paerl, H.W.; Hall, N.S.; Calandrino, E.S. Controlling harmful cyanobacterial blooms in a world experiencing anthropogenic and climatic-induced change. Sci. Total Environ. 2011, 40, 1739–1745. [Google Scholar] [CrossRef] [PubMed]
- Ibelings, B.; Havens, K. Cyanobacterial toxins: A qualitative meta-analysis of concentrations, dosage and effects in freshwater, estuarine and marine biota. Adv. Exp. Med. Biol. 2008, 619, 675–732. [Google Scholar] [PubMed]
- Meriluoto, J.; Blaha, L.; Bojadzija, G.; Bormans, M.; Brient, L.; Codd, G.A.; Drobac, D.; Faassen, E.J.; Fastner, J.; Hiskia, A.; et al. Toxic cyanobacteria and cyanotoxins in European waters—Recent progress achieved through the CYANOCOST Action and challenges for further research. Adv. Oceanogr. Limnol. 2017, 8, 161–178. [Google Scholar] [CrossRef]
- Kobos, J.; Błaszczyk, A.; Hohlfeld, N.; Toruńska-Sitarz, A.; Krakowiak, A.; Hebel, A.; Stryk, K.; Grabowska, M.; Toporowska, M.; Kokociński, M.; et al. Cyanobacteria and cyanotoxins in Polish freshwater bodies. Oceanol. Hydrobiol. Stud. 2013, 42, 358–378. [Google Scholar] [CrossRef]
- Mantzouki, E.; Lürling, M.; Fastner, J.; de Senerpont Domis, L.; Wilk-Woźniak, E.; Koreivienė, J.; Seelen, L.; Teurlincx, S.; Verstijnen, Y.; Krztoń, W.; et al. Temperature Effects Explain Continental Scale Distribution of Cyanobacterial Toxins. Toxins 2018, 10, 156. [Google Scholar] [CrossRef] [PubMed]
- Dziga, D.; Maksylewicz, A.; Maroszek, M.; Budzynska, A.; Kozak, A.; Rosinska, J.; Napiorkowska-Krzebietke, A.; Toporowska, M.; Grabowska, M.; Meriluoto, J. The biodegradation of microcystins in temperate freshwater bodies with previous cyanobacterial history. Ecotoxicol. Environ. Saf. 2017, 145, 420–430. [Google Scholar] [CrossRef]
- Krevš, A.; Koreivienė, J.; Mažeikaitė, S. Plankton food web structure during cyanobacteria bloom in the highly eutrophic Lake Gineitiškės. Ekologija 2010, 56, 47–54. [Google Scholar] [CrossRef]
- Van Eerden, M.; van Rijn, S.; Volponi, S.; Paquet, J.Y.; Carss, D.N. Cormorants and the European Environment: Exploring Cormorant Status and Distribution on a Continental Scale; INTERCAFE COST Action 635 Final Report I; NERC/Centre for Ecology & Hydrology on behalf of COST: Lancaster, UK, 2012; Available online: http://www.intercafeproject.net (accessed on 15 January 2020).
- Grémillet, D.; Nazirides, T.; Nikolaou, H.; Crivelli, A.J. Fish are not safe from great cormorants in turbid water. Aquat. Biol. 2012, 15, 187–194. [Google Scholar] [CrossRef][Green Version]
- Klimaszyk, P.; Rzymski, P. Impact of cormorant (Phalacrocorax carbo sinensis L.) colonies on microbial pollution in lakes. Limnol. Rev. 2013, 13, 139–145. [Google Scholar] [CrossRef][Green Version]
- Klimaszyk, P.; Joniak, T.; Rzymski, P. Roosting colony of cormorants (Phalacrocorax carbo sinensis L.) as a source of nutrients for the lake. Limnol. Rev. 2014, 14, 111–119. [Google Scholar]
- Klimaszyk, P.; Piotrowicz, R.; Rzymski, P. Changes in physico-chemical conditions and macrophyte abundance in a shallow soft-water lake mediated by a Great Cormorant roosting colony. J. Limnol. 2015, 74, 114–122. [Google Scholar] [CrossRef]
- Gwiazda, R.; Jarocha, K.; Szarek-Gwiazda, E. Impact of a small cormorant (Phalacrocorax carbo sinensis) roost on nutrients and phytoplankton assemblages in the littoral regions of a submontane reservoir. Biologia 2010, 65, 742–748. [Google Scholar] [CrossRef]
- Gagnon, K.; Yli-Rosti, J.; Jormalainen, V. Cormorant-induced shifts in littoral communities. Mar. Ecol. Prog. Ser. 2015, 541, 15–30. [Google Scholar] [CrossRef]
- Traczuk, P.; Kapusta, A. Great cormorant (Phalacrocorax carbo) predation on pikeperch (Sander lucioperca L.) in shallow eutrophic lakes in Poland. Arch. Pol. Fish 2017, 25, 123–130. [Google Scholar] [CrossRef][Green Version]
- Krzywosz, T.; Traczuk, P. Impact of the great cormorant in lakes in the Masurian region. In Sustainable Exploitation of Fishery Resources in Light of Their Status in 2009; Mickiewicz, M., Ed.; Wyd. IRS: Olsztyn, Poland, 2010; pp. 133–142. [Google Scholar]
- Traczuk, P.; Chybowski, Ł.; Ulikowski, D.; Kapusta, A. The great cormorant (Phalococorax carbo) in northeastern Poland—A summary of a decade of research. In Commercial and Recreational Fisheries in 2015; Mickiewicz, M., Wołos, A., Eds.; Wyd. IRS: Olsztyn, Poland, 2016; pp. 89–102. [Google Scholar]
- Utermöhl, H. Guidance on the quantitative analysis of phytoplankton—Methods. Mitt. Int. Ver. Theor. Angew. Limnol. 1958, 9, 1–38. (In German) [Google Scholar]
- Napiórkowska-Krzebietke, A.; Kobos, J. Assessment of the cell biovolume of phytoplankton widespread in coastal and inland water bodies. Water Res. 2016, 104, 532–546. [Google Scholar] [CrossRef]
- Huber-Pestalozzi, G. Das phytoplankton des Süβwassers. Systematik und Biologie. 7 Teil, 1 Häfte: Chlorophyceae (Grünalgen) Ordnung Chlorococcales. In Die Binnengewässer Einzeldarstellungen aus der Limnologie und Ihren Nachbargebieten; Thienemann, A., Ed.; E. Schweizerbart’sche Verlasbuchhandlung: Stuttgart, Germany, 1983; pp. 1–1044. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae 1. Teil: Naviculaceae. In Süβwasserflora von Mitteleuropa; Pascher, A., Ed.; VEB Gustaw Fischer Verlag: Jena, Germany, 1986; pp. 1–876. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae 2. Teil: Epithemiaceae, Surirellaceae. In Süβwasserflora von Mitteleuropa; Pascher, A., Ed.; Gustaw Fischer Verlag: Stuttgart, Germany; New York, NY, USA, 1988; pp. 1–596. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae 3. Teil: Centrales, Fragilariaceae, Eunotiaceae. In Süβwasserflora von Mitteleuropa; Pascher, A., Ed.; Gustaw Fischer Verlag: Stuttgart, Germany; Jena, Germany, 1991; pp. 1–576. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota 2. Teil: Oscillatoriales. In Süβwasserflora von Mitteleuropa; Pascher, A., Ed.; Gustaw Fischer Verlag: Stuttgart, Germany; Jena, Germany, 2005; pp. 1–759. [Google Scholar]
- Guiry, M.D.; Guiry, G.M. AlgaeBase; World-Wide Electronic Publication, National University of Ireland: Galway, Ireland, 2008; Available online: http://www.algaebase.org (accessed on 4 March 2020).
- Porter, K.G.; Feig, Y.S. The use of DAPI for identifying and counting aquatic microflora. Limnol. Oceanogr. 1980, 25, 943–948. [Google Scholar] [CrossRef]
- Foissner, W.; Berger, H.; Schaumburg, J. Identification and Ecology of Limnetic Plankton Ciliates; Informationberichte des Bayer Landesamtes für Wasserwirtschaft: München, Germany, 1999; pp. 1–793. [Google Scholar]
- Flössner, D. Krebstiere (Crustacea), Kiemen- und Blattfüßer (Branchiopoda), Fischläuse (Branchiura); VEB Gustav Fischer Verlag: Jena, Germany, 1972. [Google Scholar]
- Radwan, S.; Bielańska-Grajner, I.; Ejsmont-Karabin, J. Część ogólna, Monogononta—Część systematyczna. 32.A. In Wrotki (Rotifera). Fauna Słodkowodna Polski. 32; Radwan, S., Ed.; Polskie Towarzystwo Hydrobiologiczne, Uniwersytet Łódzki, Oficyna Wydawnicza Tercja: Łódź, Poland, 2004; pp. 1–146. [Google Scholar]
- Rybak, J.I.; Błędzki, L.A. Widłonogi, Copepoda: Cyclopoida, Klucz do oznaczania; Biblioteka Monitoringu Środowiska: Warszawa, Poland, 2005. [Google Scholar]
- Ejsmont-Karabin, J. Empirical equations for biomass calculation of planktonic rotifers. Pol. Arch. Hydrob. 1998, 45, 513–522. [Google Scholar]
- Bottrell, H.H.; Duncan, A.; Gliwicz, Z.M.; Grygierek, E.; Herzig, A.; Hillbright, I.A.; Kurasawa, H.; Larsson, P.; Weglenska, T. A review of some problems in zooplankton production studies. Norw. J. Zool. 1976, 24, 419–456. [Google Scholar]
- APHA. Standard Methods for Examination of Water & Wastewater; American Public Health Association: New York, NY, USA, 1999.
- Carlson, R.E. A trophic state index for lakes. Limnol. Oceanogr. 1977, 22, 361–369. [Google Scholar] [CrossRef]
- Kratzer, C.R.; Brezonik, P.L. A Carlson type trophic state index for nitrogen in Florida lakes. Water Res. Bull. 1981, 17, 713–715. [Google Scholar] [CrossRef]
- Carlson, R.E.; Simpson, J. A Coordinator’s Guide to Volunteer Lake Monitoring Methods; North American Lake Management Society: Madison, WI, USA, 1996; pp. 1–96.
- Carlson, R.E.; Havens, K.E. Simple Graphical Methods for the Interpretation of Relationships between Trophic State Variables. Lake Reserv. Manag. 2005, 21, 107–118. [Google Scholar] [CrossRef]
- Sakamoto, M. Primary production by phytoplankton community in some Japanese lakes and its dependence on lake depth. Arch. Hydrobiol. 1966, 62, 1–28. [Google Scholar]
- Paerl, H.W.; Fulton, R.S.; Moisander, P.H.; Dyble, J. Harmful freshwater algal blooms, with an emphasis on cyanobacterial. Sci. World 2001, 1, 76–113. [Google Scholar] [CrossRef]
- Kozak, A.; Celewicz-Gołdyn, S.; Kuczyńska-Kippen, N. Cyanobacteria in small water bodies: The effect of habitat and catchment area conditions. Sci. Total Environ. 2019, 646, 1578–1587. [Google Scholar] [CrossRef] [PubMed]
- Napiórkowska-Krzebietke, A.; Dunalska, J. Phytoplankton-based recovery requirement for urban lakes in the implementation of the Water Framework Directive’s ecological targets. Oceanol. Hydrobiol. Stud. 2015, 44, 109–119. [Google Scholar] [CrossRef]
- Napiórkowska-Krzebietke, A.; Dunalska, J.A.; Zębek, E. Taxa-specific eco-sensitivity in relation to phytoplankton bloom stability and ecologically relevant lake state. Acta Oecol. 2017, 81, 10–21. [Google Scholar]
- DeMott, W.R.; Gulati, R.D.; Van Donk, E. Daphnia food limitation in three hypertrophic Dutch lakes: Evidence for exclusion of large-bodied species by interfering filaments of cyanobacteria. Limnol. Oceanogr. 2001, 46, 2054–2060. [Google Scholar] [CrossRef]
- Bednarska, A.; Łoś, J.; Dawidowicz, P. Daphnia as a model organism in limnology and aquatic biology: Some aspects of its reproduction and development. J. Limnol. 2011, 70, 353–358. [Google Scholar] [CrossRef][Green Version]
- Dembowska, E.A.; Napiórkowski, P.; Mieszczankin, T.; Józefowicz, S. Planktonic indices in the evaluation of the ecological status and the trophic state of the longest lake in Poland. Ecol. Indic. 2015, 56, 15–22. [Google Scholar] [CrossRef]
- Harke, M.J.; Steffen, M.M.; Gobler, C.J.; Otten, T.G.; Wilhelm, S.W.; Wood, S.A.; Paerl, H.W. A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis spp. Harmful Algae 2016, 54, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Josué, I.I.P.; Cardoso, S.J.; Miranda, M.; Mucci, M.; Ger, K.A.; Roland, F.; Marinho, M.M. Cyanobacteria dominance drives zooplankton functional dispersion. Hydrobiologia 2019, 831, 149–161. [Google Scholar] [CrossRef]
- Hansson, L.-A.; Gustafsson, S.; Rengefors, K.; Bomark, L. Cyanobacterial chemical warfare affects zooplankton community composition. Freshw. Biol. 2007, 52, 1290–1301. [Google Scholar] [CrossRef]
- Tillmanns, A.R.; Wilson, A.E.; Pick, F.R.; Sarnelle, O. Meta-analysis of cyanobacterial effects on zooplankton population growth rate: Species-specific responses. Fund. Appl. Limnol. 2008, 171, 285–295. [Google Scholar] [CrossRef]
- Barrios, C.A.Z.; Nandini, S.; Sarma, S.S.S. Effect of crude extracts from cyanobacterial blooms in Lake Texcoco (Mexico) on the population growth of Brachionus calyciflorus (Rotifera). Toxicon 2017, 139, 45–53. [Google Scholar] [CrossRef]
- Von Rückert, G.; Giani, A. Biological interactions in the plankton community of a tropical eutrophic reservoir: Is the phytoplankton controlled by zooplankton? J. Plankton Res. 2008, 30, 1157–1168. [Google Scholar] [CrossRef][Green Version]
- Gołdyn, R.; Kowalczewska-Madura, K. Interactions between phytoplankton and zooplankton in the hypertrophic Swarzędzkie Lake in western Poland. J. Plankton Res. 2008, 30, 33–42. [Google Scholar] [CrossRef]
- Ger, K.A.; Panosso, R.; Lürling, M. Consequences of acclimation to Microcystis on the selective feeding behavior of the calanoid copepod Eudiaptomus gracilis. Limnol. Oceanogr. 2011, 56, 2103–2114. [Google Scholar] [CrossRef]
- Kozak, A.; Gołdyn, R.; Dondajewska, R. Phytoplankton composition and abundance in restored Maltański Reservoir under the influence of physico-chemical variables and zooplankton grazing pressure. PLoS ONE 2015, 10, e0124738. [Google Scholar] [CrossRef] [PubMed]
- Onandia, G.; Dias, J.D.; Miracle, M.R. Zooplankton grazing on natural algae and bacteria under hypertrophic conditions. Limnetica 2015, 34, 541–560. [Google Scholar]
- Nandini, S.; Sarma, S.S.S.; Amador-López, R.J.; Bolaños-Muñoz, S. Population growth and body size in five rotifer species in response to variable food concentration. J. Freshw. Ecol. 2007, 22, 1–10. [Google Scholar] [CrossRef]
- Jia, J.; Shi, W.; Chen, Q.; Lauridsen, T.L. Spatial and temporal variations reveal the response of zooplankton to cyanobacteria. Harmful Algae 2017, 64, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Kosiba, J.; Wilk-Woźniak, E.; Krztoń, W. The effect of potentially toxic cyanobacteria on ciliates (Ciliophora). Hydrobiologia 2019, 827, 325–335. [Google Scholar] [CrossRef]
- Cai, J.; Hodoki, Y.; Ushio, M.; Nakano, S. Influence of potential grazers on picocyanobacterial abundance in Lake Biwa revealed with empirical dynamic modeling. Inland Waters 2020. [Google Scholar] [CrossRef]
- Carrick, H.J.; Aldridge, F.J.; Schelske, C.L. Wind influences phytoplankton biomass and composition in a shallow, productive lake. Limnol. Oceanogr. 1993, 38, 1179–1192. [Google Scholar] [CrossRef]
- Hadas, O.; Kaplan, A.; Sukenik, A. Long-term changes in cyanobacteria populations in Lake Kinneret (Sea of Galilee), Israel: An eco-physiological outlook. Life 2015, 5, 418–431. [Google Scholar] [CrossRef]
- Klimaszyk, P.; Rzymski, P. The complexity of ecological impacts induced by great cormorants. Hydrobiologia 2016, 771, 13–30. [Google Scholar] [CrossRef]
- Schulhof, M.A.; Shurin, J.B.; Declerck, S.A.J.; Van de Waal, D.B. Phytoplankton growth and stoichiometric responses to warming, nutrient addition and grazing depend on lake productivity and cell size. Glob. Chang. Biol. 2019, 25, 2751–2762. [Google Scholar] [CrossRef]
- Napiórkowska-Krzebietke, A. Phytoplankton response to fish-induced environmental changes in a temperate shallow pond-type lake. Arch. Pol. Fish. 2017, 25, 211–264. [Google Scholar] [CrossRef][Green Version]
- Maliaka, V.; Verstijnen, Y.J.M.; Faassen, E.J.; Smolders, A.J.P.; Lürling, M. Effects of guanotrophication and warming on the abundance of green algae, cyanobacteria and microcystins in Lake Lesser Prespa, Greece. PLoS ONE 2020, 15, e0229148. [Google Scholar] [CrossRef]
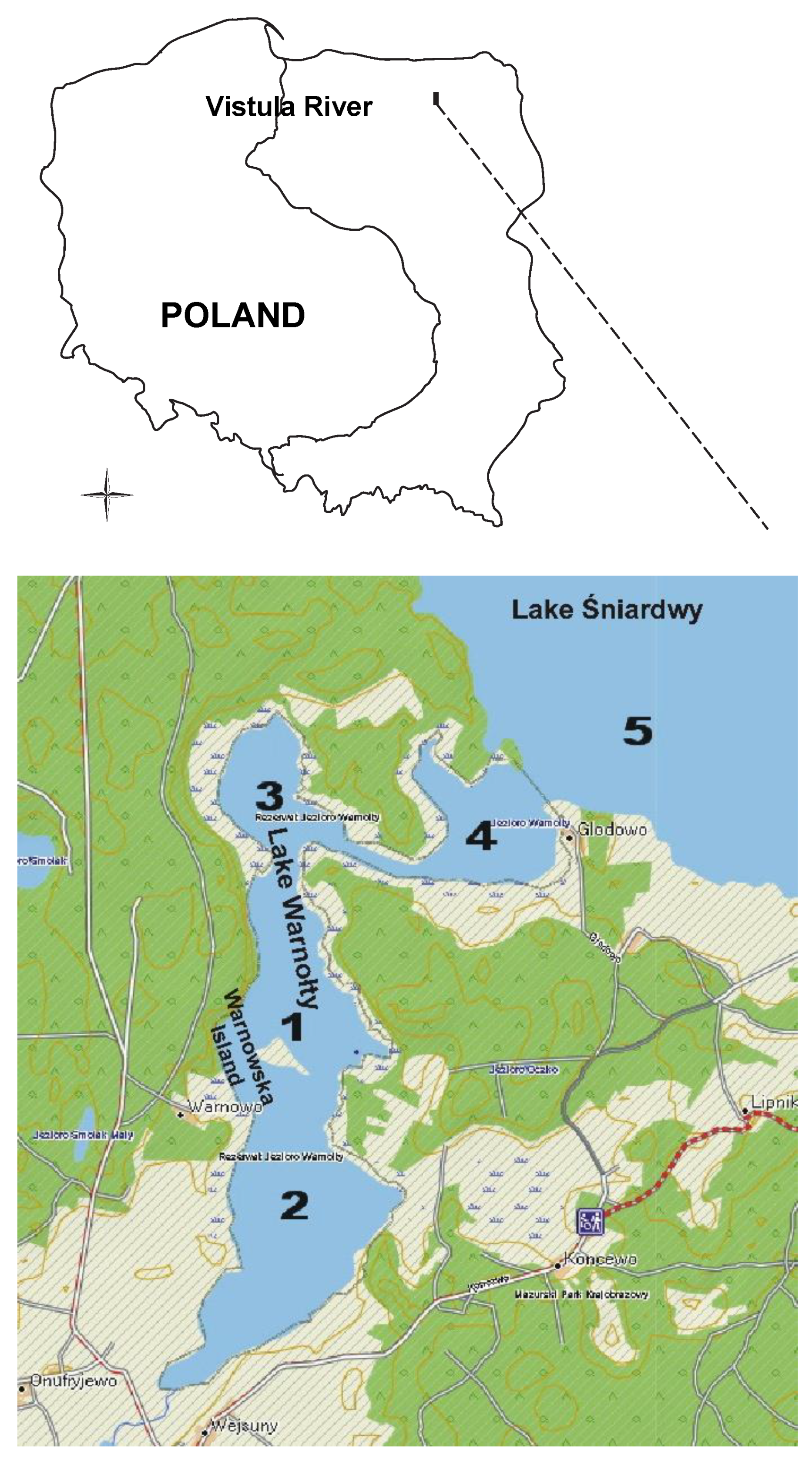
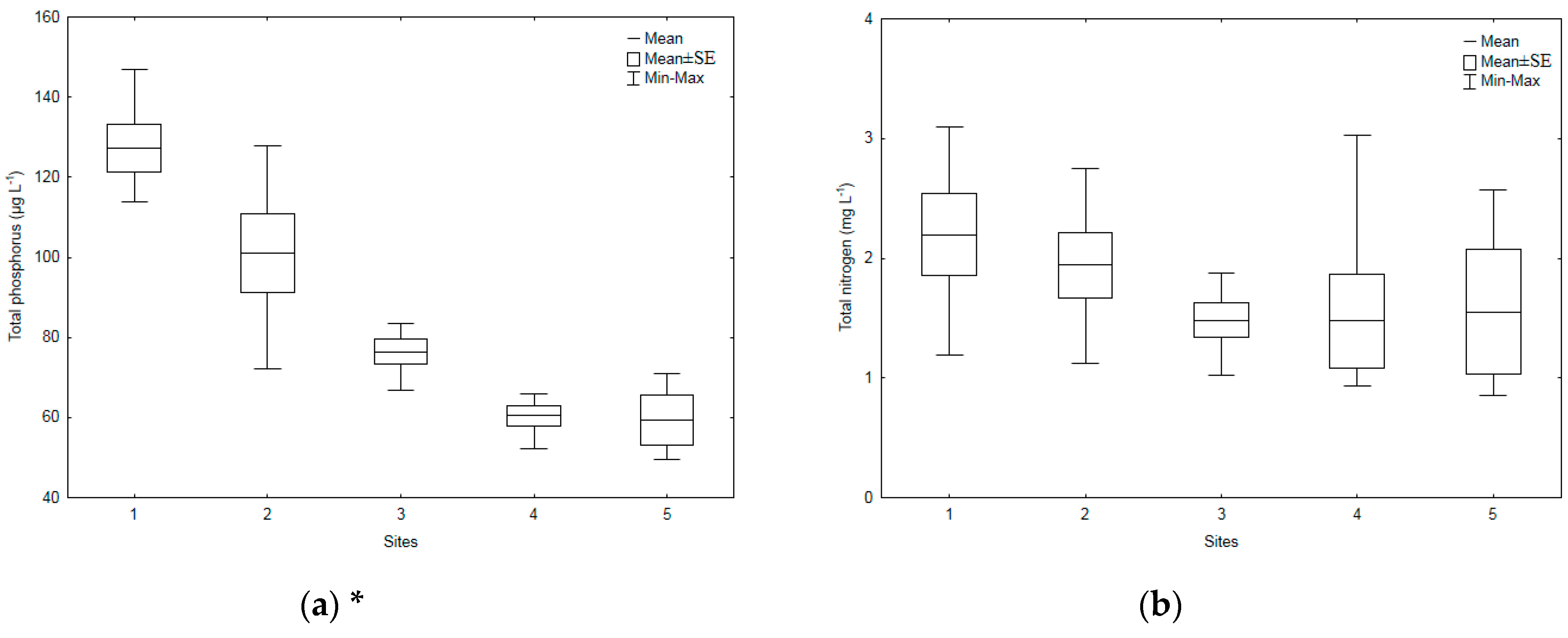
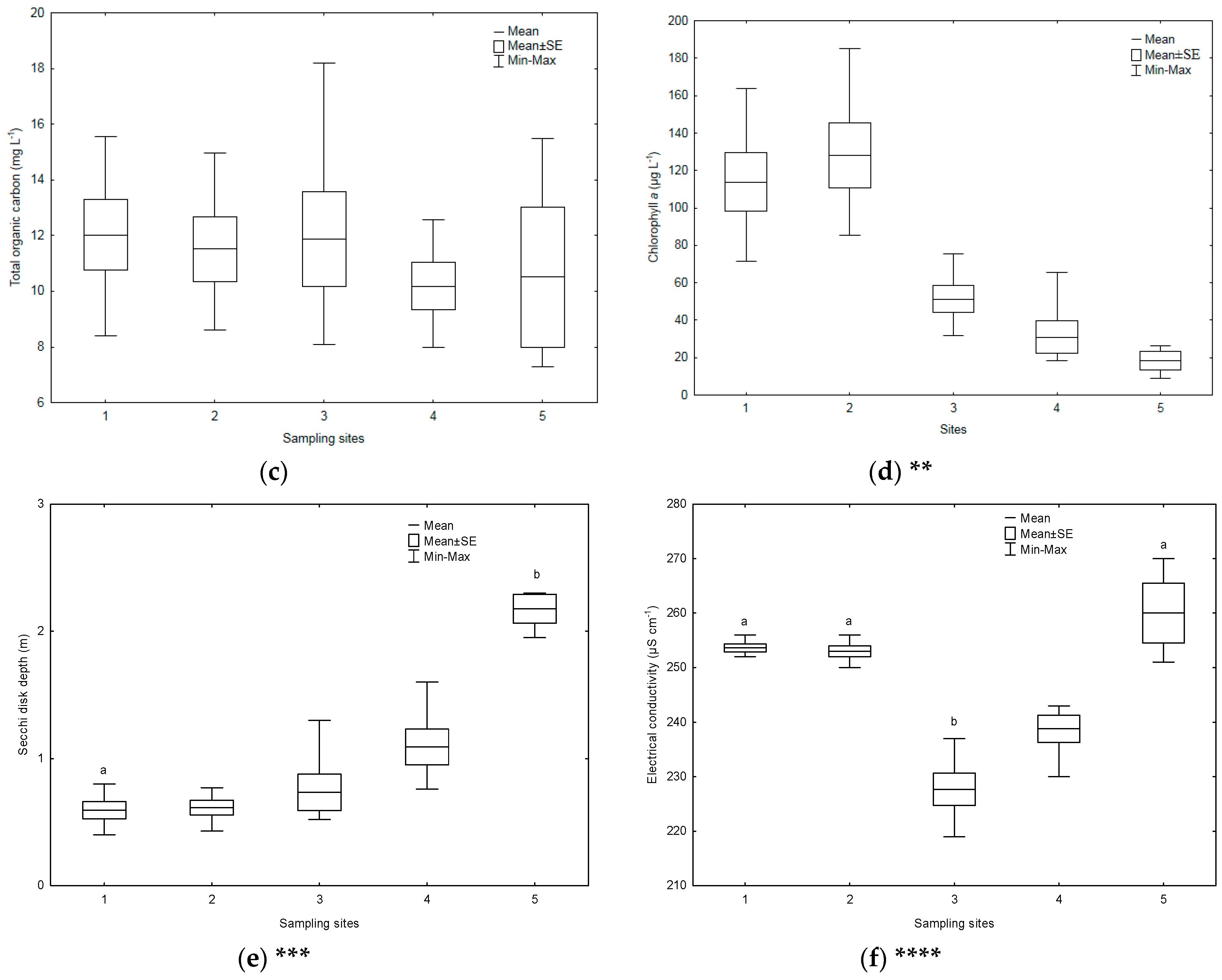
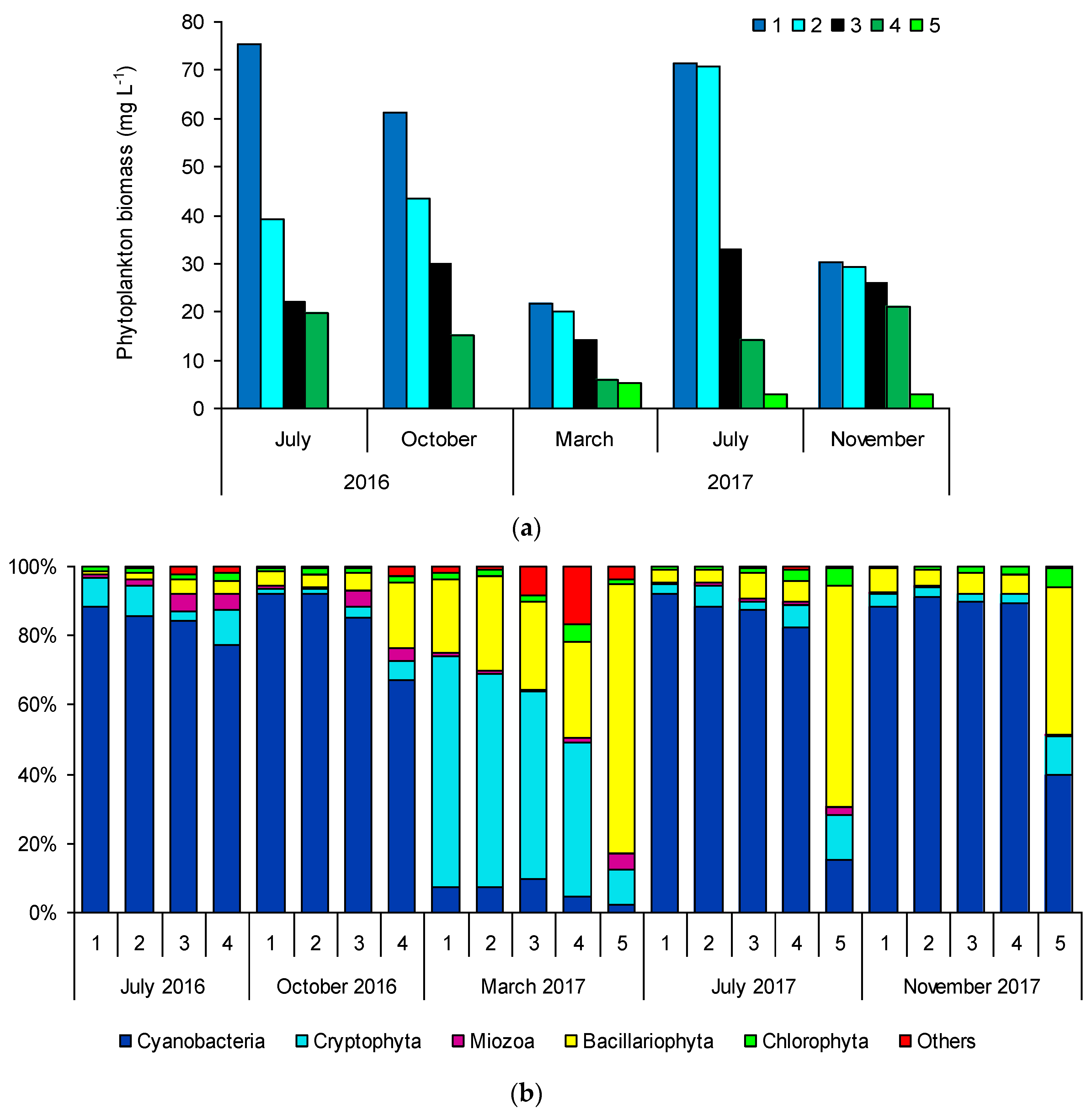
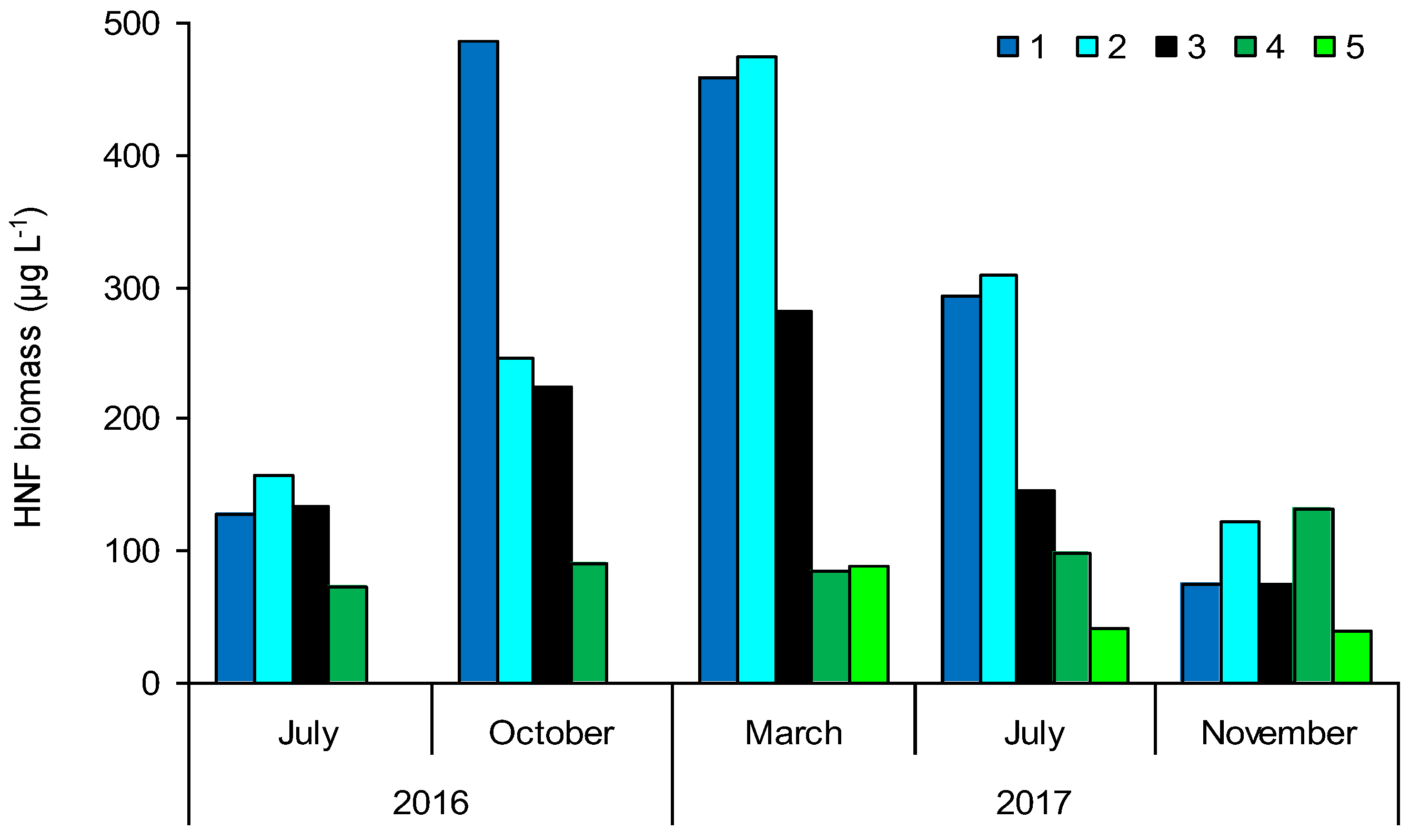

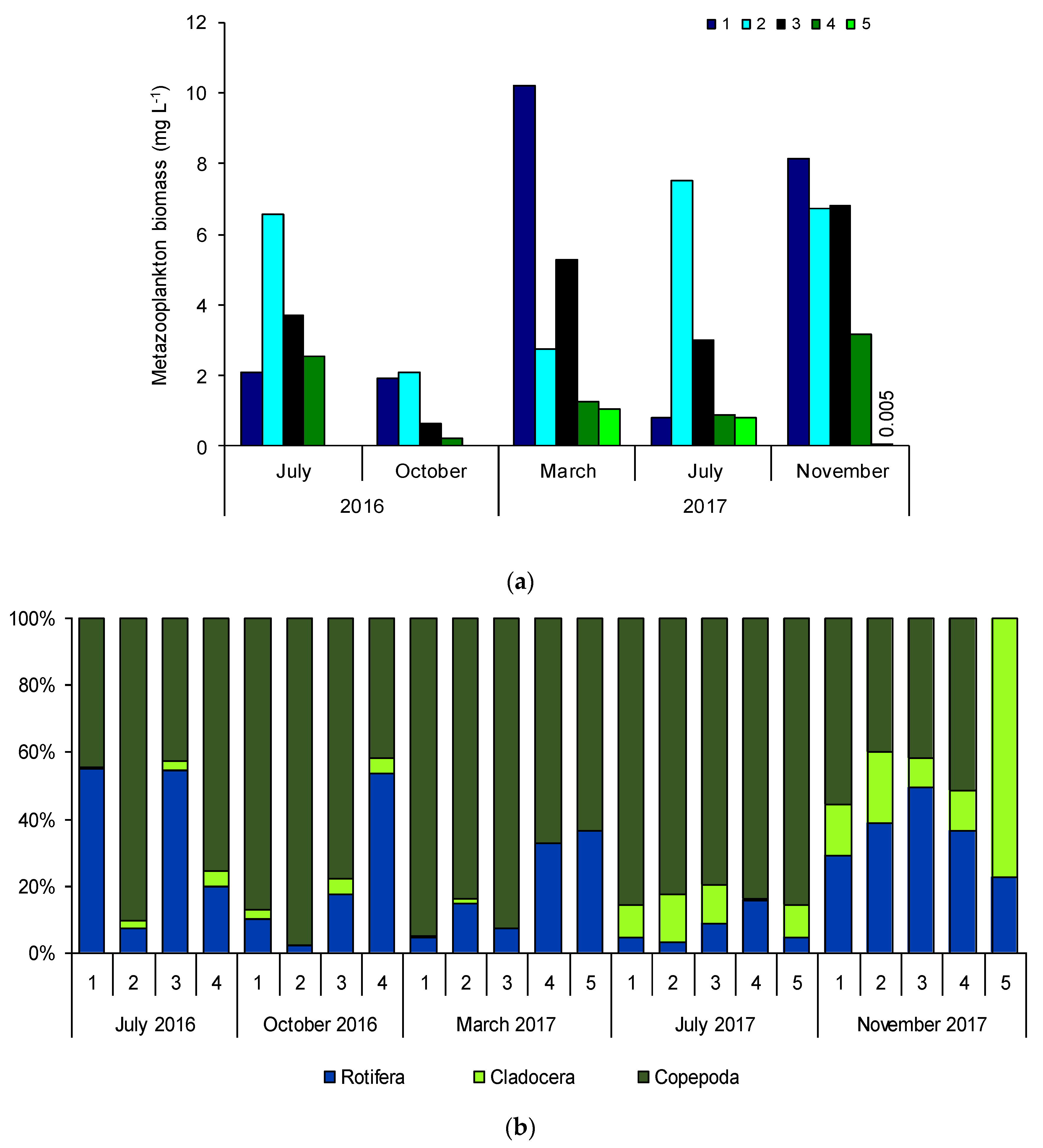
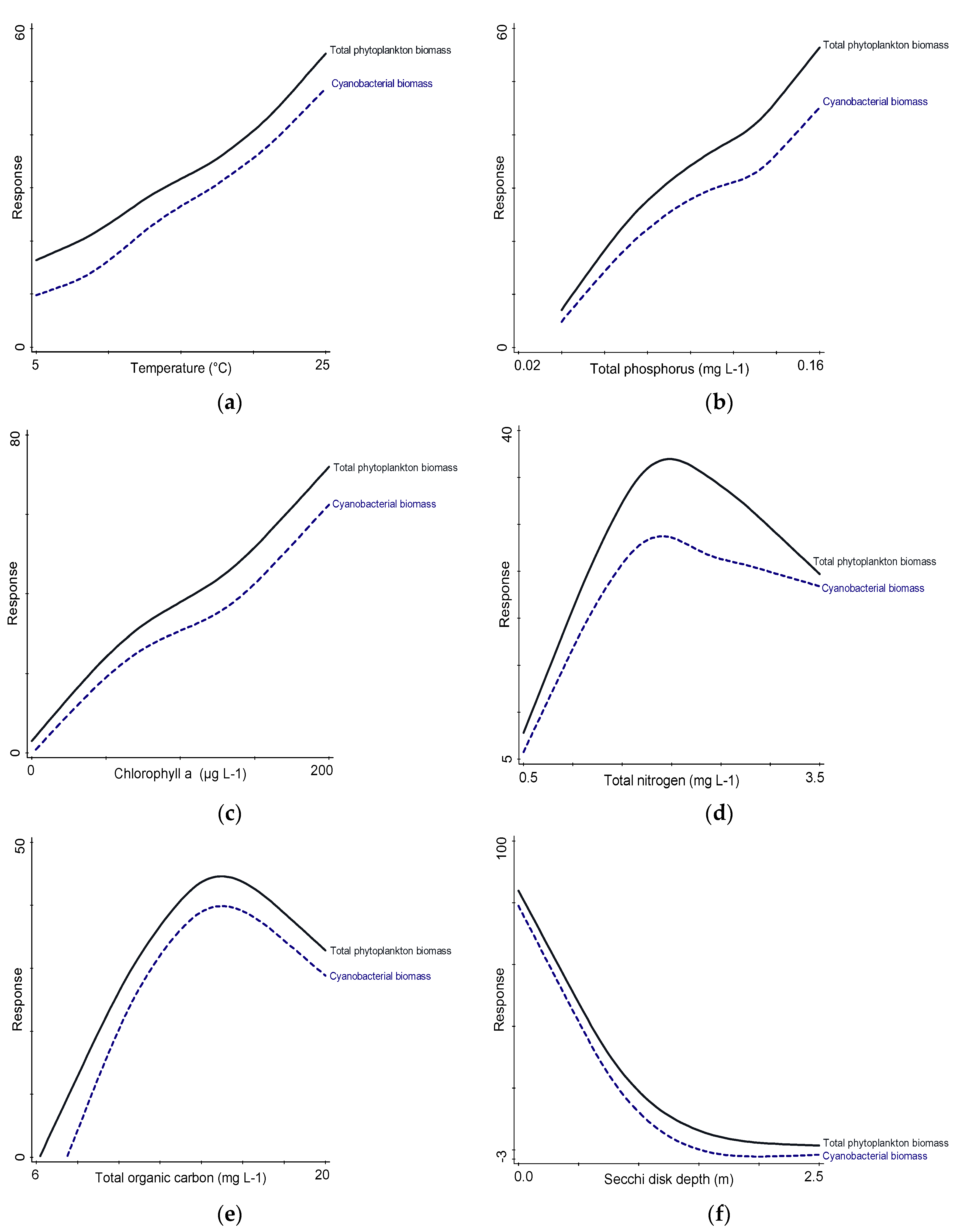
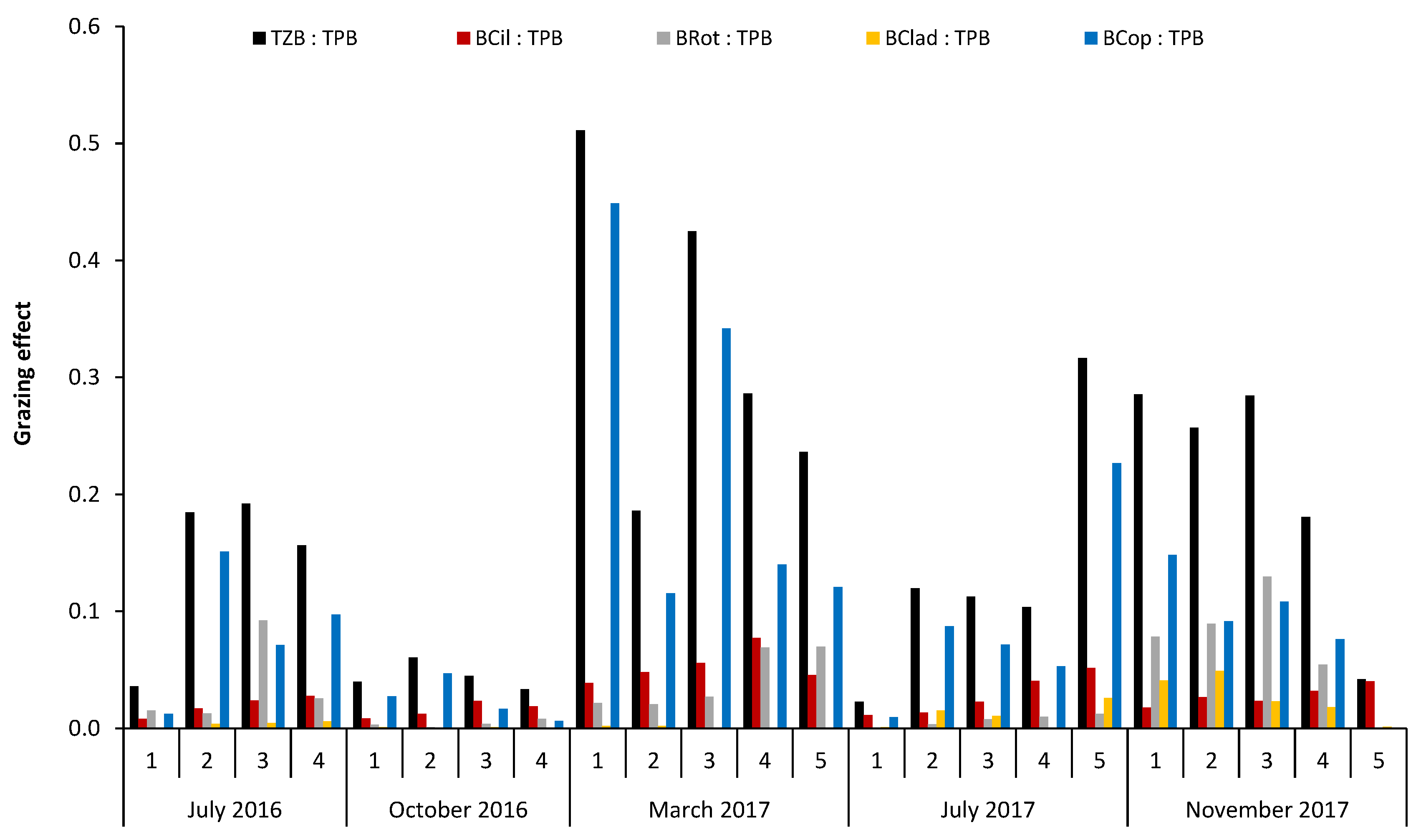
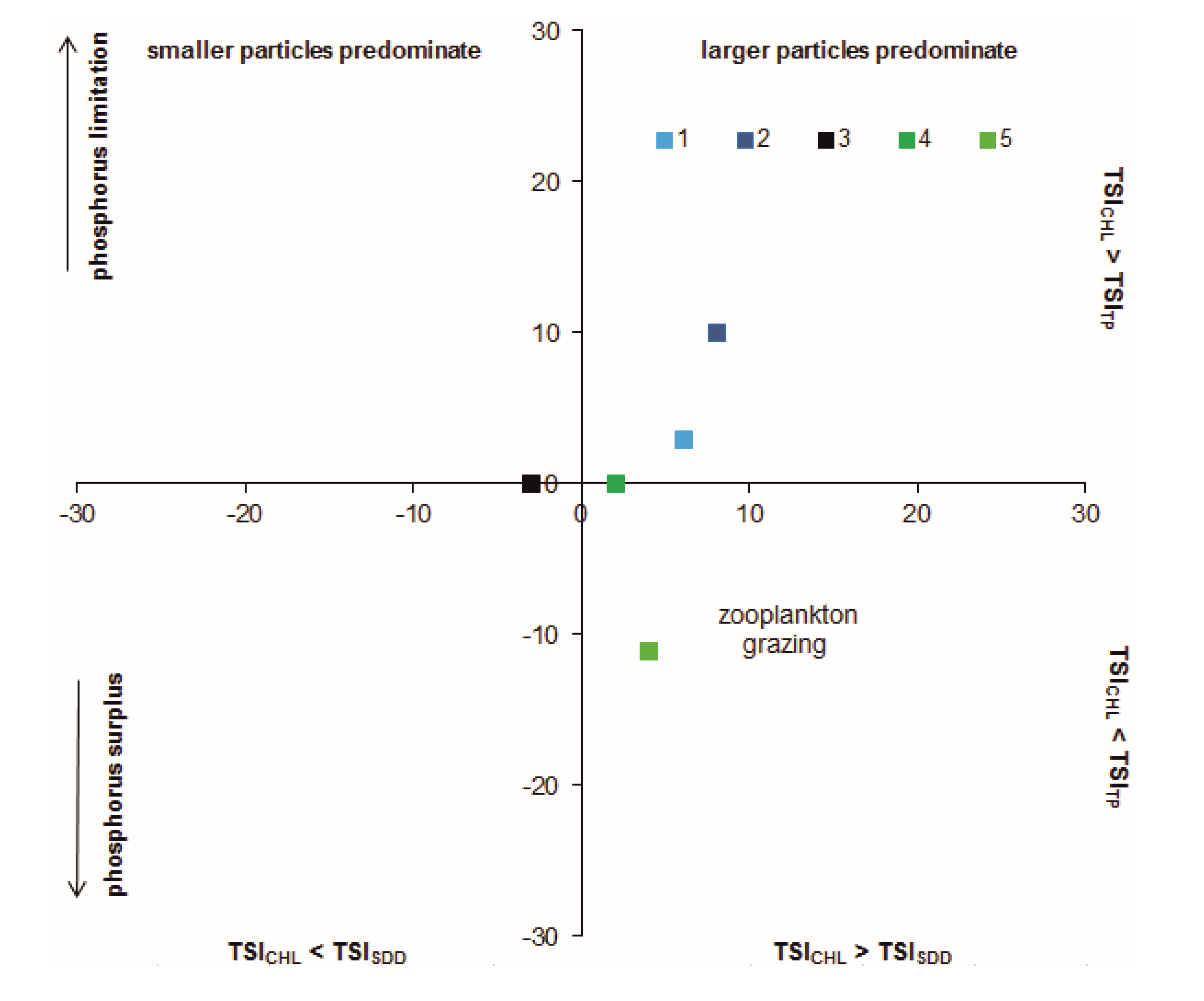
| Sampling Sites | TSICHL | TSISDD | TSITP | TSITN | TSI | State * |
|---|---|---|---|---|---|---|
| 1 | 77 | 71 | 74 | 65 | 72 | hypertrophic |
| 2 | 78 | 70 | 68 | 63 | 70 | eutrophic |
| 3 | 66 | 69 | 66 | 60 | 65 | eutrophic |
| 4 | 62 | 60 | 62 | 56 | 60 | eutrophic |
| 5 | 52 | 48 | 63 | 68 | 58 | eutrophic |
| Predictor | r | F | p |
|---|---|---|---|
| Total phytoplankton | |||
| T | 0.493 | 3.2 | 0.06157 |
| TP | 0.596 | 5.5 | 0.01238 |
| TN | 0.363 | 1.5 | 0.24410 |
| T x nutrients | 0.707 | 21.0 | 0.00016 |
| Cyanobacteria | |||
| T | 0.525 | 3.8 | 0.03985 |
| TP | 0.497 | 3.3 | 0.05846 |
| TN | 0.292 | 0.9252 | 0.58725 |
| T x nutrients | 0.669 | 16.9 | 0.00049 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Napiórkowska-Krzebietke, A.; Kalinowska, K.; Bogacka-Kapusta, E.; Stawecki, K.; Traczuk, P. Cyanobacterial Blooms and Zooplankton Structure in Lake Ecosystem under Limited Human Impact. Water 2020, 12, 1252. https://doi.org/10.3390/w12051252
Napiórkowska-Krzebietke A, Kalinowska K, Bogacka-Kapusta E, Stawecki K, Traczuk P. Cyanobacterial Blooms and Zooplankton Structure in Lake Ecosystem under Limited Human Impact. Water. 2020; 12(5):1252. https://doi.org/10.3390/w12051252
Chicago/Turabian StyleNapiórkowska-Krzebietke, Agnieszka, Krystyna Kalinowska, Elżbieta Bogacka-Kapusta, Konrad Stawecki, and Piotr Traczuk. 2020. "Cyanobacterial Blooms and Zooplankton Structure in Lake Ecosystem under Limited Human Impact" Water 12, no. 5: 1252. https://doi.org/10.3390/w12051252
APA StyleNapiórkowska-Krzebietke, A., Kalinowska, K., Bogacka-Kapusta, E., Stawecki, K., & Traczuk, P. (2020). Cyanobacterial Blooms and Zooplankton Structure in Lake Ecosystem under Limited Human Impact. Water, 12(5), 1252. https://doi.org/10.3390/w12051252






