Application of Composite Pre-Polymerized Coagulants for the Treatment of High-Strength Industrial Wastewaters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Laboratory Procedure for the Preparation of Novel Composite Coagulants
2.2. Coagulation Experiments Performed by Jar-Tests
2.3. Characterization Methods
2.3.1. Physico-Chemical Properties
2.3.2. Aluminum Species Distribution
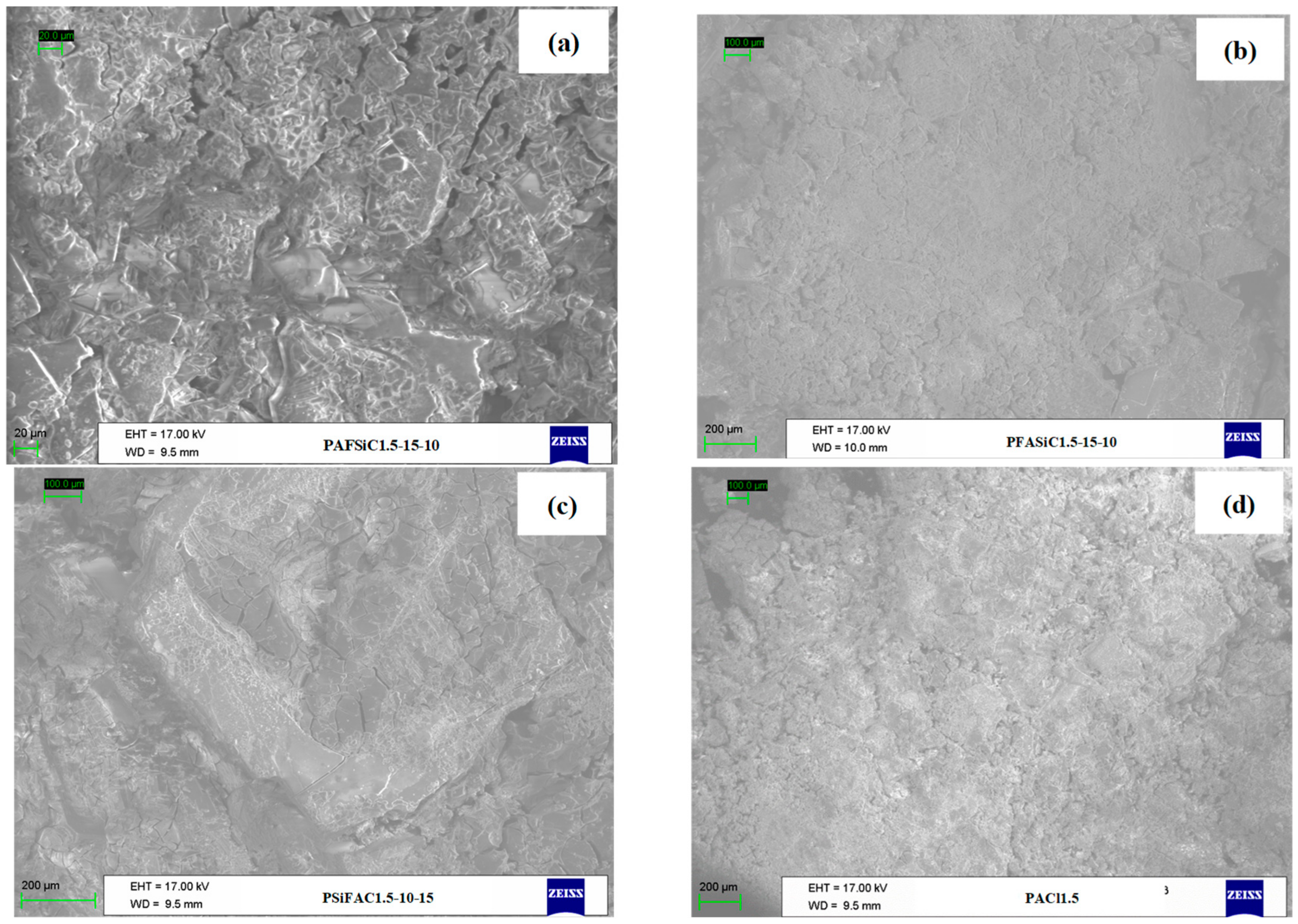
2.3.3. Scanning Electron Microscopy (SEM)
2.3.4. Fourier-Transform-Infrared Spectroscopy (FTIR)
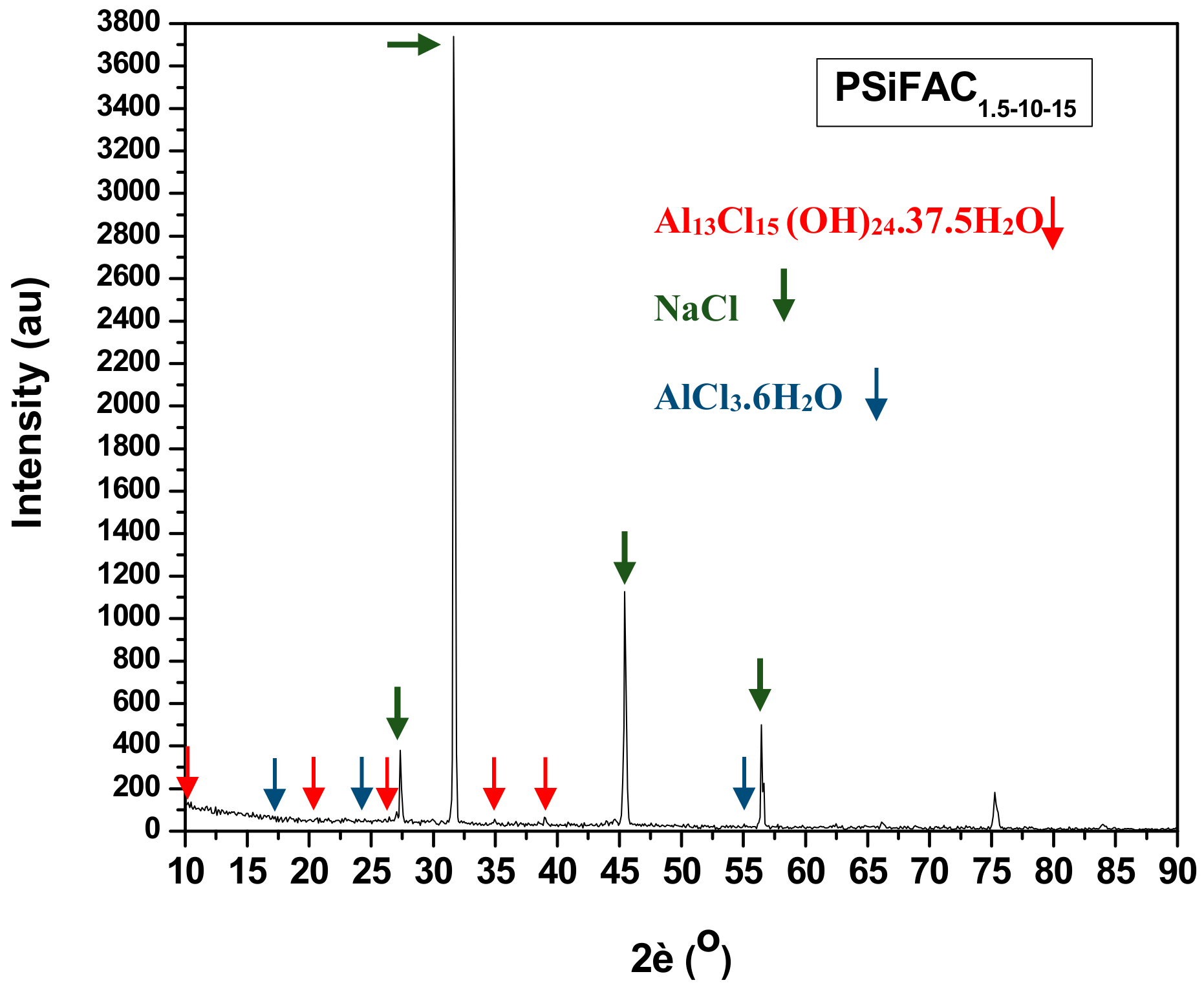
2.3.5. X-ray Diffraction Spectroscopy (XRD)
2.3.6. Zeta-Potential and Particle Size Measurements
2.4. Residual Aluminum Concentration
3. Results and Discussion
3.1. Major Physico-Chemical Properties of Prepared Coagulation Agents
3.2. Characterization of New Polymerized Composite Coagulant Agents
3.2.1. Structure Analysis
3.2.2. Morphological Analysis
3.2.3. X-ray Diffraction Spectroscopy (XRD)
3.2.4. Z-potential and Size Measurements
3.3. Coagulation Performance
3.3.1. Tannery Wastewater
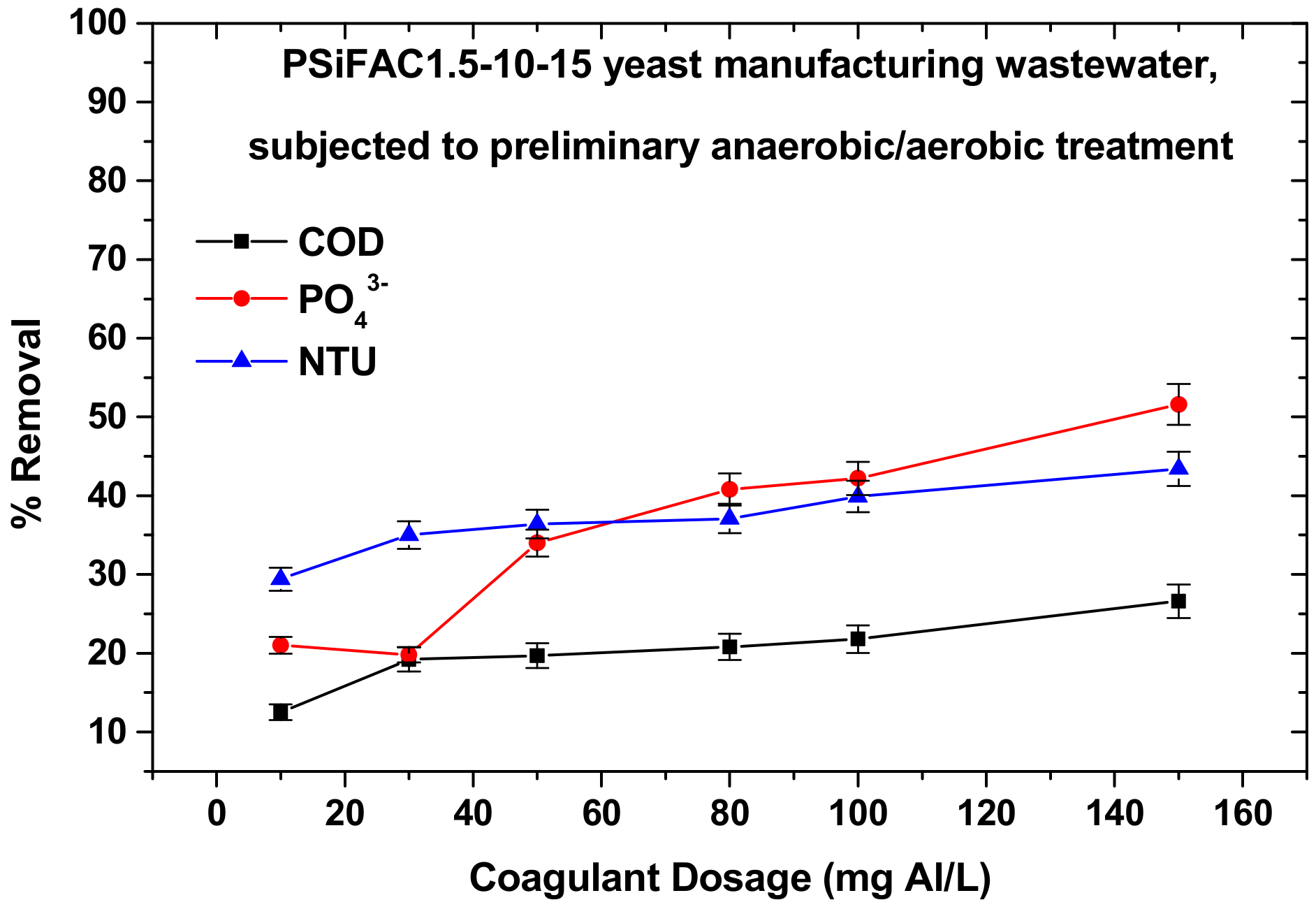
3.3.2. Yeast Production Wastewater
3.3.3. Comparison of Industrial Wastewater Treatment Results
3.3.4. Residual Aluminum Concentration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kelley, D.; Handley, R. Compositions and Methods for Wastewater Treatment. U.S. Patent 7, 931,822, 26 April 2011. [Google Scholar]
- Raptopoulou, C.; Kalaitzidou, K.; Tolkou, A.; Palasantza, P.A.; Mitrakas, M.; Zouboulis, A. Phosphate Removal from Effluent of Secondary Wastewater Treatment: Characterization of Recovered Precipitates and Potential Re-use as Fertilizer. Waste Biomass Valorization 2016, 7, 851–860. [Google Scholar] [CrossRef]
- Kalaitzidou, K.; Mitrakas, M.; Raptopoulou, C.; Tolkou, A.; Palasantza, P.A.; Zouboulis, A. Pilot-Scale Phosphate Recovery from Secondary Wastewater Effluents. Environ. Proc. 2016, 3, 5–22. [Google Scholar] [CrossRef]
- Tolkou, A.; Zouboulis, A. Review of Recent Patents on Coagulation/Flocculation (C/F) Process: Methods and Applications with Emphasis on Phosphates Removal. Recent Pat. Mater. Sci. 2014, 7, 151–163. [Google Scholar] [CrossRef]
- Kim, H.C.; Dempsey, B.A. Effects of wastewater effluent organic materials on fouling in ultrafiltration. Water Res. 2008, 42, 3379–3384. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.W.; Kim, Y.M.; Park, C.; Park, K.Y. Performance and Fouling in Pre-Denitrification Membrane Bioreactors Treating High-Strength Wastewater from Food Waste Disposers. Water 2017, 9, 512. [Google Scholar] [CrossRef] [Green Version]
- Mutamim, N.S.A.; Noor, Z.Z.; Hassan, M.A.A.; Olsson, G. Application of membrane bioreactor technology in treating high strength industrial wastewater: A performance review. Desalination 2012, 305, 1–11. [Google Scholar] [CrossRef]
- Swain, K.; Abbassi, B.; Kinsley, C. Combined Electrocoagulation and Chemical Coagulation in Treating Brewery Wastewater. Water 2020, 12, 726. [Google Scholar] [CrossRef] [Green Version]
- Akansha, J.; Nidheesh, P.V.; Gopinath, A.; Anupama, K.V.; Suresh Kumar, M. Treatment of dairy industry wastewater by combined aerated electrocoagulation and phytoremediation process. Chemosphere 2020, 253, 126652. [Google Scholar] [CrossRef]
- Saxena, G.; Purchase, D.; Bharagava, R.N. Environmental Hazards and Toxicity Profile of Organic and Inorganic Pollutants of Tannery Wastewater and Bioremediation Approaches. In Bioremediation of Industrial Waste for Environmental Safety; Saxena, G., Bharagava, R., Eds.; Springer: Singapore, 2020. [Google Scholar]
- Tolkou, A.; Zouboulis, A. Effect of climate change in WWTPs with a focus on MBR infrastructure. Desalin. Water Treat. 2016, 57, 2344–2354. [Google Scholar] [CrossRef]
- Eckenfelder, W.W. (Ed.) Industrial Water Pollution Control; McGraw-Hill Inc.: New York, NY, USA, 1999; p. 33. [Google Scholar]
- Tchobanoglous, G.; Burton, F.L.; Stensel, H.D. Metcalf & Eddy Wastewater Engineering: Treatment and Reuse; McGraw-Hill Inc.: New York, NY, USA, 2003. [Google Scholar]
- Chowdhurya, M.; Deb, A.K.; Biswas, T.K.; Bin Azam, F.A.; Hossain, D. Removal of Toxicants from Leather Industrial Wastewater Using Sawdust Filter Media and Ferric Oxide Coagulant. Orient. J. Chem. 2019, 35, 597–604. [Google Scholar] [CrossRef]
- Chung, C.Y.; Selvarajoo, A.; Sethu, V.; Koyande, A.K.; Arputhan, A.; Lim, Z.C. Treatment of palm oil mill effluent (POME) by coagulation flocculation process using peanut–okra and wheat germ–okra. Clean Technol. Environ. Policy 2018, 20, 1951–1970. [Google Scholar] [CrossRef]
- Jagaba, A.H.; Kutty, S.R.M.; Hayder, G.; Latiff, A.A.A.; Aziz, N.A.A.; Umaru, I.; Ghaleb, A.A.S.; Abubakar, S.; Lawal, I.M.; Nasara, M.A. Sustainable use of natural and chemical coagulants for contaminants removal from palm oil mill effluent: A comparative analysis. Ain Shams Eng. J. 2020. In Press, Corrected Proof. [Google Scholar] [CrossRef]
- Aleem, M.; Cao, J.; Li, C.; Rashid, H.; Wu, Y.; Nawaz, M.I.; Abbas, M.; Akram, M.W. Coagulation- and Adsorption-Based Environmental Impact Assessment and Textile Effluent Treatment. Water Air Soil Pollut. 2020, 231, 45. [Google Scholar] [CrossRef]
- Manhokwe, S.; Shoko, S.; Zvidzai, C. Optimization of biological wastewater treatment for yeast processing effluent using cultured bacteria: Application of response surface methodology. Afr. J. Microbiol. Res. 2019, 13, 430–437. [Google Scholar] [CrossRef]
- Lee, C.S.; Robinson, J.; Chong, M.F. A review on application of flocculants in wastewater treatment. Process Saf. Environ. 2014, 92, 489–508. [Google Scholar] [CrossRef]
- Sinha, S.; Yoon, Y.; Amy, G.; Yoon, J. Determining the effectiveness of conventional and alternative coagulants through effective characterization schemes. Chemosphere 2004, 57, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Crittenden, J.C.; Truddel, R.R.; Hand, D.W.; Howe, K.J.; Tchobanoglous, G. Coagulation, Mixing and Flocculation. In Water Treatment: Principles and Design, 2nd ed.; John Wiley & Sons: New Jersey, NJ, USA, 2005; pp. 664–691. [Google Scholar]
- Gao, B.; Hahn, H.; Hoffmann, E. Evaluation of aluminium–silicate polymer composite as a coagulant for water treatment. Water Res. 2002, 36, 573–3581. [Google Scholar] [CrossRef]
- Zouboulis, A.I.; Tzoupanos, N.D. Polyaluminium silicate chloride—A systematic study for the preparation and application of an efficient coagulant for water or wastewater treatment. J. Hazard. Mater. 2009, 162, 1379–1389. [Google Scholar] [CrossRef]
- Zouboulis, A.I.; Moussas, P.A. Polyferric silicate sulphate (PFSiS): Preparation, characterization and coagulation behavior. Desalination 2008, 224, 307–316. [Google Scholar] [CrossRef]
- Gao, B.Y.; Liu, L.L.; Zhou, W.Z.; Yue, Q.Y.; Li, Q. Study on the hydrolysis polymerization process of aluminum in polyaluminum silicate chloride (PASC) flocculant. Acta Sci. Circumstantiae 2005, 11, 1464–1469. [Google Scholar]
- Niu, X.; Li, X.; Zhao, J.; Ren, Y.; Yang, Y. Preparation and coagulation efficiency of polyaluminum ferric silicate chloride composite coagulant from wastewater of high-purity graphite production. J. Environ. Sci. 2011, 23, 1122–1128. [Google Scholar] [CrossRef]
- Li, R.; He, C.; He, Y. Preparation and characterization of poly-silicic-cation coagulants by synchronous-polymerization and co-polymerization. Chem. Eng. J. 2013, 223, 869–874. [Google Scholar] [CrossRef]
- Sun, T.; Sun, C.H.; Zhu, G.L.; Miao, X.J.; Wu, C.C.; Lv, S.B.; Li, W.J. Preparation and coagulation performance of poly-ferric-aluminum-silicate-sulfate from fly ash. Desalination 2011, 268, 270–275. [Google Scholar] [CrossRef]
- Tolkou, A.; Zouboulis, A. Synthesis and characterization of a novel composite pre-polymerized coagulant for water and wastewater treatment. Int. J. Environ. Eng. 2015, 2, 50–54. [Google Scholar]
- Tolkou, A.K.; Zouboulis, A.I. Synthesis and coagulation performance of composite poly-aluminum-ferric-silicate-chloride coagulants in water and wastewater. Desalin. Water Treat. 2015, 53, 3309–3318. [Google Scholar] [CrossRef]
- Katsoyiannis, I.; Tzollas, N.; Tolkou, A.; Mitrakas, M.; Ernst, M.; Zouboulis, A. Use of novel composite coagulants for arsenic removal from waters—Experimental insight for the application of polyferric sulfate (PFS). Sustainability 2017, 9, 590. [Google Scholar] [CrossRef] [Green Version]
- Tolkou, A.; Mitrakas, M.; Katsoyiannis, I.; Ernst, E.; Zouboulis, A. Fluoride removal from water by composite Al/Fe/Si/Mg pre-polymerized coagulants: Characterization and application. Chemosphere 2019, 231, 528–537. [Google Scholar] [CrossRef]
- Gkotsis, P.K.; Mitrakas, M.; Tolkou, A.K.; Zouboulis, A.I. Batch and continuous dosing of conventional and composite coagulation agents for fouling control in a pilot-scale MBR. Chem. Eng. J. 2017, 311, 255–264. [Google Scholar] [CrossRef]
- Tzoupanos, N.D.; Zouboulis, A.I.; Tsoleridis, C.A. A systematic study for the characterization of a novel coagulant (polyaluminium silicate chloride). Colloids Surf. A Physicochem. Eng. Asp. 2009, 342, 30–39. [Google Scholar] [CrossRef]
- Bolto, B.A. Soluble polymers in water treatment. Prog. Polym. Sci. 1995, 20, 987–1041. [Google Scholar] [CrossRef]
- Mortimer, D.A. Synthetic polyelectrolytes: A review. Polym. Int. 1991, 25, 29–41. [Google Scholar] [CrossRef]
- Clesceri, L.; Greenberg, A.; Trussell, R. Standard Methods for the Examination of Water and Wastewater, 17th ed.; APHA–AWWA–WEF: Washington, DC, USA, 1989. [Google Scholar]
- Duan, J.; Gregory, J. The influence of silicic acid on aluminium hydroxide precipitation and flocculation by aluminium salts. J. Inorg. Biochem. 1998, 69, 193–201. [Google Scholar] [CrossRef]
- Yan, M.; Wang, D.; Yu, J.; Ni, J.; Edwards, M.; Qu, J. Enhanced coagulation with polyaluminum chlorides: Role of pH/Alkalinity and speciation. Chemosphere 2008, 71, 1665–1673. [Google Scholar] [CrossRef] [PubMed]
- Farmer, V.C.; Fraser, A.R.; Tait, J.M. Characterization of the chemical structures of natural and synthetic aluminosilicate gels and sols by infrared spectroscopy. Geochim. Cosmochim. Acta 1979, 43, 1417–1420. [Google Scholar] [CrossRef]
- Moussas, P.A.; Zouboulis, A.I. Synthesis, Characterization and coagulation behavior of a composite coagulation reagent by the combination of Polyferric Sulphate (PFS) and cationic polyelectrolyte. Sep. Purif. Technol. 2012, 96, 263–273. [Google Scholar] [CrossRef]
- Zhou, F.S.; Wang, S.H.; Su, J.Z.; Sun, H.; Zhu, P.; Ding, J.; Xu, Y.Z.; Wu, J.G. The infrared spectra and characteristics of PMC—A multicore inorganic polymer flocculant. Fine Chem. 2003, 10, 615–618. [Google Scholar]
- Song, Z.; Williams, C.J.; Edyvean, R.G.J. Treatment of tannery wastewater by chemical coagulation. Desalination 2004, 164, 249–259. [Google Scholar] [CrossRef]
- Arukula, D.; Prem, P.; Tanwi, P.; Hariraj, S.; Vijay, L.M.; Brijesh, K.M. Treatment of tannery wastewater using aluminum formate: Influence of the formate over sulphate-based coagulant. Glob. NEST J. 2018, 20, 458–464. [Google Scholar]
- Sriwiriyarat, T.; Wongvian, R.; Kuhakaew, S. Selection of Coagulant with Consideration of Sludge Characteristics for Treatment of Industrial Wastewater Containing High Strength Mixed Surfactants. Chiang Mai J. Sci. 2017, 44, 1654–1668. [Google Scholar]






| Coagulant Type | Molar Ratios | Procedure |
|---|---|---|
| PSiFAC1.5-10-15 (poly–aluminum–ferric–silicate–chloride) | [OH]/[Al]:1.5 [Al]/[Fe]:10 [Al+Fe]/[Si]:15 | 2 mL of 0.5 M FeCl3 solution were added to 20 mL of 0.5 AlCl3 solution under vigorous stirring (0.3 mL/min, 20 rpm). Then, 1.92 mL of pSi solution were added to the resulted FpA solution and, subsequently, 30 mL of base solution were added slowly under magnetic stirring (0.1 mL/min, 70 rpm) in the mixture [30] at T = 30 °C. The composite pre-polymerized material was left under stirring to mature for approximately 1 h and then diluted with water to a final concentration of 0.1 M relative to Al. |
| PAPEFAC1.5-10-15 (poly–aluminum–ferric–silicate–chloride) | [OH]/[Al]:1.5 [Al]/[Fe]:10 [Al+Fe]/[APE]:15 | 2 mL of 0.5 M FeCl3 solution were added to 20 mL of 0.5 AlCl3 solution under vigorous stirring (0.3 mL/min, 20 rpm). Then, 0.54 mL of APE solution were added to the resulted FpA solution and, subsequently, 30 mL of base solution were added slowly under magnetic stirring (0.1 mL/min, 70 rpm) in the mixture at T = 30 °C. The composite pre-polymerized material was left under stirring to mature for about 1 h and then diluted with water to a final concentration of 0.1 M relative to Al. |
| PAFSiC1.5-15-10 (poly–aluminum–ferric–silicate–chloride) | [OH]/[Al]:1.5 [Fe]/[Si]:15 [Al]/[Si+Fe]:10 | 1.31 mL of pSi solution was added to 15 mL of 0.5 M FeCl3, under vigorous stirring (0.3 mL/min, 20 rpm). Then, the resulted FpSi solution was added to 20 mL of 0.5 M AlCl3 solution and, subsequently, 30 mL of base solution were added slowly, under magnetic stirring (20 rpm) (0.1 mL/min, 70 rpm) in the mixture at T = 30 °C. The composite pre-polymerized material was left under stirring to mature for about 1 h and then diluted with water to a final concentration of 0.1 M relative to Al. |
| PFASiC1.5-15-10 (poly–aluminum–ferric–silicate–chloride) | [OH]/[Al]:1.5 [Al]/[Si]:15 [Al+Si]/[Fe]:10 | 1.75 mL of pSi solution was added to 20 mL of 0.5 M AlCl3 solution, under vigorous stirring (0.3 mL/min, 20 rpm). Then, 2.13 mL of FeCl3 solution were added to the resulted ApSi solution, 30 mL of base solution were added slowly under magnetic stirring (0.1 mL/min, 70 rpm) in the mixture at T = 30 °C. The composite pre-polymerized material was left under stirring to mature for about 1 h and then diluted with water to a final concentration of 0.1 M relative to Al. |
| PASiC1.5-15 (polyaluminum–silicate–chloride) | [OH]/[Al]:1.5 [Al]/[Si]:15 [Al+Si]/[Fe]:0 | 1.75 mL of pSi solution was added to 20 mL of 0.5 M AlCl3 solution, under vigorous stirring (0.3 mL/min, 20 rpm). Then, 30 mL of base solution were added slowly under magnetic stirring (0.1 mL/min, 70 rpm) in the mixture at T = 30 °C [34]. The composite pre-polymerized material was left under stirring to mature for about 1 h and then diluted with water to a final concentration of 0.1 M relative to Al. |
| PACl1.5(lab) (polyaluminum–chloride) | [OH]/[Al]:1.5 | 30 mL of base solution were added slowly under magnetic stirring (0.1 mL/min, 70 rpm) to 20 mL of 0.5 M AlCl3 solution, at T = 30 °C. The composite pre-polymerized material was left under stirring to mature for about 1 h and then diluted with water to a final concentration of 0.1 M relative to Al. |
| Type of Wastewater Samples to be Treated | Turbidity (NTU) | COD (Chemical Oxygen Demand) (mg/L) | Phosphates (mg/L) |
|---|---|---|---|
| Tannery wastewater | 668 | 6800 | 1.76 |
| Yeast wastewater (after anaerobic treatment) | 418 | 11,455 | 3.49 |
| Yeast wastewater (the previous, but after the additional post-aerobic treatment as well) | 143 | 4590 | 2.40 |
| Coagulant Type | pH | Turbidity (NTU) | Conductivity (mS/cm) | Aluminum Species Distribution Al (%) | ||
|---|---|---|---|---|---|---|
| Ala | Alb | Alc | ||||
| PAFSiC1.5-15-10 | 3.9 | 117.0 | 24.0 | 68 | 22 | 10 |
| PFASiC1.5-15-10 | 3.9 | 256.0 | 24.4 | 74 | 21 | 5 |
| PSiFAC1.5-10-15 | 3.5 | 211.0 | 23.7 | 44 | 51 | 5 |
| PAPEFAC1.5-10-15 | 3.6 | 137.0 | 27.0 | 51 | 43 | 6 |
| PACl1.5(lab) | 3.8 | 2.1 | 21.4 | 47 | 39 | 14 |
| % (w/w) | PAFSiC1.5-15-10 | PFASiC1.5-15-10 | PSiFAC1.5-10-15 |
|---|---|---|---|
| Na | 11.48 | 2.96 | 2.23 |
| O | 35.46 | 44.66 | 43.89 |
| Cl | 33.29 | 28.49 | 26.91 |
| Al | 11.47 | 15.61 | 17.01 |
| Fe | 4.07 | 4.73 | 5.78 |
| Si | 0.03 | 0.67 | 1.36 |
| Coagulant | Initial pH | Initial Zeta-Potential (mV) | Initial Size (nm) | Iso-Electric Point (IEP) |
|---|---|---|---|---|
| PSiFAC15-10-15 | 4.87 | 50.5 | 189 | 9.10 |
| PAPEFAC1.5-10-15 | 2.52 | 39.2 | 245 | 9.56 |
| PASiC1.5-15 | 3.63 | 35.9 | 216 | 9.40 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tolkou, A.K.; Zouboulis, A.I. Application of Composite Pre-Polymerized Coagulants for the Treatment of High-Strength Industrial Wastewaters. Water 2020, 12, 1258. https://doi.org/10.3390/w12051258
Tolkou AK, Zouboulis AI. Application of Composite Pre-Polymerized Coagulants for the Treatment of High-Strength Industrial Wastewaters. Water. 2020; 12(5):1258. https://doi.org/10.3390/w12051258
Chicago/Turabian StyleTolkou, Athanasia K., and Anastasios I. Zouboulis. 2020. "Application of Composite Pre-Polymerized Coagulants for the Treatment of High-Strength Industrial Wastewaters" Water 12, no. 5: 1258. https://doi.org/10.3390/w12051258
APA StyleTolkou, A. K., & Zouboulis, A. I. (2020). Application of Composite Pre-Polymerized Coagulants for the Treatment of High-Strength Industrial Wastewaters. Water, 12(5), 1258. https://doi.org/10.3390/w12051258






