Characterizing Nutrient Composition and Concentration in Tomato-, Basil-, and Lettuce-Based Aquaponic and Hydroponic Systems
Abstract
:1. Introduction
2. Materials and Methods
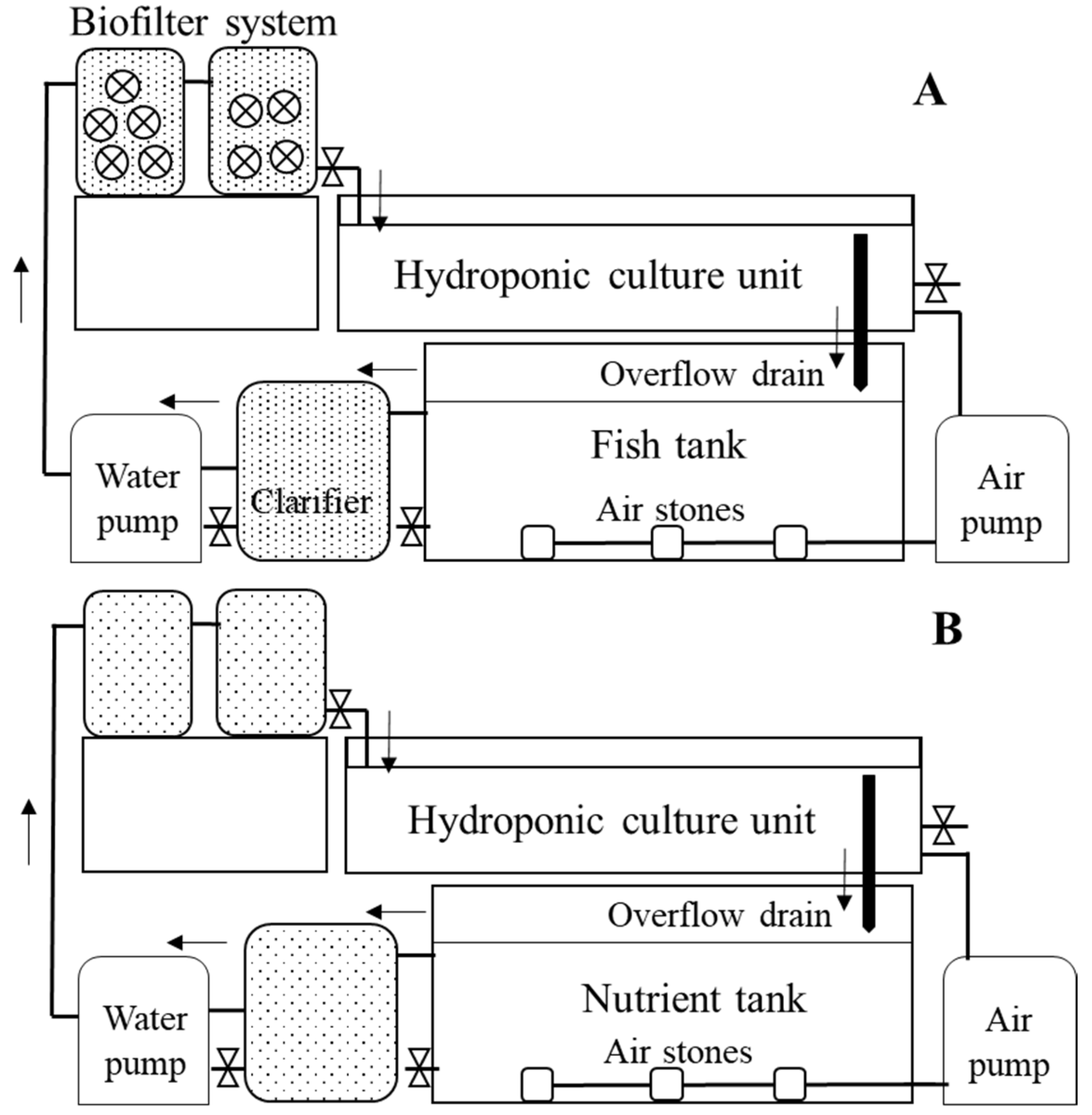
2.1. Experimental Setup and Operation
2.2. Plant and Fish Materials
2.3. Water Parameter Measurements
2.4. Daily Nutrient Release Measurements
2.5. Plant/Fish Growth and Biomass Measurements
2.6. Anion and Cation Measurements
2.7. Total N and P Measurements
2.8. Experimental Design and Data Analysis
3. Results
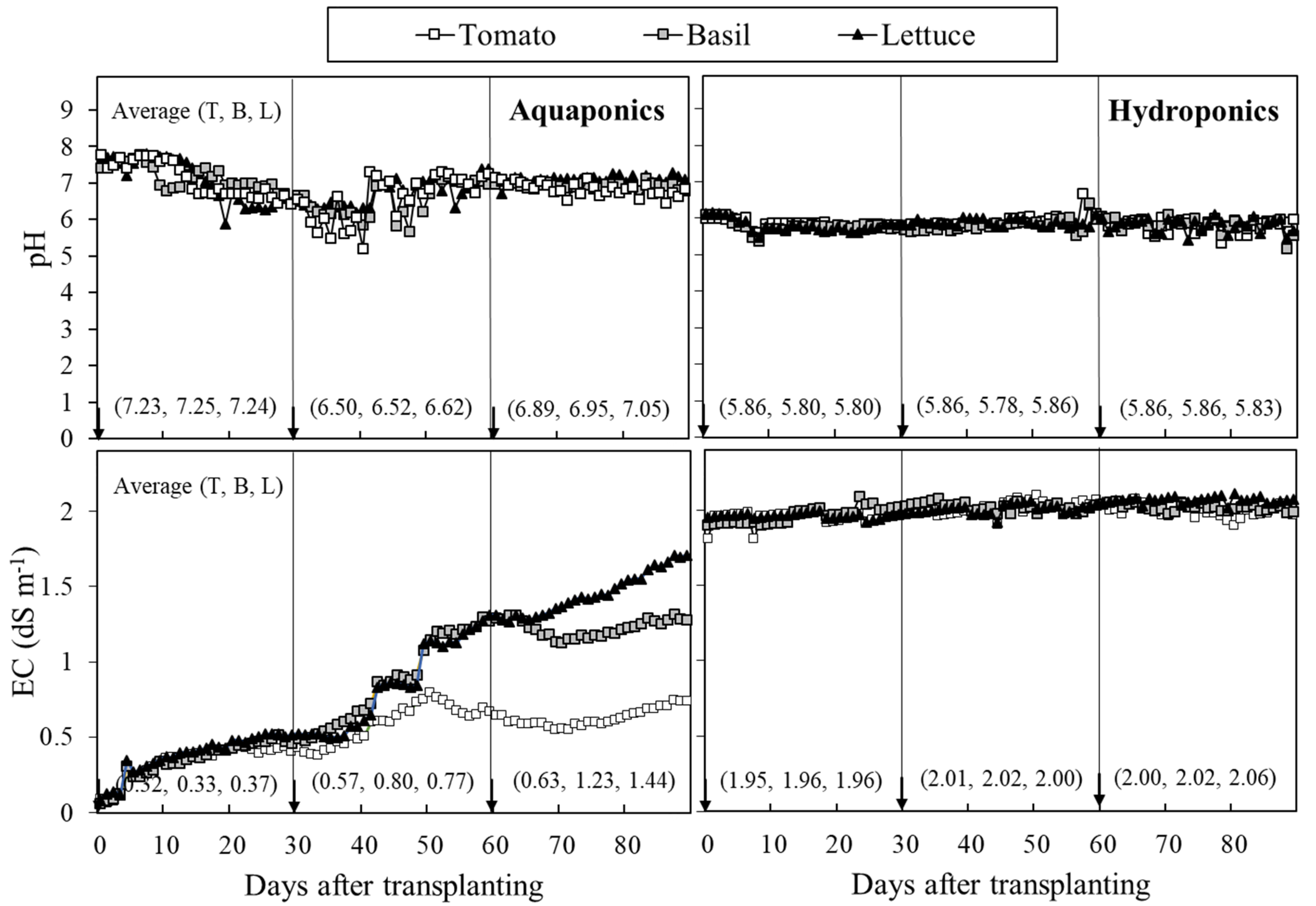
3.1. Daily Nutrient Input; Water Physical and Chemical Properties
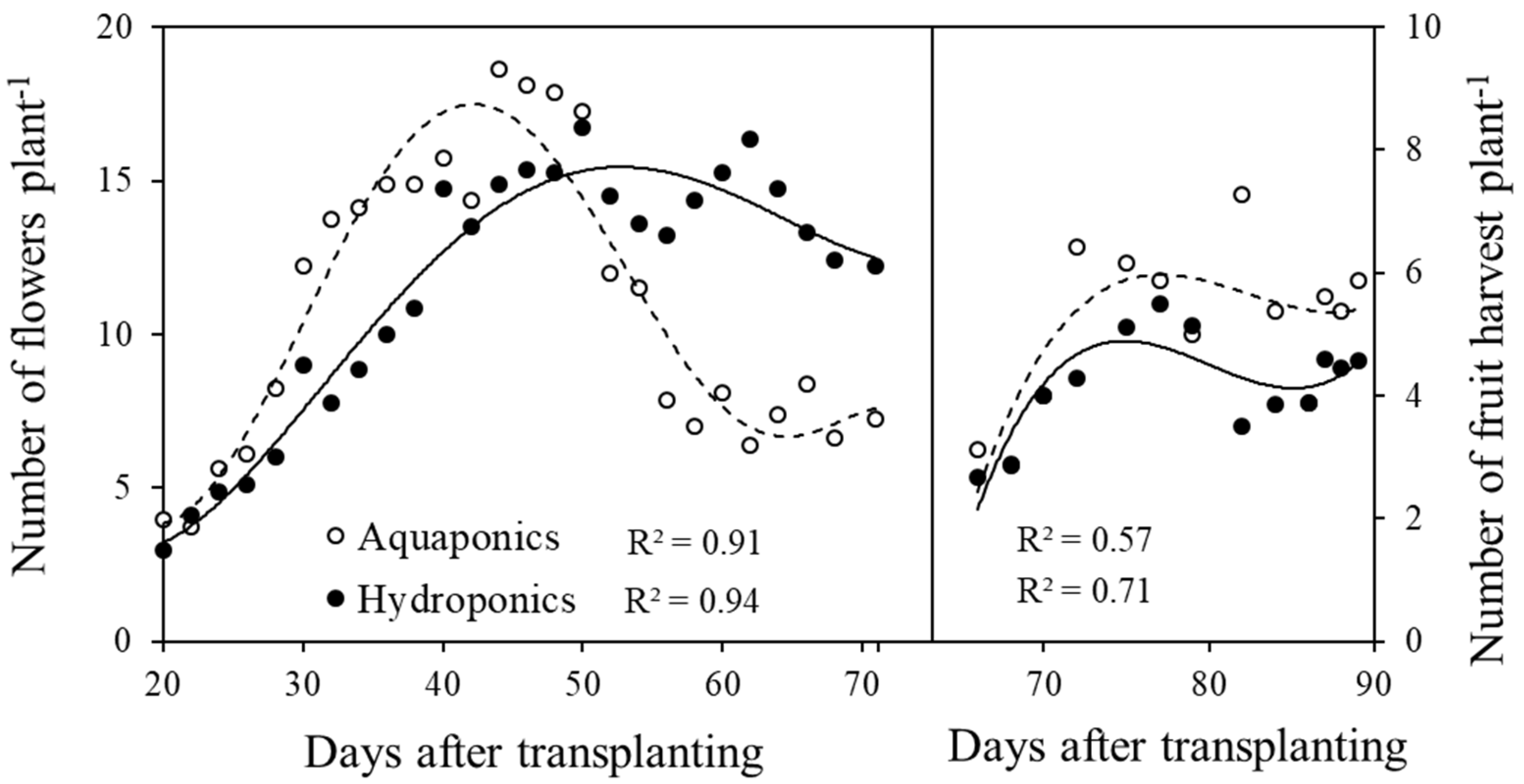
3.2. Growth and Yield of Plant and Fish Crops
3.3. Nutrient Composition and Concentration in Recirculating Water
3.4. Nutrient Composition and Concentration in Plant Tissues
4. Discussion
4.1. Daily Nutrient Input; Water Physical and Chemical Properties
4.2. Growth and Yield of Plant and Fish Crops
4.3. Mg and/or Ca Deficiency in Aquaponics
4.4. N and P Accumulation in Aquaponics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability
References
- Abdel-Raouf, N.; Al-Homaidan, A.A.; Ibraheem, I.B.M. Microalgae and wastewater treatment. Saudi J. Biol. Sci. 2012, 19, 257–275. [Google Scholar] [CrossRef] [Green Version]
- Rakocy, J.E.; Hargreaves, J.A. Integration of Vegetable Hydroponics with Fish Culture: A Review. In Techniques for Modern Aquaculture; Wang, J., Ed.; American Society for Agricultural Engineers: St. Joseph, MI, USA, 1993. [Google Scholar]
- McMurtry, M.R.; Sanders, D.C.; Cure, J.D.; Hodson, R.G.; Haning, B.C.; St Amand, P.C. Efficiency of water use of an integrated fish/vegetable co-culture system. J. World Aquac. Soc. 1997, 28, 420–428. [Google Scholar] [CrossRef]
- Yang, T.; Kim, H.J. Nutrient management regime affects water quality, crop growth, and nitrogen use efficiency of aquaponic systems. Sci. Hortic. 2019, 256. [Google Scholar] [CrossRef]
- Goddek, S.; Delaide, B.; Mankasingh, U.; Ragnarsdottir, K.V.; Jijakli, H.; Thorarinsdottir, R. Challenges of sustainable and commercial aquaponics. Sustainability 2015, 7, 4199–4224. [Google Scholar] [CrossRef] [Green Version]
- Al-Hafedh, Y.S.; Alam, A.; Beltagi, M.S. Food production and water conservation in a recirculating aquaponic system in Saudi Arabia at different ratios of fish feed to plants. J. World Aquac. Soc. 2008, 39, 510–520. [Google Scholar] [CrossRef]
- Rakocy, J.E.; Bailey, D.S.; Shultz, R.C.; Thoman, E.S. Update on Tilapia and Vegetable Production in the UVI Aquaponic System. In Proceedings of the 6th International Symposium on Tilapia in Aquaculture, New Dimensions on Farmed Tilapia, Manila, Philippines, 12–16 September 2004; pp. 676–690. [Google Scholar]
- Resh, H.M. Hydroponic Food Production: A Definitive Guidebook for the Advanced Home Gardener and the Commercial Hydroponic Grower, 7th ed.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Pantanella, E.; Cardarelli, M.; Colla, G.; Rea, E.; Marcucci, A. Aquaponics vs. hydroponics: Production and quality of lettuce crop. Acta Hortic. 2012, 927, 887–893. [Google Scholar] [CrossRef]
- Somerville, C.; Cohen, M.; Pantanella, E.; Stankus, A.; Lovatelli, A. Small-Scale Aquaponic Food Production Integrated Fish and Plant Farming; FAO Fisheries and Aquaculture Technical Paper; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2014; Volume 589, pp. 1–262. [Google Scholar]
- Roosta, H.R. Comparison of the vegetative growth, eco-physiological characteristics and mineral nutrient content of basil plants in different irrigation ratios of hydroponic: Aquaponic solutions. J. Plant Nutr. 2014, 37, 1782–1803. [Google Scholar] [CrossRef]
- Wortman, S.E. Crop physiological response to nutrient solution electrical conductivity and pH in an ebb-and-flow hydroponic system. Sci. Hortic. 2015, 194, 34–42. [Google Scholar] [CrossRef]
- Alcarraz, E.; Flores, M.; Tapia, M.L.; Bustamante, A.; Wacyk, J.; Escalona, V. Quality of lettuce (Lactuca sativa L.) grown in aquaponic and hydroponic systems. Acta Hortic. 2018, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Savidov, N.A.; Hutchings, E.; Rakocy, J.E. Fish and plant production in a recirculating aquaponic system: A new approach to sustainable agriculture in Canada. Acta Hortic. 2007, 209–221. [Google Scholar] [CrossRef]
- Delaide, B.; Goddek, S.; Gott, J.; Soyeurt, H.; Jijakli, M.H. Lettuce (Lactuca sativa L. var. Sucrine) growth performance in complemented aquaponic solution outperforms hydroponics. Water 2016, 8, 467. [Google Scholar] [CrossRef]
- Graber, A.; Junge, R. Aquaponic systems: Nutrient recycling from fish wastewater by vegetable production. Desalination 2009, 246, 147–156. [Google Scholar] [CrossRef]
- Roosta, H.R.; Hamidpour, M. Mineral nutrient content of tomato plants in aquaponic and hydroponic systems: Effect of foliar application of some macro-and micro-nutrients. J. Plant Nutr. 2013, 36, 2070–2083. [Google Scholar] [CrossRef]
- Suhl, J.; Dannehl, D.; Kloas, W.; Baganz, D.; Jobs, S.; Scheibe, G.; Schmidt, U. Advanced aquaponics: Evaluation of intensive tomato production in aquaponics vs. conventional hydroponics. Agric. Water Manag. 2016, 178, 335–344. [Google Scholar] [CrossRef]
- Rakocy, J.E.; Shultz, R.C.; Bailey, D.S.; Thoman, E.S. Aquaponic production of tilapia and basil: Comparing a batch and staggered cropping system. Acta Hortic. 2004, 648, 63–69. [Google Scholar] [CrossRef]
- Rakocy, J.E.; Masser, M.P.; Losordo, T.M. Recirculating aquaculture tank production systems: Aquaponics-Integrating fish and plant culture. SRAC Publ. South. Reg. Aquac. Cent. Publ. 2006, 454, 1–16. Available online: http://pods.dasnr.okstate.edu/docushare/dsweb/Get/Document-10215/SRAC-454web.pdf (accessed on 1 August 2019).
- Roosta, H.R.; Hamidpour, M. Effects of foliar application of some macro-and micro-nutrients on tomato plants in aquaponic and hydroponic systems. Sci. Hortic. 2011, 129, 396–402. [Google Scholar] [CrossRef]
- Krom, M.D.; Ben David, A.; Ingall, E.D.; Benning, L.G.; Clerici, S.; Bottrell, S.; Davies, C.; Potts, N.J.; Mortimer, R.J.G.; van Rijn, J. Bacterially mediated removal of phosphorus and cycling of nitrate and sulfate in the waste stream of a “zero-discharge” recirculating mariculture system. Water Res. 2014, 56, 109–121. [Google Scholar] [CrossRef]
- Seawright, D.E.; Stickney, R.R.; Walker, R.B. Nutrient dynamics in integrated aquaculture-hydroponics systems. Aquaculture 1998, 160, 215–237. [Google Scholar] [CrossRef]
- Wongkiew, S.; Popp, B.N.; Kim, H.J.; Khanal, S.K. Fate of nitrogen in floating-raft aquaponic systems using natural abundance nitrogen isotopic compositions. Int. Biodeterior. Biodegrad. 2017, 125, 24–32. [Google Scholar] [CrossRef]
- Kim, H.J.; Yang, T.; Lin, M.Y.; Langenhoven, P. Plant propagation for successful hydroponic production. Acta Hortic. 2018, 1212, 109–116. [Google Scholar] [CrossRef]
- Basta, N.T.; Tabatabai, M.A. Determination of total potassium, sodium, calcium, and magnesium in plant materials by ion chromatography. Soil Sci. Soc. Am. J. 1985, 49, 76–81. [Google Scholar] [CrossRef]
- Beke, G.J.; Selles, F. Comparison of ion chromatography and a continuous-flow technique for analysis of chloride and sulfate in plant-samples. Commun. Soil Sci. Plant Anal. 1993, 24, 973–978. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for determination of phosphate in natural waters. Anal. Chim. Acta 1962, 26, 31–36. [Google Scholar] [CrossRef]
- Danaher, J.J.; Shultz, R.C.; Rakocy, J.E.; Bailey, D.S.; Knight, L. Effect of a parabolic screen filter on water quality and production of Nile tilapia (Oreochromis niloticus) and water spinach (Ipomoea aquatica) in a recirculating raft aquaponic system. Int. J. Recirc. Aquac. 2011, 12, 35–53. [Google Scholar] [CrossRef]
- Rakocy, J.E. Integrating Tilapia Culture with Vegetable Hydroponics in Recirculating Systems. In Tilapia Aquaculture in the Americas; Costa Pierce, B.A., Rakocy, J.E., Eds.; The World Aquaculture Society: Baton Rouge, LA, USA, 1997. [Google Scholar]
- Yildiz, H.Y.; Robaina, L.; Pirhonen, J.; Mente, E.; Dominguez, D.; Parisi, G. Fish welfare in aquaponic systems: Its relation to water quality with an emphasis on feed and faeces—A review. Water 2017, 9, 13. [Google Scholar] [CrossRef] [Green Version]
- Boyd, C.E. Water Quality Management for Pond Fish Culture; Elsevier Scientific Publishing Co.: Amsterdam, The Netherlands, 1982. [Google Scholar]
- Fondriest Environmental, Inc. “Dissolved Oxygen”. Fundamentals of Environmental Measurements. 2013. Available online: https://www.fondriest.com/environmental-measurements/parameters/water-quality/dissolved-oxygen/ (accessed on 18 July 2019).
- Schroder, F.G.; Lieth, J.H. Irrigation Control in Hydroponics. In Hydroponic Production of Vegetables and Ornamentals; Savvas, D., Passam, H., Eds.; Embryo Publishing: Athens, Greece, 2002; pp. 265–296. [Google Scholar]
- Wurts, W.A.; Durborow, R.M. Interactions of pH, carbon dioxide, alkalinity and hardness in fish ponds. South. Reg. Aquac. Cent. Publ. 1992, 464, 1–4. [Google Scholar]
- Ebeling, J.M.; Timmons, M.B. Recirculating Aquaculture Systems. In Aquaculture Production Systems; Tidwell, J.H., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 245–277. [Google Scholar]
- New, M.B. Feed and Feeding of Fish and Shrimp. A Manual on the Preparation and Presentation of Compound Feeds for Shrimp and FISH in Aquaculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 1987; Available online: http://www.fao.org/3/S4314E/S4314E00.htm (accessed on 25 March 2020).
- Kim, H.J.; Lin, M.-Y.; Mitchell, C.A. Light spectral and thermal properties govern biomass allocation in tomato through morphological and physiological changes. Environ. Exp. Bot. 2019, 157, 228–240. [Google Scholar] [CrossRef]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Elsevier/Academic Press: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Yang, T.; Kim, H.J. Effects of hydraulic loading rate on spatial and temporal water quality characteristics and crop growth and yield in aquaponic systems. Horticulturae 2020, 6, 9. [Google Scholar] [CrossRef] [Green Version]
- Nozzi, V.; Graber, A.; Schmautz, Z.; Mathis, A.; Junge, R. Nutrient management in aquaponics: Comparison of three approaches for cultivating lettuce, mint and mushroom herb. Agron. Basel 2018, 8, 27. [Google Scholar] [CrossRef] [Green Version]
- Maathuis, F.J.M. Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol. 2009, 12, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Wege, S.; Gilliham, M.; Henderson, S.W. Chloride: Not simply a ‘cheap osmoticum’, but a beneficial plant macronutrient. J. Exp. Bot. 2017, 68, 3057–3069. [Google Scholar] [CrossRef] [PubMed]
- Halevy, J. Growth-Rate and nutrient-uptake of 2 cotton cultivars grown under irrigation. Agron. J. 1976, 68, 701–705. [Google Scholar] [CrossRef]
- Azoulay, A.; Garzon, P.; Eisenberg, M.J. Comparison of the mineral content of tap water and bottled waters. J. Gen. Intern. Med. 2001, 16, 168–175. [Google Scholar] [CrossRef]
- Pehrsson, P.; Patterson, K. The Mineral Content of U.S. Drinking and Municipal Water. In Proceedings of the 32nd National Nutrient Databank Conference, Ottawa, ON, Canada, 12–14 May 2008. [Google Scholar]
- Grattan, S.R.; Grieve, C.M. Mineral element acquisition and growth-response of plants grown in saline environments. Agric. Ecosyst. Environ. 1992, 38, 275–300. [Google Scholar] [CrossRef]
- Cakmak, I.; Kirkby, E.A. Role of magnesium in carbon partitioning and alleviating photooxidative damage. Physiol. Plant. 2008, 133, 692–704. [Google Scholar] [CrossRef]
- Kim, H.J.; Fonseca, J.M.; Choi, J.H.; Kubota, C.; Kwon, D.Y. Salt in irrigation water affects the nutritional and visual properties of romaine lettuce (Lactuca sativa L.). J. Agric. Food Chem. 2008, 56, 3772–3776. [Google Scholar] [CrossRef]
- Vandendriessche, R. Prediction of mineral nutrient status of trees by foliar analysis. Bot. Rev. 1974, 40, 347–394. [Google Scholar] [CrossRef]
- Colla, G.; Kim, H.J.; Kyriacou, M.C.; Rouphael, Y. Nitrate in fruits and vegetables. Sci. Hortic. 2018, 237, 221–238. [Google Scholar] [CrossRef]
- Delaide, B.; Delhaye, G.; Dermience, M.; Gott, J.; Soyeurt, H.; Jijakli, M.H. Plant and fish production performance, nutrient mass balances, energy and water use of the PAFF Box, a small-scale aquaponic system. Aquac. Eng. 2017, 78, 130–139. [Google Scholar] [CrossRef]
- Goddek, S.; Schmautz, Z.; Scott, B.; Delaide, B.; Keesman, K.J.; Wuertz, S.; Junge, R. The effect of anaerobic and aerobic fish sludge supernatant on hydroponic lettuce. Agron. Basel 2016, 6, 37. [Google Scholar] [CrossRef] [Green Version]
- Lennard, W.A.; Leonard, B.V. A comparison of three different hydroponic sub-systems (gravel bed, floating and nutrient film technique) in an Aquaponic test system. Aquac. Int. 2006, 14, 539–550. [Google Scholar] [CrossRef]
- Rakocy, J.E.; Bailey, D.S.; Shultz, R.C.; Danaher, J.J. Preliminary evaluation of organic waste from two aquaculture systems as a source of inorganic nutrients for hydroponics. Acta Hortic. 2007, 742, 201–207. [Google Scholar] [CrossRef]
- Kim, H.J.; Lynch, J.P.; Brown, K.M. Ethylene insensitivity impedes a subset of responses to phosphorus deficiency in tomato and petunia. Plant Cell Environ. 2008, 31, 1744–1755. [Google Scholar] [CrossRef]
- Santos, B.M.; Dusky, J.A.; Stall, W.M.; Bewick, T.A.; Shilling, D.G.; Gilreath, J.P. Phosphorus absorption in lettuce, smooth pigweed (Amaranthus hybridus), and common purslane (Portulaca oleracea) mixtures. Weed Sci. 2004, 52, 389–394. [Google Scholar] [CrossRef]
- Kim, H.J.; Li, X. Effects of phosphorus on shoot and root growth, partitioning, and phosphorus utilization efficiency in lantana. HortScience 2016, 51, 1001–1009. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Choi, S.; Fan, J.; Kim, H.J. Biomass and phosphorus accumulation and partitioning of geranium and coleus in response to phosphorus availability and growth phase. Agron. Basel 2019, 9, 813. [Google Scholar] [CrossRef] [Green Version]
- Clement, C.R.; Hopper, M.J.; Jones, L.H.P. Uptake of nitrate by Lolium perenne from flowing nutrient solution: 1. Effect of NO3-concentration. J. Exp. Bot. 1978, 29, 453–464. [Google Scholar] [CrossRef]
- Edwards, J.H.; Barber, S.A. Nitrogen uptake characteristics of corn roots at low n-concentration as influenced by plant age. Agronomy J. 1976, 68, 17–19. [Google Scholar] [CrossRef]
- Warncke, D.D.; Barber, S.A. Nitrate uptake effectiveness of 4 plant species. J. Environ. Qual. 1974, 3, 28–30. [Google Scholar] [CrossRef]
- Letey, J.; Jarrell, W.M.; Valoras, N. Nitrogen and water-uptake patterns and growth of plants at various minimum solution nitrate concentrations. J. Plant Nutr. 1982, 5, 73–89. [Google Scholar] [CrossRef]



| Macronutrient | RO Water a (mg L−1) | Aquaponics | Hydroponics | ||||
|---|---|---|---|---|---|---|---|
| Daily Release Rate b (mg L−1) | Daily Replenishment Rate d (mg L−1 day−1) | Initial Concentration (mg L−1) | |||||
| Tomato | Basil | Lettuce | Tomato | Basil/Lettuce | |||
| NO3–N | 0.42 | 1.04 (1.6%) c | 9.29 | 5.00 | 4.52 | 178.7 | 161.4 |
| NO2–N | – | 0.13 (0.2%) | 0.37 | 0.16 | 0.14 | 7.1 | 5.1 |
| NH4–N | 0.02 | 0.40 (0.6%) | 1.15 | 0.44 | 0.39 | 22.2 | 14.1 |
| PO4–P | 0.44 | 2.41 (25.7%) | 4.92 | 2.80 | 2.53 | 94.6 | 90.2 |
| K | 0.34 | 0.82 (7.8%) | 8.41 | 5.59 | 5.05 | 161.7 | 180.3 |
| SO4–S | 1.24 | 15.7 (35.3%) | 21.5 | 14.6 | 13.2 | 413.1 | 471.7 |
| Ca | 2.45 | 0.20 (0.7%) | 5.39 | 3.53 | 3.19 | 103.7 | 113.9 |
| Mg | 0.59 | 0.02 (0.9%) | 1.38 | 0.90 | 0.81 | 26.6 | 29.0 |
| Na | 2.76 | 0.11 (0.4%) | – | – | – | – | – |
| Cl | 2.30 | 0.50 (2.2%) | – | – | – | – | – |
| EC (dS m−1) | 0.03 | 0.1 | 2.0 | 2.0 | |||
| pH | 7.3 | 6.9 | 6.0 | 6.0 | |||
| Parameter | Aquaponics a | Hydroponics b | |
|---|---|---|---|
| Tomato | Basil/Lettuce | ||
| Macronutrient (%) | |||
| Total nitrogen (N) | >6.88 | 0.044 | 0.043 |
| P2O5–P | >1.10 | 0.130 | 0.093 |
| K2O–K | 0.99 | 0.034 | 0.035 |
| SO4–S | 0.43 | – | – |
| Ca | 2.25–2.75 | 0.075 | 0.075 |
| Mg | 0.23 | 0.037 | 0.039 |
| Micronutrient (mg kg−1) | |||
| B | – | 2.75 | 2.00 |
| Cu | 10 | 0.95 | 1.05 |
| Fe | 40 | 10.00 | 21.00 |
| Mn | 80 | 8.00 | 1.90 |
| Mo | – | 0.40 | 0.42 |
| Zn | 153 | 2.70 | 2.10 |
| Crop | Production System | DO (mg L−1) | Water Temperature (°C) | pH | EC (dS m−1) | Feed Conversion Ratio (FCR) | Fish Biomass Increment a (%) | pH Correction Solution (L) | Cumulative Water Use b (L) |
|---|---|---|---|---|---|---|---|---|---|
| Tomato | Aquaponics | 7.2 b | 26.9 b | 6.9 a | 0.54 c | 1.3 | 39 a | 5.92 b | 466 |
| Hydroponics | 9.4 a | 22.1 c | 5.8 b | 1.95 a | – | – | 0.50 c | 366 | |
| Basil | Aquaponics | 7.1 b | 26.6 b | 6.7 a | 0.84 b | 1.5 | 33 a | 4.44 b | 418 |
| Hydroponics | 9.3 a | 22.1 c | 5.8 b | 1.94 a | – | – | 0.36 c | 276 | |
| Lettuce | Aquaponics | 7.1 b | 27.5 a | 6.8 a | 0.92 b | 1.8 | 27 b | 8.86 a | 437 |
| Hydroponics | 9.3 a | 22.2 c | 5.8 b | 1.96 a | – | – | 0.40 c | 239 | |
| ANOVA | |||||||||
| System | *** | *** | *** | *** | – | – | – | * | |
| Crop | * | *** | * | * | * | * | *** | ns | |
| System × Crop | ns | *** | ** | * | – | – | – | ns | |
| Crop | Production System | Plant Height (cm) | Leaf Length (cm) | Leaf Number (plant−1) | SPAD | Time to Flowering (DAT) | Time to Harvest (DAT) | Fruit Number (plant−1) | Individual Fruit Fresh Mass (g fruit−1) |
|---|---|---|---|---|---|---|---|---|---|
| Tomato | Aquaponics | 87.2 b | 39.5 b | 21.0 c | 24.5 c | 27.5 b | 30.1 b | 61.4 | 19.2 |
| Hydroponics | 103.7 a | 46.3 a | 21.6 c | 38.6 a | 29.4 a | 32.3 a | 51.4 | 21.8 | |
| Basil | Aquaponics | 39.9 c | 11.4 d | 118.3 b | 23.2 c | – | – | – | – |
| Hydroponics | 49.8 c | 12.9 d | 140.7 a | 31.7 b | – | – | – | – | |
| Lettuce | Aquaponics | 16.3 d | 20.5 c | 16.7 c | 24.6 c | – | – | – | – |
| Hydroponics | 18.1 d | 22.1 c | 18.1c | 25.7 c | – | – | – | – | |
| ANOVA | |||||||||
| System | ns | * | ns | *** | – | – | – | – | |
| Crop | ** | *** | ns | *** | ** | ** | ns | ns | |
| System × Crop | *** | *** | *** | *** | – | – | – | – | |
| Crop | Production System | Fresh Mass (g plant−1) | Dry Mass (g plant−1) | Root-to-Shoot Ratio a | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Shoots | Roots | Fruits | Total | Shoots | Roots | Fruits | |||
| Tomato | Aquaponics | 1318.5 b | 601.7 b | 316.9 a | 399.9 | 104.7 b | 66.2 b | 13.8 b | 24.8 | 0.18 ab |
| Hydroponics | 2032.8 a | 1300.7 a | 337.0 a | 395.1 | 179.5 a | 130.8 a | 23.2 a | 25.5 | 0.17 ab | |
| Basil | Aquaponics | 306.3 d | 213.9 de | 92.4 c | – | 29.8 d | 25.7 d | 4.1 d | – | 0.19 a |
| Hydroponics | 545.9 c | 385.2 c | 160.7 b | – | 52.4 c | 46.6 c | 5.8 c | – | 0.15 b | |
| Lettuce | Aquaponics | 181.0 d | 152.1 e | 29.0 d | – | 7.0 e | 5.8 e | 1.2 e | – | 0.21 a |
| Hydroponics | 263.5 d | 228.3 d | 35.2 d | – | 9.3 e | 8.2 e | 1.1 e | – | 0.14 b | |
| ANOVA | ||||||||||
| System | *** | *** | *** | ns | *** | *** | *** | ns | *** | |
| Crop | *** | *** | *** | – | *** | *** | *** | – | ns | |
| System × Crop | *** | *** | *** | – | *** | *** | *** | – | *** | |
| Harvest Time | System | Marketable Yield (g plant−1) | Root Fresh Mass (g plant−1) | Total Yield (g plant−1) |
|---|---|---|---|---|
| First | Aquaponics | 89.7 c | 18.2 d | 107.9 c |
| Hydroponics | 210.3 a | 31.2 b | 241.5 a | |
| Second | Aquaponics | 97.1 c | 26.4 c | 123.5 c |
| Hydroponics | 148.4 b | 26.1 c | 174.5 b | |
| Third | Aquaponics | 177.3 b | 26.9 c | 204.2 b |
| Hydroponics | 233.6 a | 37.0 a | 270.6 a | |
| ANOVA | ||||
| System | *** | *** | *** | |
| Time | *** | *** | *** | |
| System × Time | ns | ns | ns | |
| Crop | Production System | NO3–N | NO2–N | NH4–N | PO4–P | K | Ca | Mg | SO4–S | Na | Cl |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (mg L−1) | |||||||||||
| Tomato | Aquaponics | 60.5 d | 0.8 a | 1.8 a | 30.1 c0 | 29.2 c | 12.1 d0 | 0.6 do | 218.1 b | 61.9 a | 0 c |
| Hydroponics | 207.9 a | 0.07 b | 0.4 bc | 122.8 ab | 334.0 a | 141.9 b0 | 39.4 ab | 627.5 a | 8.6 b | 0 c | |
| Basil | Aquaponics | 108.0 c | 0.9 a | 1.7 ab | 33.0 co | 82.3 b | 22.6 c0 | 1.9 co | 267.8 b | 76.7 a | 1.7 b |
| Hydroponics | 193.8 a | 0.1 b | 0.3 bc | 107.4 b | 326.8 a | 145.8 ab | 40.5 a | 674.5 a | 14.0 b | 0 c | |
| Lettuce | Aquaponics | 161.6 b | 0.8 a | 1.8 a | 27.1 c0 | 114.1 b | 20.4 c0 | 2.4 co | 235.8 b | 70.0 a | 11.2 a |
| Hydroponics | 198.6 a | 0.05 b | 0.2 c | 125.5 a0 | 339.0 a | 148.8 a0 | 39.0 b | 727.8 a | 10.7 b | 0 c | |
| ANOVA | |||||||||||
| System | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | |
| Crop | *** | ns | ns | ns | ns | *** | ** | ns | ns | *** | |
| System × Crop | *** | ns | ns | ** | ns | *** | *** | ns | ns | *** | |
| Aquaponics | 110.0 b | 0.8 a | 1.8 a | 30.1 b | 75.2 b | 18.4 b | 1.6 b | 242.3 b | 69.5 a | 4.3 a | |
| Hydroponics | 197.6 a | 0.1 b | 0.3 b | 118.6 a | 333.3 a | 145.5 a | 39.6 a | 676.6 a | 11.1 b | 0 b | |
| Cherry Tomato | 134.2 b | 0.5 | 1.6 | 76.5 | 181.6 | 75.6 | 20.0 | 376.6 | 35.2 | 0 b | |
| Basil | 150.9 ab | 0.5 | 1.6 | 70.2 | 204.5 | 81.5 | 20.4 | 403.4 | 45.3 | 0.9 b | |
| Lettuce | 176.0 a | 0.4 | 1.6 | 76.3 | 226.6 | 83.2 | 20.3 | 399.8 | 40.4 | 5.6 a | |
| Total N | Total P | K | Ca | Mg | SO4–S | Na | Cl | ||
|---|---|---|---|---|---|---|---|---|---|
| (mg g−1) | |||||||||
| Tomato | Aquaponics | 25.7 c | 15.6 c | 60.6 | 4.6 b | 5.9 ab | 1.2 b | 8.9 a | 5.6 b |
| Hydroponics | 33.0 b | 18.0 bc | 54.2 | 6.6 ab | 8.0 a | 6.1 b | 1.7 b | 2.5 c | |
| Basil | Aquaponics | 30.2 bc | 19.8 b | 53.1 | 6.2 ab | 2.2 b | 11.0 b | 4.8 b | 2.6 c |
| Hydroponics | 33.6 b | 19.9 b | 44.9 | 7.7 a | 4.1 ab | 38.2 a | 2.9 b | 3.2 bc | |
| Lettuce | Aquaponics | 42.7 a | 21.2 b | 66.7 | 3.5 b | 2.0 b | 0 b | 11.8 a | 10.8 a |
| Hydroponics | 45.3 a | 26.9 a | 69.3 | 3.4 b | 2.6 b | 0 b | 3.6 b | 1.3 c | |
| ANOVA | |||||||||
| System | * | ** | ns | ns | ns | ** | *** | *** | |
| Crop | *** | *** | * | *** | *** | *** | *** | *** | |
| System × Crop | ns | * | ns | ns | ns | ns | *** | *** | |
| Aquaponics | 31.1 b | 18.1 b | 59.5 | 4.9 b | 3.8 b | 6.9 b | 8.2 a | 5.7 a | |
| Hydroponics | 36.4 a | 20.8 a | 54.5 | 6.2 a | 5.5 a | 23.8 a | 2.5 b | 2.4 b | |
| Cherry Tomato | 29.3 b | 16.9 c | 57.4 ab | 5.6 a | 7.0 a | 9.9 b | 5.3 ab | 3.9 ab | |
| Basil | 32.2 b | 19.8 b | 49.0 b | 6.9 a | 3.1 b | 33.7 a | 3.8 b | 2.9 b | |
| Lettuce | 44.0 a | 24.6 a | 68.0 a | 3.4 b | 2.3 b | 0 b | 7.7 a | 6.1 a | |
| Treatment | Regression Coefficient | ||||
|---|---|---|---|---|---|
| Crop | Variables | Total N | Ca | Mg | SPAD |
| Tomato | Total N | — | |||
| Ca | 0.93 *** | — | |||
| Mg | 0.86 *** | 0.93 *** | — | ||
| SPAD | 0.82 *** | 0.78 ** | 0.65 * | — | |
| Basil | Total N | — | |||
| Ca | −0.19 ns | — | |||
| Mg | 0.55 ns a | −0.04 ns | — | ||
| SPAD | 0.80 ** | −0.54 ns | 0.48 ns a | — | |
| Lettuce | Total N | — | |||
| Ca | −0.22 ns | — | |||
| Mg | −0.22 ns | 0.84 *** | — | ||
| SPAD | 0.59 * | −0.51 ns | −0.31 ns | — | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; Kim, H.-J. Characterizing Nutrient Composition and Concentration in Tomato-, Basil-, and Lettuce-Based Aquaponic and Hydroponic Systems. Water 2020, 12, 1259. https://doi.org/10.3390/w12051259
Yang T, Kim H-J. Characterizing Nutrient Composition and Concentration in Tomato-, Basil-, and Lettuce-Based Aquaponic and Hydroponic Systems. Water. 2020; 12(5):1259. https://doi.org/10.3390/w12051259
Chicago/Turabian StyleYang, Teng, and Hye-Ji Kim. 2020. "Characterizing Nutrient Composition and Concentration in Tomato-, Basil-, and Lettuce-Based Aquaponic and Hydroponic Systems" Water 12, no. 5: 1259. https://doi.org/10.3390/w12051259
APA StyleYang, T., & Kim, H.-J. (2020). Characterizing Nutrient Composition and Concentration in Tomato-, Basil-, and Lettuce-Based Aquaponic and Hydroponic Systems. Water, 12(5), 1259. https://doi.org/10.3390/w12051259






