Characterization of Aquifer Hydrochemistry from the Operation of a Shallow Geothermal System
Abstract
:1. Introduction
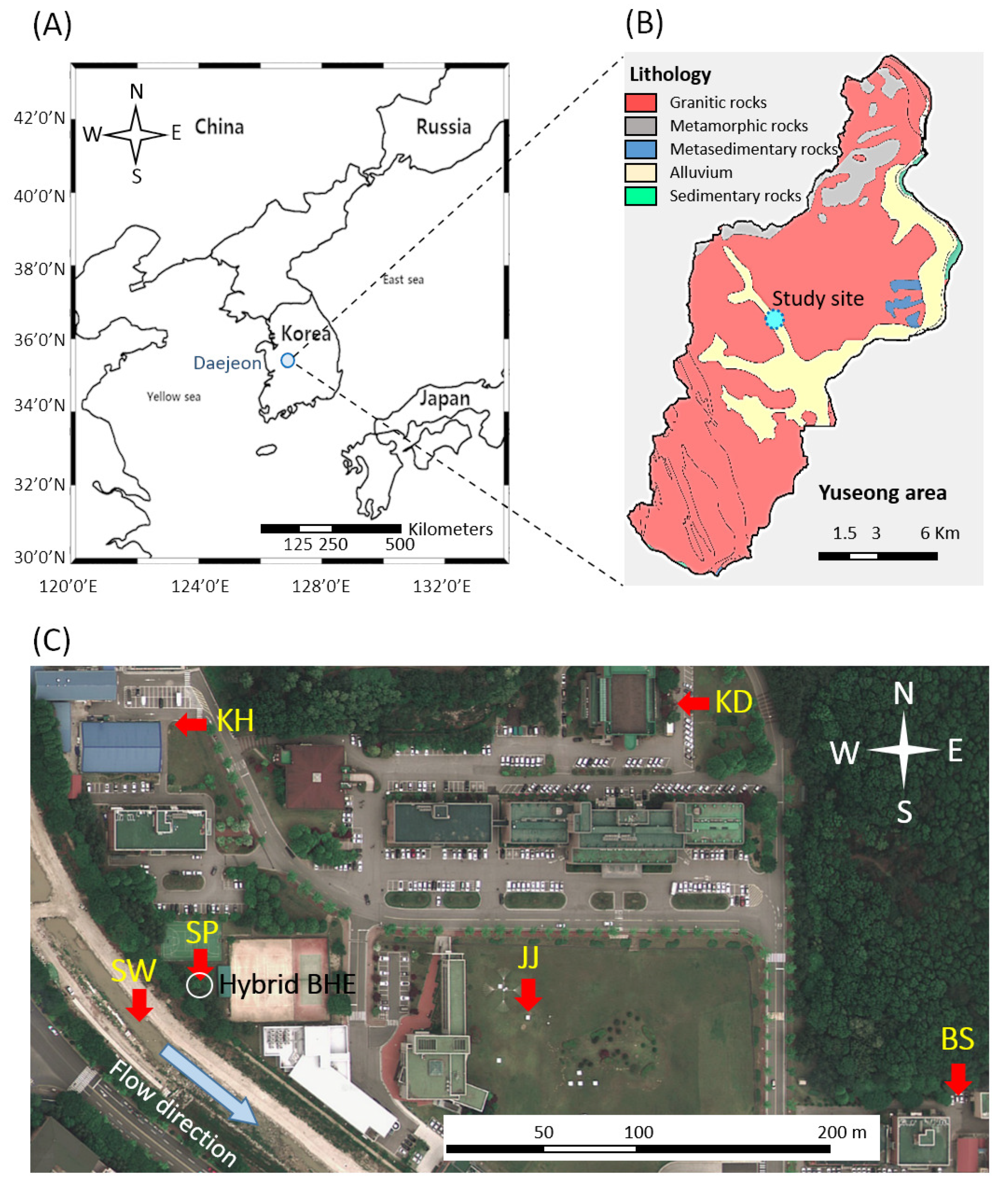
2. Site Description
3. Materials and Methods
3.1. Sampling and Analysis
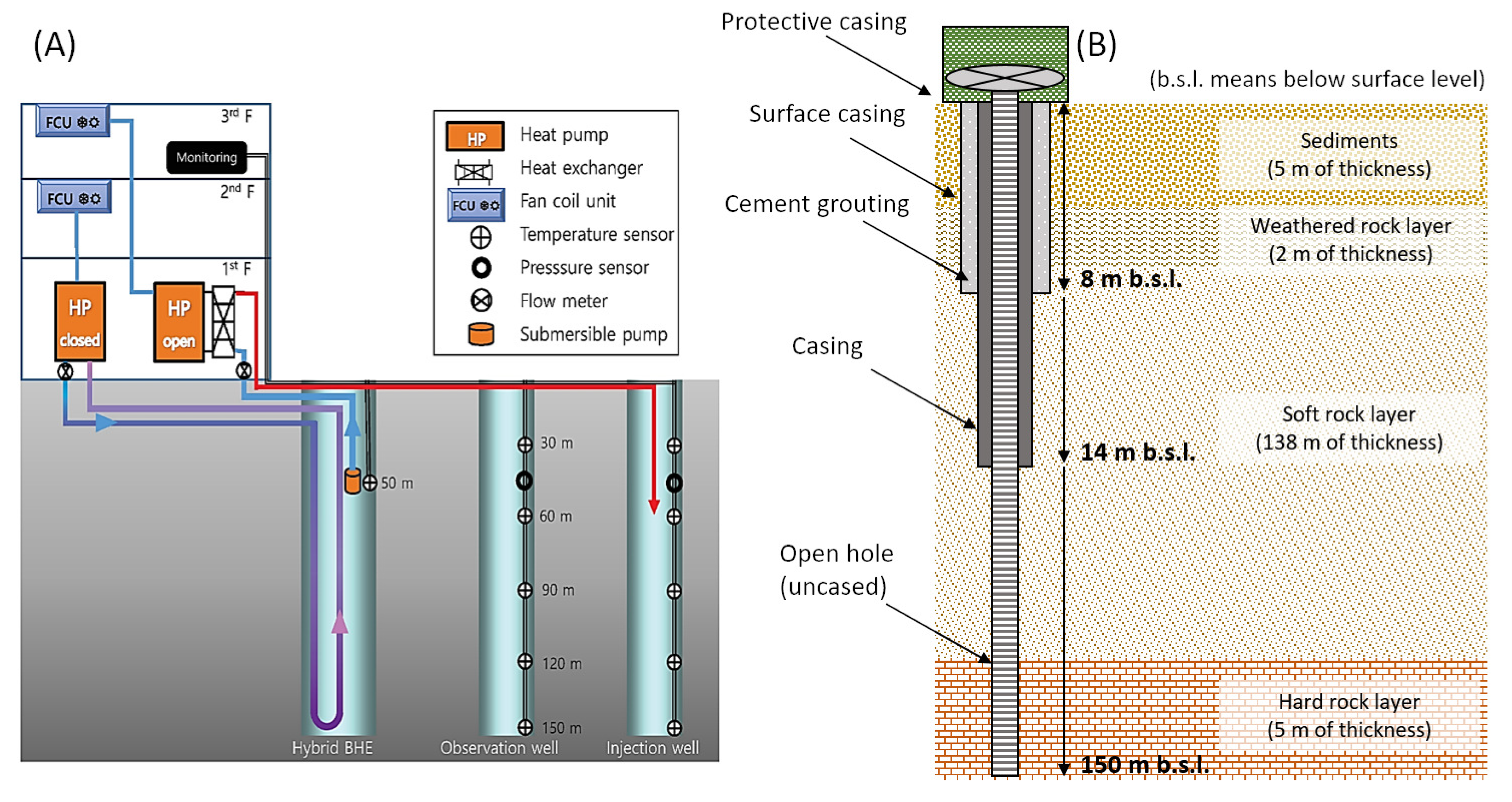
3.2. Hydrothermal Variation Monitoring
4. Results and Discussions
4.1. Temperature Variation of the Aquifer
4.2. Field Measurements and Hydrochemical Composition
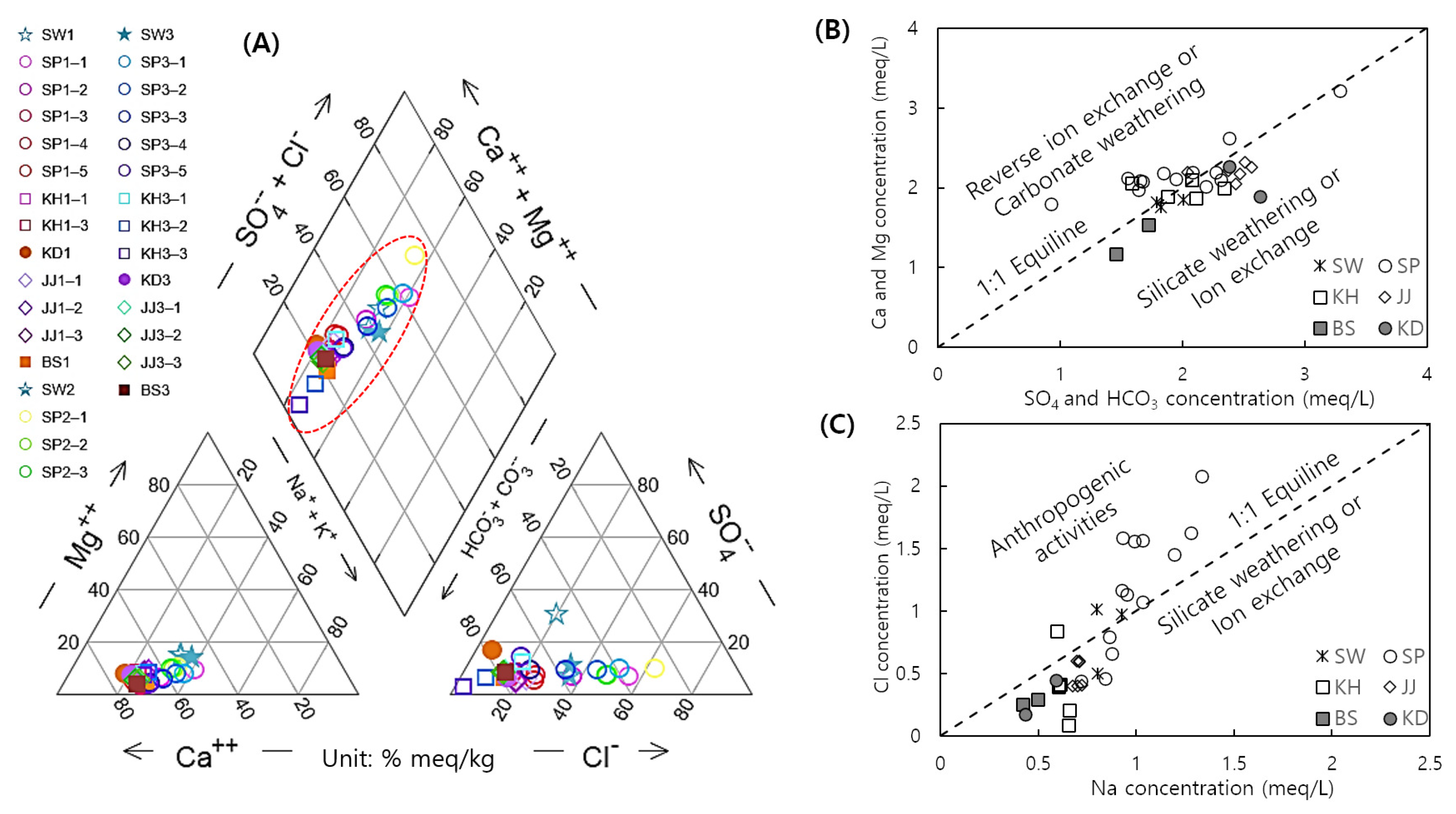
4.3. Source of the Solute and Mixing
4.4. Mixing Ratio (Cl, Na, and Sr)
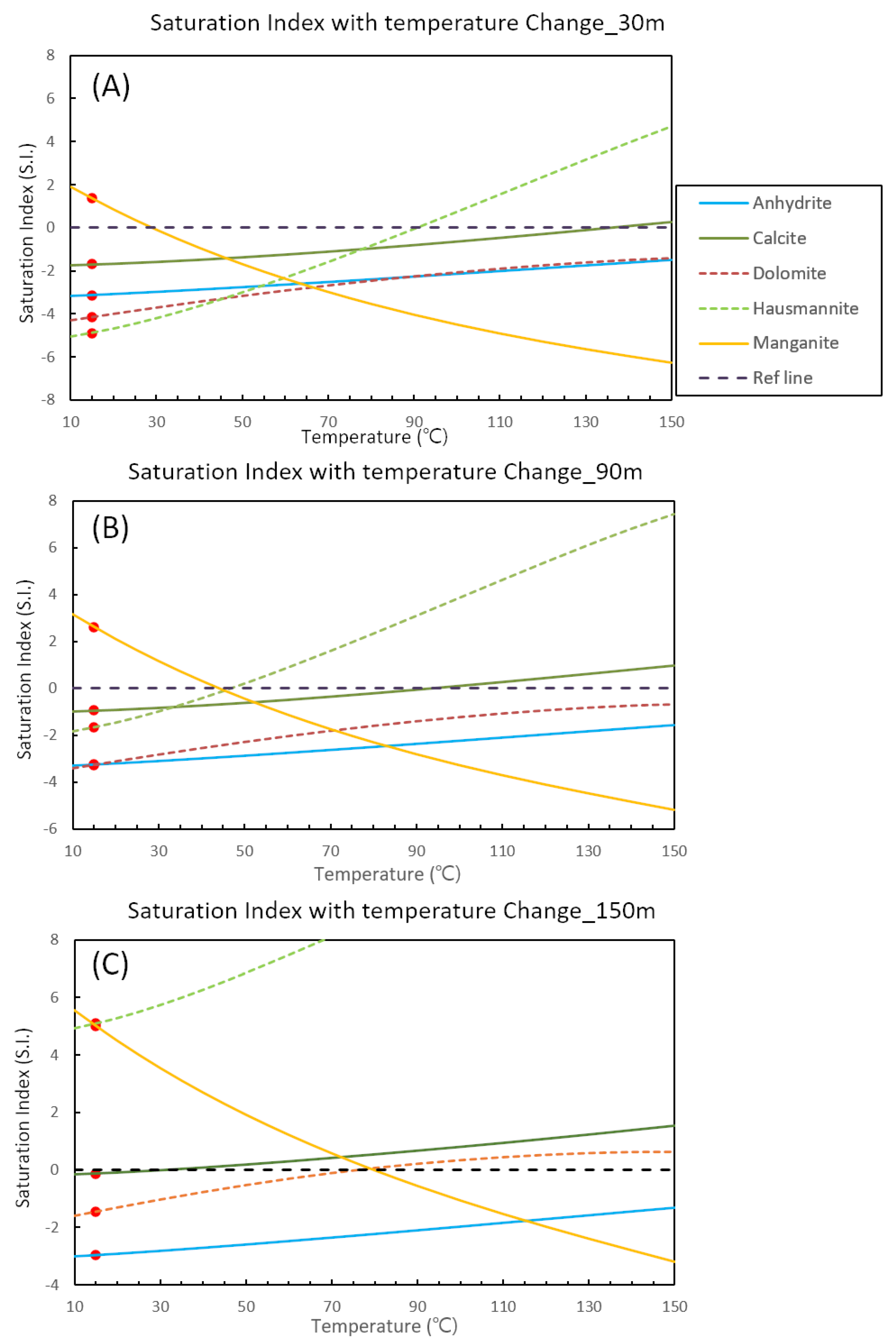
4.5. Variation of Saturation Index with Increasing Temperature
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| GWHP | Groundwater Heat Pump |
| GSHP | Ground Source Heat Pump |
| ATES | Aquifer Thermal Energy Storage |
| BHE | Borehole Heat Exchanger |
| LSI | Langelier Saturation Index |
| RSI | Ryznar Stability Index |
Appendix A
| Site | Altitude (MASL) | Depth to Water (m) | Groundwater Level (m) | ||||
|---|---|---|---|---|---|---|---|
| January 2019 | February 2019 | June 2019 | January 2019 | February 2019 | June 2019 | ||
| SP | 46 | 5.52 | 15.2 | 5.58 | 40.48 | 30.8 | 40.42 |
| KH | 49 | 6.04 | 2.58 | 42.96 | 46.42 | ||
| KD | 53 | 2.26 | - | 50.74 | - | ||
| JJ | 48 | 5.24 | 5.64 | 42.76 | 42.36 | ||
| BS | 46 | 3.11 | 3.43 | 42.89 | 42.57 | ||
| SW | 45 | 0 | 0 | 0 | 45 | 45 | |
References
- Cuthbert, M.; Gleeson, T.; Moosdorf, N.; Befus, K.; Schneider, A.; Hartmann, J.; Lehner, B. Global patterns and dynamics of climate–Groundwater interactions. Nat. Clim. Chang. 2019, 9, 137–141. [Google Scholar] [CrossRef]
- Benz, S.A.; Bayer, P.; Blum, P. Global patterns of shallow groundwater temperatures. Environ. Res. Lett. 2017, 12, 034005. [Google Scholar] [CrossRef]
- Sørensen, B. A history of renewable energy technology. Energy Policy 1991, 19, 8–12. [Google Scholar] [CrossRef]
- Kim, J.S.; Song, S.H.; Jeong, G.C.; Cha, J.H. Analysis of Economic Feasibility and Reductions of Carbon Dioxide Emission of Geothermal Heating and Cooling System using Groundwater. J. Eng. Geol. 2015, 25, 599–612. [Google Scholar] [CrossRef]
- Karytsas, S.; Choropanitis, I. Barriers against and actions towards renewable energy technologies diffusion: A Principal Component Analysis for residential ground source heat pump (GSHP) systems. Renew. Sustain. Energy Rev. 2017, 78, 252–271. [Google Scholar] [CrossRef]
- Savaresi, A. The Paris Agreement: A new beginning? J. Energy Nat. Resour. Law 2016, 34, 16–26. [Google Scholar] [CrossRef] [Green Version]
- Blum, P.; Campillo, G.; Münch, W.; Kölbel, T. CO2 savings of ground source heat pump systems—A regional analysis. Renew. Energy 2010, 35, 122–127. [Google Scholar] [CrossRef]
- Bayer, P.; Saner, D.; Bolay, S.; Rybach, L.; Blum, P. Greenhouse gas emission savings of ground source heat pump systems in Europe: A review. Renew. Sustain. Energy Rev. 2012, 16, 1256–1267. [Google Scholar] [CrossRef]
- Müller, J.; Galgaro, A.; Dalla Santa, G.; Cultrera, M.; Karytsas, C.; Mendrinos, D.; Pera, S.; Perego, R.; O’Neill, N.; Pasquali, R.; et al. Generalized Pan-European Geological Database for Shallow Geothermal Installations. Geosciences 2018, 8, 32. [Google Scholar] [CrossRef] [Green Version]
- Rybach, L.; Eugster, W.J. Sustainability aspects of geothermal heat pump operation, with experience from Switzerland. Geothermics 2010, 39, 365–369. [Google Scholar] [CrossRef]
- Rees, S. Advances in Ground-Source Heat Pump Systems; Woodhead Publishing: Sawston, UK, 2016. [Google Scholar]
- Chiasson, A.D. Geothermal Heat Pump and Heat Engine Systems: Theory and Practice; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Busby, J.; Lewis, M.; Reeves, H.; Lawley, R. Initial geological considerations before installing ground source heat pump systems. Q. J. Eng. Geol. Hydrogeol. 2009, 42, 295–306. [Google Scholar] [CrossRef]
- García-Gil, A.; Vázquez-Suñe, E.; Schneider, E.G.; Sánchez-Navarro, J.Á.; Mateo-Lázaro, J. Relaxation factor for geothermal use development–Criteria for a more fair and sustainable geothermal use of shallow energy resources. Geothermics 2015, 56, 128–137. [Google Scholar] [CrossRef]
- Liu, X.; Hughes, P.; Spitler, J.; Anderson, A. Updated assessment of the technical potential of geothermal heat pump applications in the United States. In Proceedings of the IGSHPA Technical/Research Conference and Expo 2017; International Ground Source Heat Pump Association: Stillwater, OK, USA, 2017. [Google Scholar]
- Lund, J.W.; Boyd, T.L. Direct utilization of geothermal energy 2015 worldwide review. Geothermics 2016, 60, 66–93. [Google Scholar] [CrossRef]
- Zheng, K.; Mo, Y.; Chen, L. Twenty years of geothermal heat pumps in China. In Proceedings of the World Geothermal Congress 2015, Melbourne, Australia, 16–24 April 2015. [Google Scholar]
- Abesser, C.; Lewis, M.A.; Marchant, A.P.; Hulbert, A.G. Mapping suitability for open-loop ground source heat pump systems: A screening tool for England and Wales, UK. Q. J. Eng. Geol. Hydrogeol. 2014, 47, 373–380. [Google Scholar] [CrossRef] [Green Version]
- Farabi-Asl, H.; Chapman, A.; Itaoka, K.; Noorollahi, Y. Ground source heat pump status and supportive energy policies in Japan. Energy Procedia 2019, 158, 3614–3619. [Google Scholar] [CrossRef]
- Lee, J.Y. Current status of ground source heat pumps in Korea. Renew. Sustain. Energy Rev. 2009, 13, 1560–1568. [Google Scholar] [CrossRef]
- Lu, Q.; Narsilio, G.A.; Aditya, G.R.; Johnston, I.W. Economic analysis of vertical ground source heat pump systems in Melbourne. Energy 2017, 125, 107–117. [Google Scholar] [CrossRef]
- Santos, A.F.; de Souza, H.J.; Cantao, M.P.; Gaspar, P.D. Analysis of geothermal temperatures for heat pumps application in Paraná (Brasil). Open Eng. 2016, 6. [Google Scholar] [CrossRef]
- Raymond, J.; Malo, M.; Tanguay, D.; Grasby, S.; Bakhteyar, F. Direct utilization of geothermal energy from coast to coast: A review of current applications and research in Canada. In Proceedings of the World Geothermal Congress 2015, Melbourne, Australia, 16–24 April 2015. [Google Scholar]
- Hecht-Méndez, J.; Molina-Giraldo, N.; Blum, P.; Bayer, P. Evaluating MT3DMS for heat transport simulation of closed geothermal systems. Groundwater 2010, 48, 741–756. [Google Scholar] [CrossRef]
- Kim, H.; Nam, Y.; Jeoun, O.; mu Bae, S. Development of a Multi-Well Pairing System for Groundwater Heat Pump Systems. Energies 2018, 11, 3485. [Google Scholar] [CrossRef] [Green Version]
- Haehnlein, S.; Bayer, P.; Blum, P. International legal status of the use of shallow geothermal energy. Renew. Sustain. Energy Rev. 2010, 14, 2611–2625. [Google Scholar] [CrossRef]
- Lee, J.Y.; Won, J.H.; Hahn, J.S. Evaluation of hydrogeologic conditions for groundwater heat pumps: Analysis with data from national groundwater monitoring stations. Geosci. J. 2006, 10, 91. [Google Scholar] [CrossRef]
- Saito, T.; Hamamoto, S.; Ueki, T.; Ohkubo, S.; Moldrup, P.; Kawamoto, K.; Komatsu, T. Temperature change affected groundwater quality in a confined marine aquifer during long-term heating and cooling. Water Res. 2016, 94, 120–127. [Google Scholar] [CrossRef]
- Vienken, T.; Kreck, M.; Dietrich, P. Monitoring the impact of intensive shallow geothermal energy use on groundwater temperatures in a residential neighborhood. Geotherm. Energy 2019, 7, 8. [Google Scholar] [CrossRef]
- Fleuchaus, P.; Godschalk, B.; Stober, I.; Blum, P. Worldwide application of aquifer thermal energy storage—A review. Renew. Sustain. Energy Rev. 2018, 94, 861–876. [Google Scholar] [CrossRef]
- Rad, F.M.; Fung, A.S.; Rosen, M.A. An integrated model for designing a solar community heating system with borehole thermal storage. Energy Sustain. Dev. 2017, 36, 6–15. [Google Scholar] [CrossRef]
- Hähnlein, S.; Bayer, P.; Ferguson, G.; Blum, P. Sustainability and policy for the thermal use of shallow geothermal energy. Energy Policy 2013, 59, 914–925. [Google Scholar] [CrossRef]
- Belitz, K.; Jurgens, B.; Johnson, T. Potential Corrosivity of Untreated Groundwater in the United States; Scientific Investigations Report 2016-5092; US Geological Survey: Reston, VA, USA, 2016. [Google Scholar]
- Brons, H.; Griffioen, J.; Appelo, C.; Zehnder, A. (Bio) geochemical reactions in aquifer material from a thermal energy storage site. Water Res. 1991, 25, 729–736. [Google Scholar] [CrossRef]
- Ferguson, G. Unfinished business in geothermal energy. GroundWater 2009, 47, 167. [Google Scholar] [CrossRef]
- Bonte, M. Impacts of Shallow Geothermal Energy on Groundwater Quality; Iwa Publishing: London, UK, 2015. [Google Scholar]
- Bonte, M.; Stuyfzand, P.; Van den Berg, G.; Hijnen, W. Effects of aquifer thermal energy storage on groundwater quality and the consequences for drinking water production: A case study from the Netherlands. Water Sci. Technol. 2011, 63, 1922–1931. [Google Scholar] [CrossRef] [Green Version]
- Sowers, L.; York, K.P.; Stiles, L. Impact of Thermal Buildup on Groundwater Chemistry and Aquifer Microbes. Proceedings of Ecostock. 2006. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.572.673&rep=rep1&type=pdf (accessed on 13 May 2020).
- Zuurbier, K.G.; Hartog, N.; Valstar, J.; Post, V.E.; van Breukelen, B.M. The impact of low-temperature seasonal aquifer thermal energy storage (SATES) systems on chlorinated solvent contaminated groundwater: Modeling of spreading and degradation. J. Contam. Hydrol. 2013, 147, 1–13. [Google Scholar] [CrossRef]
- Possemiers, M.; Huysmans, M.; Batelaan, O. Influence of Aquifer Thermal Energy Storage on groundwater quality: A review illustrated by seven case studies from Belgium. J. Hydrol. Reg. Stud. 2014, 2, 20–34. [Google Scholar] [CrossRef]
- Freedman, V.L.; Waichler, S.R.; Mackley, R.D.; Horner, J.A. Assessing the thermal environmental impacts of an groundwater heat pump in southeastern Washington State. Geothermics 2012, 42, 65–77. [Google Scholar] [CrossRef]
- Griffioen, J.; Appelo, C.A.J. Nature and extent of carbonate precipitation during aquifer thermal energy storage. Appl. Geochem. 1993, 8, 161–176. [Google Scholar] [CrossRef]
- Jesußek, A.; Grandel, S.; Dahmke, A. Impacts of subsurface heat storage on aquifer hydrogeochemistry. Environ. Earth Sci. 2013, 69, 1999–2012. [Google Scholar] [CrossRef]
- Holm, T.R.; Eisenreich, S.J.; Rosenberg, H.L.; Holm, N.P. Groundwater geochemistry of short-term aquifer thermal energy storage test cycles. Water Resour. Res. 1987, 23, 1005–1019. [Google Scholar] [CrossRef]
- Perlinger, J.A.; Almendinger, J.E.; Urban, N.R.; Eisenreich, S.J. Groundwater geochemistry of aquifer thermal energy storage: Long-term test cycle. Water Resour. Res. 1987, 23, 2215–2226. [Google Scholar] [CrossRef]
- Bonte, M. Impacts of Shallow Geothermal Energy on Groundwater Quality: A Hydrochemical and Geomicrobial Study of the Effects of Ground Source Heat Pumps and Aquifer Thermal Energy Storage. Ph.D. Thesis, Vrije Universiteit, Amsterdam, The Netherlands, 2013. [Google Scholar]
- Rafferty, K. Scaling in Geothermal Heat Pump Systems; Geo-Heat Center: Klamath Falls, OR, USA, 1999. [Google Scholar]
- Dehghani, M.; Kashtgar, L.; Davoodi, S.; Shamsedini, N.; Zaravar, F. Data on the trend of corrosivity and scale formation potential of Shiraz groundwater drinking water resources during 2001–2007. Data Brief 2019, 23, 103736. [Google Scholar] [CrossRef]
- Watzlaf, G.R.; Ackman, T.E. Underground mine water for heating and cooling using geothermal heat pump systems. Mine Water Environ. 2006, 25, 1–14. [Google Scholar] [CrossRef]
- Abesser, C. Open-Loop Ground Source Heat Pumps and Groundwater Systems: A Literature Review of Current Applications, Regulations and Problems; British Geological Survey: Nottingham, UK, 2010; 23p. [Google Scholar]
- Park, Y.; Kim, N.; Lee, J.Y. Geochemical properties of groundwater affected by open loop geothermal heat pump systems in Korea. Geosci. J. 2015, 19, 515–526. [Google Scholar] [CrossRef]
- Négrel, P.; Fouillac, C.; Brach, M. A strontium isotopic study of mineral and surface waters from the Cézallier (Massif Central, France): Implications for mixing processes in areas of disseminated emergences of mineral waters. Chem. Geol. 1997, 135, 89–101. [Google Scholar] [CrossRef]
- Christensen, J.N.; Dafflon, B.; Shiel, A.E.; Tokunaga, T.K.; Wan, J.; Faybishenko, B.; Dong, W.; Williams, K.H.; Hobson, C.; Brown, S.T.; et al. Using strontium isotopes to evaluate the spatial variation of groundwater recharge. Sci. Total Environ. 2018, 637, 672–685. [Google Scholar] [CrossRef] [Green Version]
- Marques, J.; Carreira, P.; Goff, F.; Eggenkamp, H.; da Silva, M.A. Input of 87Sr/86Sr ratios and Sr geochemical signatures to update knowledge on thermal and mineral waters flow paths in fractured rocks (N-Portugal). Appl. Geochem. 2012, 27, 1471–1481. [Google Scholar] [CrossRef]
- Shand, P.; Darbyshire, D.; Love, A.; Edmunds, W. Sr isotopes in natural waters: Applications to source characterisation and water–rock interaction in contrasting landscapes. Appl. Geochem. 2009, 24, 574–586. [Google Scholar] [CrossRef]
- Casasso, A.; Sethi, R. Assessment and Minimization of Potential Environmental Impacts of Ground Source Heat Pump (GSHP) Systems. Water 2019, 11, 1573. [Google Scholar] [CrossRef] [Green Version]
- Holm, T.R.; Lu, X.; Larson, D.R. Feasibility of Groundwater Source Heat Pumps for Space Heating and Cooling in Mason County and the American Bottoms Area, Illinois; Technical Report; Illinois Sustainable Technology Center: Champaign, IL, USA, 2015. [Google Scholar]
- Park, H.; Lee, J.; Cheong, J. Explanatory Text of the Geological Map of Yuseong Sheet; Korea Research Institute of Geoscience and Mineral Resources: Daejeon, Korea, 1977. [Google Scholar]
- Appelo, C.A.J.; Postma, D. Geochemistry, Groundwater and Pollution; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Rajesh, R.; Brindha, K.; Murugan, R.; Elango, L. Influence of hydrogeochemical processes on temporal changes in groundwater quality in a part of Nalgonda district, Andhra Pradesh, India. Environ. Earth Sci. 2012, 65, 1203–1213. [Google Scholar] [CrossRef]
- Sajil Kumar, P.; James, E. Identification of hydrogeochemical processes in the Coimbatore district, Tamil Nadu, India. Hydrol. Sci. J. 2016, 61, 719–731. [Google Scholar] [CrossRef]
- Karunanidhi, D.; Aravinthasamy, P.; Deepali, M.; Subramani, T.; Roy, P.D. The effects of geochemical processes on groundwater chemistry and the health risks associated with fluoride intake in a semi-arid region of South India. RSC Adv. 2020, 10, 4840–4859. [Google Scholar] [CrossRef] [Green Version]
- Raiber, M.; Webb, J.A.; Bennetts, D.A. Strontium isotopes as tracers to delineate aquifer interactions and the influence of rainfall in the basalt plains of southeastern Australia. J. Hydrol. 2009, 367, 188–199. [Google Scholar] [CrossRef]
- Cheong, W.S.; Kim, Y.S.; Na, K.C. SHRIMP zircon U-Pb geochronology, geochemistry and Sr-Nd isotopic study of the Cheongju granitoid rocks. J. Petrol. Soc. Korea 2011, 20, 191–206. [Google Scholar] [CrossRef] [Green Version]
- Harrington, G.; Herczeg, A. The importance of silicate weathering of a sedimentary aquifer in arid Central Australia indicated by very high 87Sr/86Sr ratios. Chem. Geol. 2003, 199, 281–292. [Google Scholar] [CrossRef]
- Bullen, T.D.; Krabbenhoft, D.P.; Kendall, C. Kinetic and mineralogic controls on the evolution of groundwater chemistry and 87Sr/86Sr in a sandy silicate aquifer, northern Wisconsin, USA. Geochim. Cosmochim. Acta 1996, 60, 1807–1821. [Google Scholar] [CrossRef]
- Jeon, S.R.; Nakano, T. Geochemical comparison of stream water, rain water, and watershed geology in Central Korea. Water Air Soil Pollut. 2001, 130, 739–744. [Google Scholar] [CrossRef]
- Walter, J.; Chesnaux, R.; Cloutier, V.; Gaboury, D. The influence of water/rock- water/clay interactions and mixing in the salinization processes of groundwater. J. Hydrol. Reg. Stud. 2017, 13, 168–188. [Google Scholar] [CrossRef]
- Kouadra, R.; Demdoum, A.; Chabour, N.; Benchikh, R. The use of hydrogeochemical analyses and multivariate statistics for the characterization of thermal springs in the Constantine area, Northeastern Algeria. Acta Geochim. 2019, 38, 292–306. [Google Scholar] [CrossRef]
- Ogrinc, N.; Tamše, S.; Zavadlav, S.; Vrzel, J.; Jin, L. Evaluation of geochemical processes and nitrate pollution sources at the Ljubljansko polje aquifer (Slovenia): A stable isotope perspective. Sci. Total Environ. 2019, 646, 1588–1600. [Google Scholar] [CrossRef]
- Gunnlaugsson, E.; Ármannsson, H.; Thorhallsson, S.; Steingrímsson, B. Problems in Geothermal Operation–Scaling and Corrosion; Goethermal Training Program: Iceland, 2014; pp. 1–18. [Google Scholar]
- Boch, R.; Leis, A.; Haslinger, E.; Goldbrunner, J.E.; Mittermayr, F.; Fröschl, H.; Hippler, D.; Dietzel, M. Scale-fragment formation impairing geothermal energy production: Interacting H2S corrosion and CaCO3 crystal growth. Geotherm. Energy 2017, 5, 4. [Google Scholar] [CrossRef] [Green Version]
- Ryznar, J.W. A new index for determining amount of calcium carbonate scale formed by a water. J. Am. Water Works Assoc. 1944, 36, 472–483. [Google Scholar] [CrossRef]
- Allison, J.D.; Brown, D.S.; Novo-Gradac, K.J. MINTEQA2/PRODEFA2, a Geochemical Assessment Model for Environmental Systems: Version 3.0 User’s Manual; US Environmental Protection Agency: Washington, DC, USA, 1991.
- Parkhurst, D.L.; Appelo, C. Description of Input and Examples for PHREEQC Version 3: A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; Technical Report; US Geological Survey: Reston, VA, USA, 2013. [Google Scholar]




| Condition | ID | Depth | pH | EC | DO | Ca | Mg | Na | K | SiO2 | Sr | Fe | Mn | HCO3 | F | Cl | Br | NO3 | SO4 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (m) | S/cm | mg/L | (mg/L) | (g/L) | mg/L | ||||||||||||||
| Before heating operation January 2019 | SW1 | - | 7.97 | 317 | 12.5 | 28.8 | 5.03 | 18.5 | 4.90 | 5.95 | 224 | BDL * | BDL | 75.9 | 0.40 | 17.8 | 0.98 | 7.86 | 36.8 |
| SP1-1 | 30 | 6.40 | 395 | 9.06 | 33.6 | 3.66 | 30.7 | 2.24 | 14.4 | 314 | 4.46 | 2.56 | 85.5 | 0.26 | 73.7 | 0.19 | 10.2 | 11.4 | |
| SP1-2 | 60 | 6.92 | 344 | 4.10 | 38.6 | 2.32 | 21.4 | 1.24 | 13.7 | 183 | 1.74 | 2.33 | 107 | 0.92 | 41.2 | 0.58 | 8.57 | 9.44 | |
| SP1-3 | 90 | 7.64 | 279 | 3.72 | 38.9 | 1.00 | 16.5 | 0.74 | 12.0 | 111 | 3.38 | 1.29 | 124 | 1.09 | 15.6 | 0.86 | 6.79 | 7.51 | |
| SP1-4 | 120 | 7.52 | 359 | 6.95 | 50.0 | 1.62 | 19.8 | 1.21 | 12.0 | 164 | 0.83 | 3.63 | 132 | 0.85 | 28.2 | 0.68 | 7.74 | 10.4 | |
| SP1-5 | 150 | 7.40 | 412 | 7.95 | 60.7 | 2.36 | 21.9 | 1.85 | 12.4 | 220 | 2.21 | 15.4 | 187 | 0.86 | 40.2 | 0.41 | 7.06 | 10.5 | |
| KH1-1 | 30 | 7.19 | 306 | 5.91 | 37.8 | 2.61 | 13.9 | 1.11 | 10.9 | 115 | BDL | BDL | 114 | 0.84 | 13.9 | 0.92 | 1.75 | 10.1 | |
| KH1-2 | 60 | 7.23 | 302 | 3.61 | 37.1 | 2.59 | 13.6 | 1.15 | 10.2 | 115 | BDL | BDL | 67.9 | 1.34 | 29.8 | 1.98 | 3.05 | 22.8 | |
| KH1-3 | 120 | 7.42 | 305 | 2.58 | 37.9 | 2.63 | 13.9 | 1.13 | 11.9 | 118 | BDL | BDL | 114 | 0.91 | 14.3 | 0.90 | 2.20 | 10.2 | |
| KD1 | - | 7.65 | 281 | 20.4 | 34.7 | 2.08 | 10.0 | 0.89 | 11.8 | 157 | BDL | BDL | 133 | 0.69 | 6.07 | 0.07 | 5.04 | 22.0 | |
| JJ1-1 | 30 | 7.48 | 359 | 4.50 | 39.4 | 2.79 | 16.0 | 1.15 | 11.7 | 180 | 75.6 | 34.2 | 115 | 1.00 | 21.2 | 0.05 | 5.50 | 7.19 | |
| JJ1-2 | 60 | 7.34 | 325 | 4.53 | 36.5 | 2.85 | 16.4 | 1.27 | 10.6 | 175 | 191 | 43.1 | 141 | 0.92 | 21.2 | 0.04 | 4.90 | 6.45 | |
| JJ1-3 | 120 | 7.22 | 320 | 4.00 | 39.0 | 2.86 | 16.3 | 1.28 | 11.8 | 186 | 202 | 47.1 | 141 | 0.93 | 21.0 | 0.05 | 5.48 | 7.20 | |
| BS1 | 60 | 8.10 | 190 | 6.59 | 22.4 | 0.67 | 9.72 | 1.31 | 8.23 | 127 | 39.9 | 20.0 | 82.7 | 0.77 | 8.99 | ND ** | 2.39 | 4.98 | |
| Heating operation February 2019 | SW2 | - | 8.21 | 500 | 12.5 | 28.1 | 5.07 | 18.4 | 4.88 | 7.43 | 223 | BDL | BDL | 97.1 | 0.17 | 36.0 | 0.03 | 13.4 | 9.45 |
| SP2-1 | 60 | 6.77 | 355 | 8.76 | 30.7 | 3.30 | 21.4 | 1.85 | 13.2 | 251 | 14.9 | 3.55 | 42.4 | 0.23 | 56.2 | 0.30 | 9.64 | 11.2 | |
| SP2-2 | 90 | 6.60 | 352 | 8.75 | 35.8 | 3.71 | 23.7 | 1.96 | 14.9 | 288 | BDL | BDL | 86.6 | 0.23 | 55.5 | 0.30 | 9.55 | 11.1 | |
| SP2-3 | 120 | 6.46 | 380 | 8.97 | 35.8 | 3.67 | 22.7 | 1.94 | 14.6 | 292 | BDL | BDL | 88.2 | 0.31 | 55.2 | 0.31 | 9.52 | 11.1 | |
| Before cooling operation June 2019 | SW3 | - | 7.63 | 310 | 9.32 | 27.5 | 4.79 | 21.3 | 5.99 | 6.47 | 200 | BDL | BDL | 92.6 | 0.38 | 34.5 | BDL | 9.78 | 14.7 |
| SP3-1 | 30 | 6.29 | 376 | 3.98 | 37.2 | 3.24 | 29.5 | 1.80 | 14.7 | 260 | BDL | BDL | 75.9 | 0.43 | 57.6 | 0.34 | 9.73 | 14.7 | |
| SP3-2 | 60 | 6.32 | 369 | 3.77 | 38.9 | 3.02 | 27.5 | 1.67 | 14.6 | 240 | BDL | BDL | 94.2 | 0.54 | 51.6 | 0.38 | 9.25 | 14.4 | |
| SP3-3 | 90 | 6.52 | 343 | 2.64 | 40.3 | 2.24 | 23.7 | 1.34 | 13.4 | 190 | BDL | BDL | 110 | 0.84 | 38.0 | 0.48 | 8.19 | 13.5 | |
| SP3-4 | 120 | 6.80 | 313 | 2.39 | 41.6 | 1.52 | 20.1 | 1.01 | 12.3 | 150 | BDL | BDL | 123 | 1.08 | 23.4 | 0.58 | 7.77 | 12.5 | |
| SP3-5 | 150 | 5.44 | 328 | 1.79 | 40.3 | 1.21 | 19.3 | 0.85 | 11.7 | 130 | 310 | BDL | 118 | 2.61 | 16.2 | 0.60 | 3.55 | 18.7 | |
| KH3-1 | 30 | 7.14 | 267 | 5.69 | 34.2 | 2.37 | 14.1 | 0.98 | 15.8 | 110 | BDL | BDL | 97.9 | 0.84 | 14.6 | 0.77 | 6.71 | 13.3 | |
| KH3-2 | 60 | 6.80 | 285 | 3.94 | 33.5 | 2.47 | 15.2 | 1.09 | 15.1 | 110 | BDL | BDL | 120 | 0.41 | 7.25 | 0.38 | 3.32 | 6.58 | |
| KH3-3 | 120 | 6.99 | 269 | 6.72 | 36.2 | 2.34 | 15.1 | 1.10 | 15.7 | 110 | BDL | BDL | 139 | 0.17 | 2.94 | 0.18 | 1.11 | 2.66 | |
| KD3 | 7.81 | 278 | 0.30 | 41.6 | 2.44 | 13.6 | 1.08 | 12.1 | 180 | BDL | BDL | 135 | 0.61 | 15.9 | BDL | 0.01 | 8.20 | ||
| JJ3-1 | 30 | 7.44 | 331 | 3.90 | 41.5 | 1.87 | 15.6 | 1.08 | 13.2 | 180 | BDL | BDL | 131 | 1.13 | 14.2 | BDL | 5.28 | 11.0 | |
| JJ3-2 | 60 | 7.49 | 292 | 4.33 | 43.4 | 1.89 | 16.2 | 1.08 | 13.3 | 180 | BDL | BDL | 139 | 1.15 | 14.3 | BDL | 5.30 | 11.3 | |
| JJ3-3 | 120 | 7.51 | 329 | 3.13 | 42.2 | 1.91 | 16.6 | 1.09 | 13.2 | 180 | BDL | BDL | 142 | 1.17 | 14.3 | BDL | 5.28 | 11.3 | |
| BS3 | 60 | 8.03 | 213 | 5.22 | 29.5 | 0.86 | 11.5 | 0.69 | 12.7 | 160 | BDL | BDL | 95.2 | 1.06 | 10.5 | BDL | 3.83 | 7.87 |
| End Member (EM) Mixing | Mixing Indicator | Mixing Ratio | Period | ||||
|---|---|---|---|---|---|---|---|
| EM 1 | EM 2 | Target Site | EM 1 | EM 2 | |||
| Scenario 1 | SP1-1 | KH1-2 | SP1-2 | Before heating operation | |||
| Shallow GW | 73.7 | 13.9 | 41.2 | Cl (mg/L) | 45.7 | 54.3 | |
| mixing at | 0.715785 | 0.716644 | 0.716070 | Sr/Sr | 34.2 | 65.8 | |
| 60 m | 0.314231 | 0.115052 | 0.183277 | Sr (mg/L) | |||
| Scenario 2 | SP1-1 | KH1-3 | SP1-4 | ||||
| Deep GW | 73.7 | 14.3 | 28.2 | Cl (mg/L) | 23.4 | 76.6 | |
| mixing at | 0.715785 | 0.716661 | 0.716288 | Sr/Sr | 23.3 | 76.7 | |
| 120 m | 0.314231 | 0.117828 | 0.163594 | Sr (mg/L) | |||
| Scenario 3 | SP2-1 | SP2-3 | SP2-2 | During heating operation | |||
| Vertical mixing | 56.2 | 55.2 | 55.5 | Cl (mg/L) | 30.0 | 70.0 | |
| 0.715884 | 0.715899 | 0.715889 | Sr/Sr | 10.2 | 89.8 | ||
| 0.251343 | 0.291917 | 0.287779 | Sr (mg/L) | ||||
| Scenario 4 | SP3-1 | KH3-2 | SP3-2 | Cl (mg/L) | 88.1 | 11.9 | Before cooling operation |
| Mixing at 60 m | 57.6 | 7.25 | 51.6 | ||||
| Scenario 5 | SP3-1 | KH3-3 | SP3-4 | Cl (mg/L) | 37.4 | 62.6 | |
| Mixing at 120 m | 57.6 | 2.94 | 23.4 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.; Kim, J.; Shim, B.O.; Kim, D.-h. Characterization of Aquifer Hydrochemistry from the Operation of a Shallow Geothermal System. Water 2020, 12, 1377. https://doi.org/10.3390/w12051377
Choi H, Kim J, Shim BO, Kim D-h. Characterization of Aquifer Hydrochemistry from the Operation of a Shallow Geothermal System. Water. 2020; 12(5):1377. https://doi.org/10.3390/w12051377
Chicago/Turabian StyleChoi, Hanna, Jaeyeon Kim, Byoung Ohan Shim, and Dong-hun Kim. 2020. "Characterization of Aquifer Hydrochemistry from the Operation of a Shallow Geothermal System" Water 12, no. 5: 1377. https://doi.org/10.3390/w12051377
APA StyleChoi, H., Kim, J., Shim, B. O., & Kim, D.-h. (2020). Characterization of Aquifer Hydrochemistry from the Operation of a Shallow Geothermal System. Water, 12(5), 1377. https://doi.org/10.3390/w12051377






