Characterizing the Reactivity of Metallic Iron for Water Treatment: H2 Evolution in H2SO4 and Uranium Removal Efficiency
Abstract
1. Introduction
2. Experimental Section
2.1. Iron Materials
2.2. Experimental Methods
2.2.1. Hydrogen Evolution
2.2.2. Uranium Removal
2.3. Analytical Methods
2.4. Expression of the Experimental Results
3. Results and Discussion
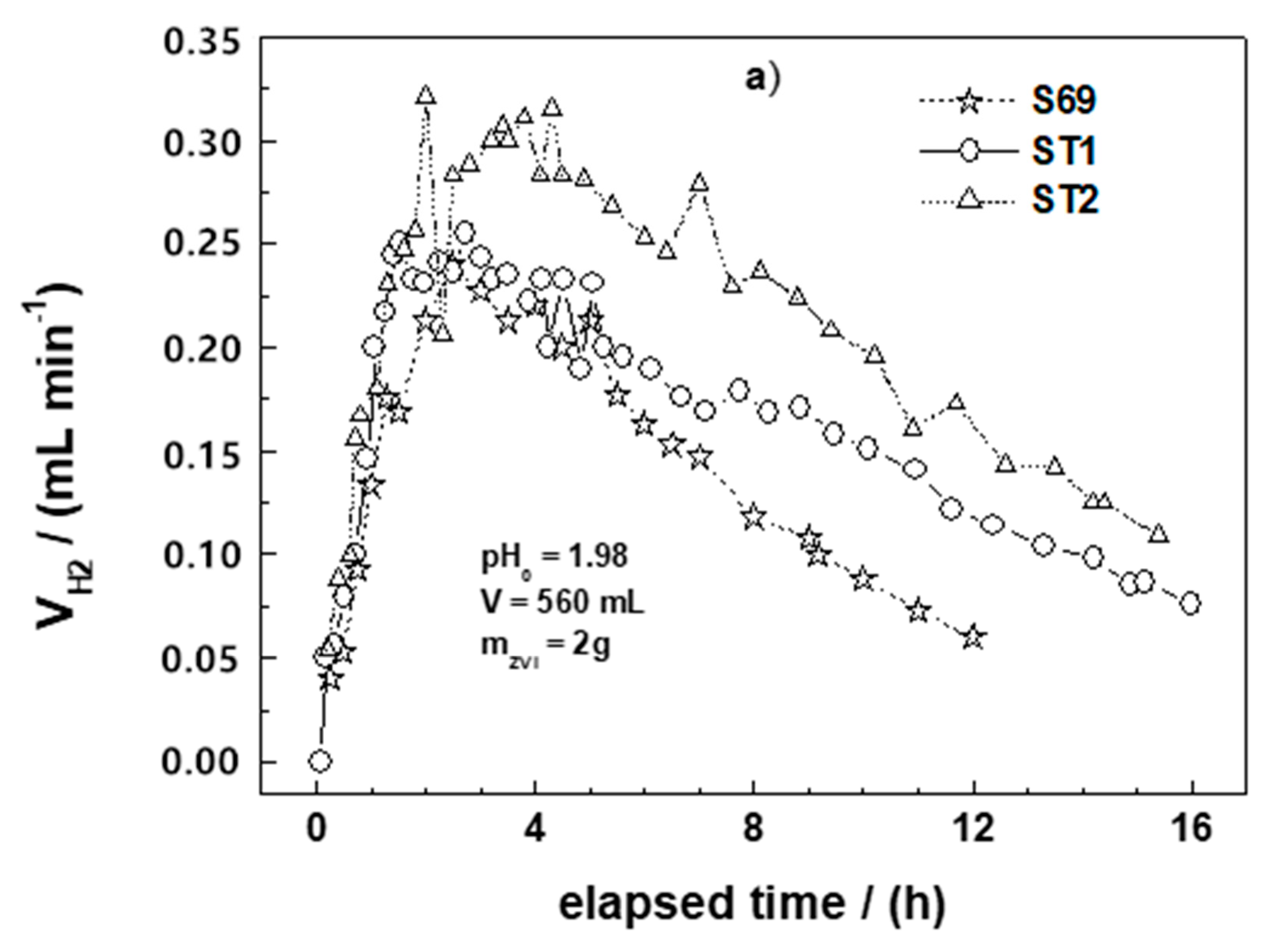
3.1. Molecular Hydrogen Production
3.2. Uranium Removal
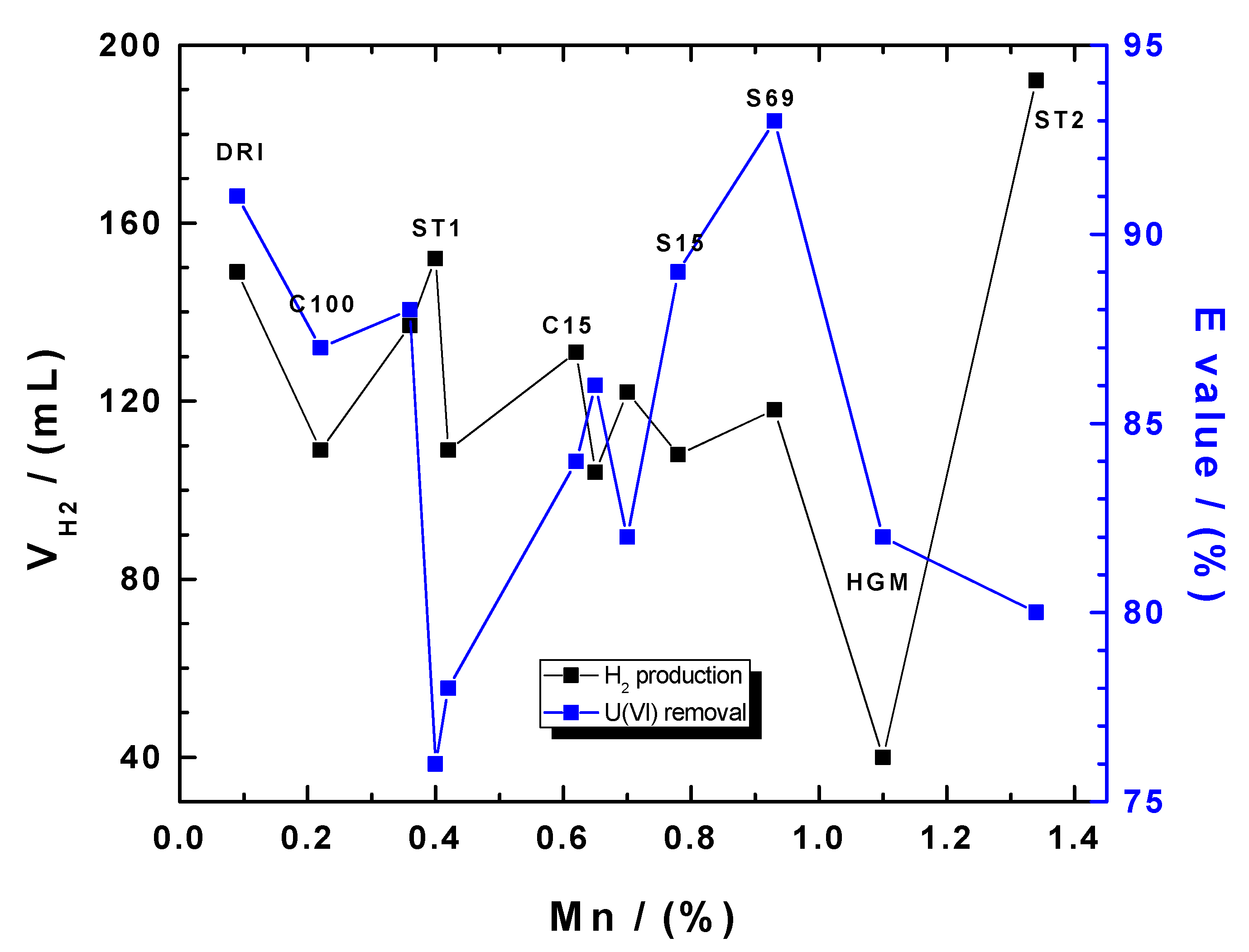
3.3. Comparing H2 Production and Uranium Removal
4. Fe0 Materials for Water Treatment
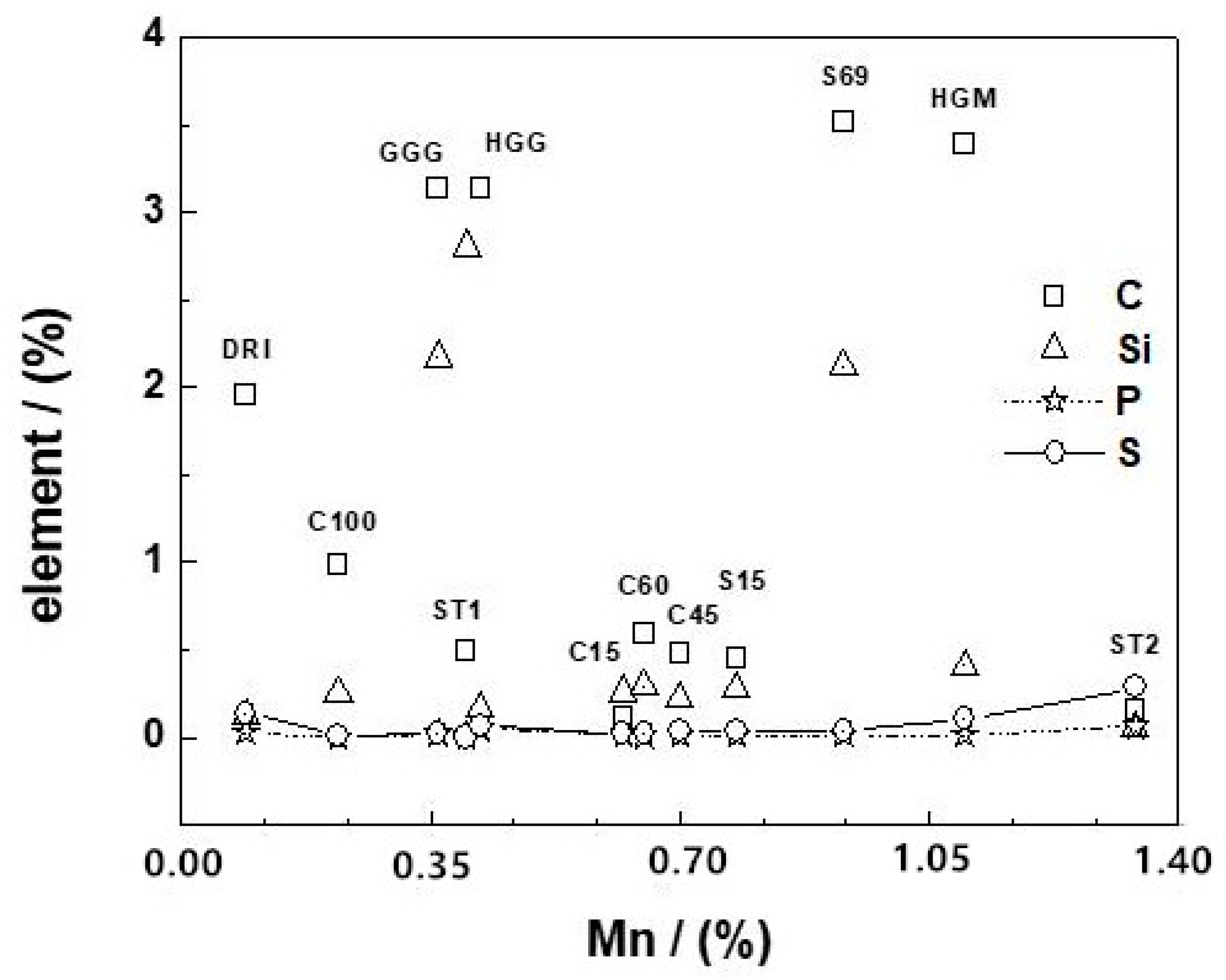
4.1. Fe0 Materials
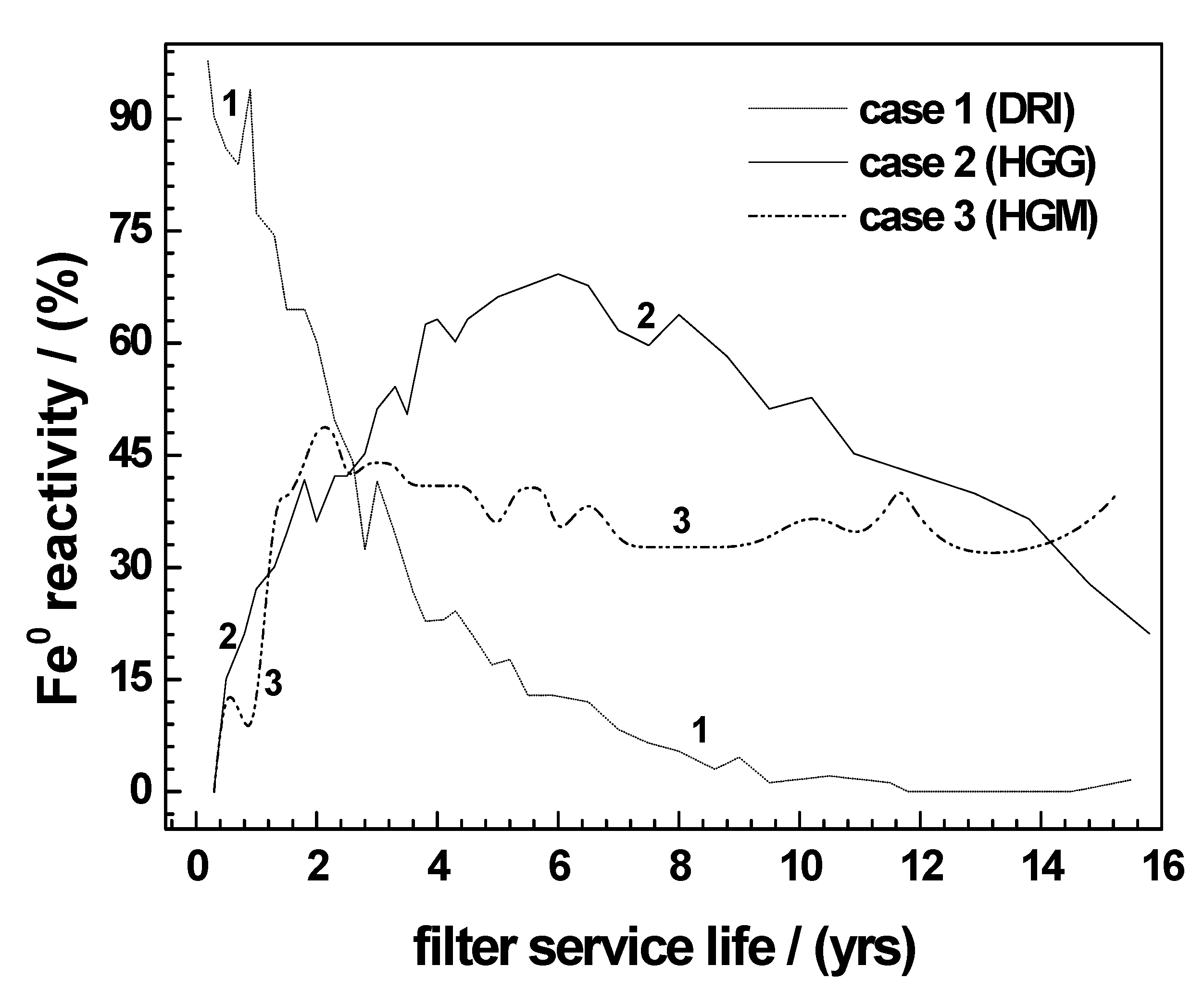
4.2. Long-Term Performance of Fe0 Materials
4.3. Future Perspectives
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Devonshire, E. The purification of water by means of metallic iron. J. Frankl. Inst. 1890, 129, 449–461. [Google Scholar] [CrossRef]
- Brown, G.E., Jr.; Henrich, V.E.; Casey, W.H.; Clark, D.L.; Eggleston, C.; Felmy, A.; Goodman, D.W.; Grätzel, M.; Maciel, G.; McCarthy, M.I.; et al. Metal oxide surfaces and their interactions with aqueous solutions and microbial organisms. Chem. Rev. 1999, 99, 77–174. [Google Scholar] [CrossRef]
- You, Y.; Han, J.; Chiu, P.C.; Jin, Y. Removal and inactivation of waterborne viruses using zerovalent iron. Environ. Sci. Technol. 2005, 39, 9263–9269. [Google Scholar] [CrossRef] [PubMed]
- Bradley, I.; Straub, A.; Maraccini, P.; Markazi, S.; Nguyen, T.H. Iron oxide amended biosand filters for virus removal. Water Res. 2011, 45, 4501–4510. [Google Scholar] [CrossRef] [PubMed]
- Tepong-Tsindé, R.; Ndé-Tchoupé, A.I.; Noubactep, C.; Nassi, A.; Ruppert, H. Characterizing a newly designed steel-wool-based household filter for safe drinking water provision: Hydraulic conductivity and efficiency for pathogen removal. Processes 2019, 7, 966. [Google Scholar] [CrossRef]
- Bischof, G. The Purification of Water: Embracing the Action of Spongy Iron on Impure Water; Bell and Bain: Glasgow, UK, 1873; p. 19. [Google Scholar]
- Lauderdale, R.A.; Emmons, A.H. A method for decontaminating small volumes of radioactive water. J. Am. Water Work. Assoc. 1951, 43, 327–331. [Google Scholar] [CrossRef]
- Gheju, M. Hexavalent chromium reduction with zero-valent iron (ZVI) in aquatic systems. Water Air Soil Pollut. 2011, 222, 103–148. [Google Scholar] [CrossRef]
- Obiri-Nyarko, F.; Grajales-Mesa, S.J.; Malina, G. An overview of permeable reactive barriers for in situ sustainable groundwater remediation. Chemosphere 2014, 111, 243–259. [Google Scholar] [CrossRef]
- Ghauch, A. Iron-based metallic systems: An excellent choice for sustainable water treatment. Freib. Online Geosci. 2015, 38, 1–80. [Google Scholar]
- Guan, X.; Sun, Y.; Qin, H.; Li, J.; Lo, I.M.C.; He, D.; Dong, H. The limitations of applying zero-valent iron technology in contaminants sequestration and the corresponding countermeasures: The development in zero-valent iron technology in the last two decades (1994–2014). Water Res. 2015, 75, 224–248. [Google Scholar] [CrossRef]
- Banerji, T.; Chaudhari, S. A cost-effective technology for arsenic removal: Case study of zerovalent iron-based IIT Bombay arsenic filter in West Bengal. In Water and Sanitation in the New Millennium; Nath, K., Sharma, V., Eds.; Springer: New Delhi, India, 2017. [Google Scholar]
- Wakatsuki, T.; Esumi, H.; Omura, S. High performance and N, P removable on-site domestic wastewater treatment system by multi-soil-layering method. Water Sci. Technol. 1993, 27, 31–40. [Google Scholar] [CrossRef]
- Hering, J.G.; Maag, S.; Schnoor, J.L. A call for synthesis of water research to achieve the sustainable development goals by 2030. Environ. Sci. Technol. 2016, 50, 6122–6123. [Google Scholar] [CrossRef] [PubMed]
- Nanseu-Njiki, C.P.; Gwenzi, W.; Pengou, M.; Rahman, M.A.; Noubactep, C. Fe0/H2O filtration systems for decentralized safe drinking water: Where to from here? Water 2019, 11, 429. [Google Scholar] [CrossRef]
- Hu, R.; Yang, H.; Tao, R.; Cui, X.; Xiao, M.; Amoah, B.K.; Cao, V.; Lufingo, M.; Soppa-Sangue, N.P.; Ndé-Tchoupé, A.I.; et al. Metallic iron for environmental remediation: Starting an overdue progress in knowledge. Water 2020, 12, 641. [Google Scholar] [CrossRef]
- Btatkeu-K, B.D.; Miyajima, K.; Noubactep, C.; Caré, S. Testing the suitability of metallic iron for environmental remediation: Discoloration of methylene blue in column studies. Chem. Eng. J. 2013, 215–216, 959–968. [Google Scholar] [CrossRef]
- Kim, H.; Yang, H.; Kim, J. Standardization of the reducing power of zero-valent iron using iodine. J. Environ. Sci. Health Part A 2014, 49, 514–523. [Google Scholar] [CrossRef]
- Li, S.; Ding, Y.; Wang, W.; Lei, H. A facile method for determining the Fe0 content and reactivity of zero-valent iron. Anal. Methods 2016, 8, 1239–1248. [Google Scholar] [CrossRef]
- Hu, R.; Cui, X.; Xiao, M.; Qiu, P.; Lufingo, M.; Gwenzi, W.; Noubactep, C. Characterizing the suitability of granular Fe0 for the water treatment industry. Processes 2019, 7, 652. [Google Scholar] [CrossRef]
- Li, J.; Dou, X.; Qin, H.; Sun, Y.; Yin, D.; Guan, X. Characterization methods of zerovalent iron for water treatment and remediation. Water Res. 2019, 148, 70–85. [Google Scholar] [CrossRef]
- Lufingo, M.; Ndé-Tchoupé, A.I.; Hu, R.; Njau, K.N.; Noubactep, C. A novel and facile method to characterize the suitability of metallic iron for water treatment. Water 2019, 11, 2465. [Google Scholar] [CrossRef]
- Hildebrant, B.; Ndé-Tchoupé, A.I.; Lufingo, M.; Licha, T.; Noubactep, C. Steel wool for water treatment: Intrinsic reactivity and defluoridation efficiency. Processes 2020, 8, 265. [Google Scholar] [CrossRef]
- Lavine, B.K.; Auslander, G.; Ritter, J. Polarographic studies of zero valent iron as a reductant for remediation of nitroaromatics in the environment. Microchem. J. 2001, 70, 69–83. [Google Scholar] [CrossRef]
- Furukawa, Y.; Kim, J.-W.; Watkins, J.; Wilkin, R.T. Formation of ferrihydrite and associated iron corrosion products in permeable reactive barriers of zero-valent iron. Environ. Sci. Technol. 2002, 36, 5469–5475. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Bailey, E.H.; Mooney, S.J. Quantification of changes in zero valent iron morphology using X-ray computed tomography. J. Environ. Sci. 2013, 25, 2344–2351. [Google Scholar] [CrossRef]
- Btatkeu-K, B.D.; Tchatchueng, J.B.; Noubactep, C.; Caré, S. Designing metallic iron based water filters: Light from methylene blue discoloration. J. Environ. Manag. 2016, 166, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C. Metallic iron for environmental remediation: Prospects and limitations. In A Handbook of Environmental Toxicology: Human Disorders and Ecotoxicology; D’Mello, J.P.F., Ed.; CAB International: Wallingford, UK, 2020; Chapter 36; pp. 531–544. [Google Scholar]
- Noubactep, C. A framework for technology development in Africa: The case of metallic iron (Fe0) water filters for safe drinking water provision. In Science and Biotechnology in Africa: Proceedings of a Conference on Scientific Advancement; Kapalanga, J., Raphael, D., Mutesa, L., Eds.; Cambridge Scholars Publishing: New Castle, UK, 2020; pp. 111–139. [Google Scholar]
- El-Meligi, A.A.; Ismail, N. Hydrogen evolution reaction of low carbon steel electrode in hydrochloric acid as a source for hydrogen production. Int. J. Hydrogen Energy 2009, 34, 91–97. [Google Scholar] [CrossRef]
- Reardon, J.E. Zerovalent irons: Styles of corrosion and inorganic control on hydrogen pressure buildup. Environ. Sci. Technol. 2005, 39, 7311–7317. [Google Scholar] [CrossRef]
- Noubactep, C. Untersuchungen zur Passiven In-Situ-Immobilisierung von U(VI) aus Wasser. Ph.D. Thesis, TU Bergakademie Freiberg, Freiberg, Germany, 2003; pp. 21, 140. [Google Scholar]
- Klas, H.; Steinrath, H. Die Korrosion des Eisens und ihre Verhütung; Stahleisen: Düsseldorf, Germany, 1974; p. 632. [Google Scholar]
- Whitman, G.W.; Russel, R.P.; Altieri, V.J. Effect of hydrogen-ion concentration on the submerged corrosion of steel. Ind. Eng. Chem. 1924, 16, 665–670. [Google Scholar] [CrossRef]
- Stratmann, M.; Müller, J. The mechanism of the oxygen reduction on rust-covered metal substrates. Corros. Sci. 1994, 36, 327–359. [Google Scholar] [CrossRef]
- Noubactep, C. A critical review on the mechanism of contaminant removal in Fe0–H2O systems. Environ. Technol. 2008, 29, 909–920. [Google Scholar] [CrossRef]
- Xiao, M.; Hu, R.; Cui, X.; Gwenzi, W.; Noubactep, C. Understanding the operating mode of Fe0/Fe-sulfide/H2O systems for water treatment. Processes 2020, 8, 409. [Google Scholar] [CrossRef]
- Noubactep, C.; Meinrath, G.; Dietrich, P.; Sauter, M.; Merkel, B. Testing the suitability of zerovalent iron materials for reactive Walls. Environ. Chem. 2005, 2, 71–76. [Google Scholar] [CrossRef]
- Jiao, Y.; Qiu, C.; Huang, L.; Wu, K.; Ma, H.; Chen, S.; Ma, L.; Wu, L. Reductive dechlorination of carbon tetrachloride by zero-valent iron and related iron corrosion. Appl. Catal. B Environ. 2009, 91, 434–440. [Google Scholar] [CrossRef]
- Ndé-Tchoupé, A.I. Design and Construction of Fe0-Based Filters for Households. Ph.D. Thesis, University of Douala, Douala, Cameroon, 2019; p. 198. (In French). [Google Scholar]
- Reardon, J.E. Anaerobic corrosion of granular iron: Measurement and interpretation of hydrogen evolution rates. Environ. Sci. Technol. 1995, 29, 2936–2945. [Google Scholar] [CrossRef] [PubMed]
- Miehr, R.; Tratnyek, G.P.; Bandstra, Z.J.; Scherer, M.M.; Alowitz, J.M.; Bylaska, J.E. Diversity of contaminant reduction reactions by zerovalent iron: Role of the reductate. Environ. Sci. Technol. 2004, 38, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Birke, V.; Schuett, C.; Burmeier, H.; Friedrich, H.-J. Impact of trace elements and impurities in technical zero-valent iron brands on reductive dechlorination of chlorinated ethenes in groundwater. In Permeable Reactive Barrier Sustainable Groundwater Remediation; Naidu, R., Birke, V., Eds.; CRC Press: Boca Raton, FL, USA, 2015; ISBN 978-1-4822-2447-4. [Google Scholar]
- Hussam, A.; Munir, A.K.M. A simple and effective arsenic filter based on composite iron matrix: Development and deployment studies for groundwater of Bangladesh. J. Environ. Sci. Health 2007, 42, 1869–1878. [Google Scholar] [CrossRef]
- Meinrath, G.; Volke, P.; Helling, C.; Dudel, E.G.; Merkel, P. Determination and interpretation of environmental water samples contaminated by uranium mining activities. Fresenius J. Anal. Chem. 1999, 364, 191–202. [Google Scholar] [CrossRef]
- HACH DR/2000: UV-Vis Spectrophotometer (Procedures and Reference): Parameters, Methods, and Ranges. Available online: https://www.hach.com/dr-2000-spectrophotometer/product-downloads?id=7640439022 (accessed on 1 May 2020).
- Meinrath, G.; Spitzer, P. Uncertainties in determination of pH. Mikrochim. Acta 2000, 135, 155–168. [Google Scholar] [CrossRef]
- Buck, R.P.; Rondinini, S.; Covington, A.K.; Baucke, F.G.K.; Brett, C.M.A.; Camoes, M.F.; Milton, M.J.T.; Mussini, T.; Naumann, R.; Pratt, K.W.; et al. Measurement of pH: Definition, standards, and procedures (IUPAC recommendations 2002). Pure Appl. Chem. 2002, 74, 2169–2200. [Google Scholar] [CrossRef]
- Ahaneka, I.E.; Kamal, A.R.; Ogunjirin, O.A. Effects of heat treatment on the properties of mild steel using quenchants. Front. Sci. 2012, 2, 153–158. [Google Scholar] [CrossRef][Green Version]
- Seikh, A.H. Influence of heat treatment on the corrosion of microalloyed steel in sodium chloride solution. J. Chem. 2013, 2013. [Google Scholar] [CrossRef]
- Scendo, M.; Szczerba, K. influence of heat treatment on corrosion of mild steel coated with WC-Co-Al2O3 cermet composite produced by electrospark deposition. Int. J. Electrochem. Sci. 2019, 14, 1009–1023. [Google Scholar] [CrossRef]
- Noubactep, C.; Meinrath, G.; Dietrich, P.; Merkel, B. Mitigating uranium in groundwater: Prospects and limitations. Environ. Sci. Technol. 2003, 37, 4304–4308. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C.; Schöner, A.; Meinrath, G. Mechanism of uranium (VI) fixation by elemental iron. J. Hazard. Mater. 2006, 132, 202–212. [Google Scholar] [CrossRef]
- Velimirovic, M.; Larsson, P.-O.; Simons, Q.; Bastiaens, L. Reactivity screening of microscale zerovalent irons and iron sulphides towards different CAHs under standardized experimental conditions. J. Hazard. Mater. 2013, 252–253, 204–212. [Google Scholar] [CrossRef]
- Velimirovic, M.; Larsson, P.-O.; Simons, Q.; Bastiaens, L. Impact of carbon, oxygen and sulphur content of microscale zerovalent iron particles on its reactivity towards CAHs. Chemosphere 2013, 93, 2040–2045. [Google Scholar] [CrossRef]
- Piwowarsky, E. Gußeisen; Springer: Berlin/Heidelberg, Germany, 1951; p. 1070. [Google Scholar]
- Uhlig, H.H. Korrosion und Korrosionsschutz; Akademie Verlag: Berlin, Germany, 1975; p. 412. [Google Scholar]
- Mercer, A.D.; Lumbard, E.A. Corrosion of mild steel in water. Br. Corros. J. 1995, 30, 43–55. [Google Scholar] [CrossRef]
- Noubactep, C.; Schöner, A. Fe0-based alloys for environmental remediation: Thinking outside the box. J. Hazard. Mater. 2009, 165, 1210–1214. [Google Scholar] [CrossRef]
- Talbot, D.; Talbot, J. Corrosion Science & Technology; CRC Press LLC: Boca Raton, FL, USA; New York, NY, USA; Washington, DC, USA; London, UK, 1998; p. 406. [Google Scholar]
- Támara, M.L.; Butler, E.C. Effects of iron purity and groundwater characteristics on rates and products in the degradation of carbon tetrachloride by iron metal. Environ. Sci. Technol. 2004, 38, 1866–1876. [Google Scholar] [CrossRef]
- Rodenhäuser, J. Iron Material for the Remediation of DNAPL-Polluted Groundwater. Masters’s Thesis, The Royal Institute of Technology Stockholm, Stockholm, Sweden, 2003; p. 37. [Google Scholar]
- Lipczynska-Kochany, E.; Harms, S.; Milburn, R.; Sprah, G.; Nadarajah, N. Degradation of carbon tetrachloride in the presence of iron and sulphur containing compounds. Chemosphere 1994, 29, 1477–1489. [Google Scholar] [CrossRef]
- Butler, C.E.; Hayes, F.K. Factors influencing rates and products in the transformation of trichloroethylene by iron sulfide and iron metal. Environ. Sci. Technol. 2001, 35, 3884–3891. [Google Scholar] [CrossRef] [PubMed]
- Allred, B.J. Laboratory evaluation of zero valent iron and sulfur-modified iron for agricultural drainage water treatment. Ground Water Monit. Remediat. 2012, 32, 81–95. [Google Scholar] [CrossRef]
- Allred, B.J.; Tost, B.C. Laboratory comparison of four iron-based filter materials for water treatment of trace element contaminants. Water Environ. Res. 2014, 86, 2221–2232. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Ndé-Tchoupé, A.I.; Lufingo, M.; Xiao, M.; Nassi, A.; Noubactep, C.; Njau, K.N. The impact of selected pre-treatment procedures on iron dissolution from metallic iron specimens used in water treatment. Sustainability 2019, 11, 671. [Google Scholar] [CrossRef]
- Landis, R.L.; Gillham, R.W.; Reardon, E.J.; Fagan, R.; Focht, R.M.; Vogan, J.L. An examination of zero-valent iron sources used in permeable reactive barriers. In Proceedings of the 3rd International Containment Technology Conference, Tallahassee, FL, USA, 10–13 June 2001; 5p. [Google Scholar]
- Lee, G.; Rho, S.; Jahng, D. Design considerations for groundwater remediation using reduced metals. Korean J. Chem. Eng. 2004, 21, 621–628. [Google Scholar] [CrossRef]
- Noubactep, C. Processes of contaminant removal in “Fe0–H2O” systems revisited. The importance of co-precipitation. Open Environ. J. 2007, 1, 9–13. [Google Scholar] [CrossRef]
- Domga, R.; Togue-Kamga, F.; Noubactep, C.; Tchatchueng, J.B. Discussing porosity loss of Fe0 packed water filters at ground level. Chem. Eng. J. 2015, 263, 127–134. [Google Scholar] [CrossRef]
- Moraci, N.; Lelo, D.; Bilardi, S.; Calabrò, P.S. Modelling long-term hydraulic conductivity behaviour of zero valent iron column tests for permeable reactive barrier design. Can. Geotech. J. 2016, 53, 946–961. [Google Scholar] [CrossRef]
- Noubactep, C. Predicting the hydraulic conductivity of metallic iron filters: Modeling gone astray. Water 2016, 8, 162. [Google Scholar] [CrossRef]
- Henderson, A.D.; Demond, A.H. Long-term performance of zero-valent iron permeable reactive barriers: A critical review. Environ. Eng. Sci. 2007, 24, 401–423. [Google Scholar] [CrossRef]
- Khudenko, B.M. Feasibility evaluation of a novel method for destruction of organics. Water Sci. Technol. 1991, 23, 1873–1881. [Google Scholar] [CrossRef]
- Naseri, E.; Ndé-Tchoupé, A.I.; Mwakabona, H.T.; Nanseu-Njiki, C.P.; Noubactep, C.; Njau, K.N.; Wydra, K.D. Making Fe0-based filters a universal solution for safe drinking water provision. Sustainability 2017, 9, 1224. [Google Scholar] [CrossRef]
- Huang, Y.H.; Zhang, T.C.; Shea, P.J.; Comfort, S.D.J. Effects of oxide coating and selected cations on nitrate reduction by iron metal. J. Environ. Qual. 2003, 32, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Briehl, H. Chemie der Werkstoffe; B.G. Teubner: Stuttgart, Germany, 1995; p. 334. [Google Scholar]
- Souvent, P.; Pirc, S. Pollution caused by metallic fragments introduced into soils because of World War I activities. Environ. Geol. 2001, 40, 317–323. [Google Scholar] [CrossRef]
- Noubactep, C. The suitability of metallic iron for environmental remediation. Environ. Prog. 2010, 29, 286–291. [Google Scholar] [CrossRef]
- Noubactep, C. The fundamental mechanism of aqueous contaminant removal by metallic iron. Water SA 2010, 36, 663–670. [Google Scholar] [CrossRef]
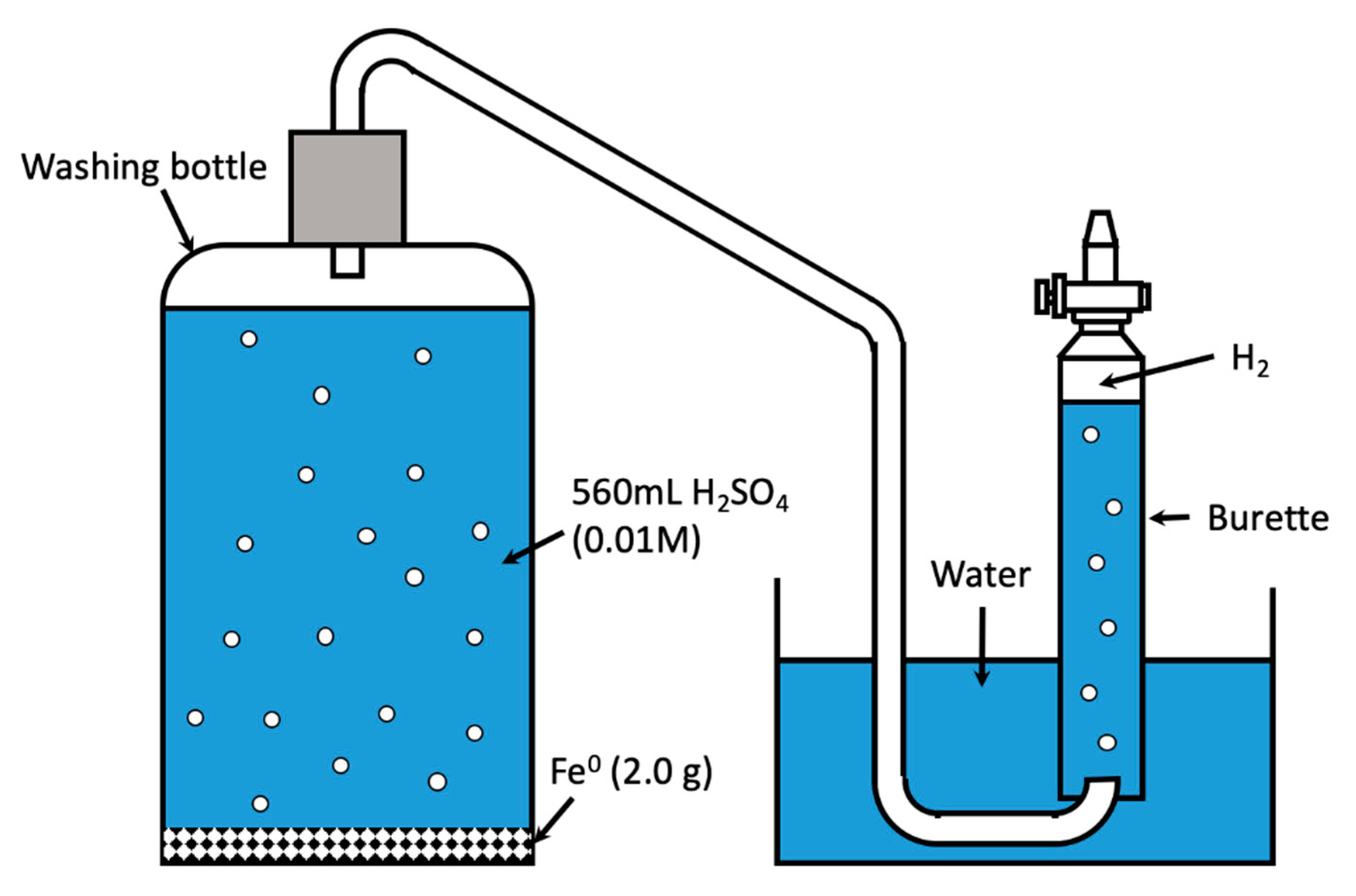

| Process | Reaction | ||
|---|---|---|---|
| Iron oxidation | Fe0 + 2 H+ | ⇔ | Fe2+ + H2 |
| Formation of Fe(OH)2 | Fe2+ + 2 OH− | ⇔ | Fe(OH)2 |
| Formation of Fe(OH)3 | 2 Fe(OH)2 + ½ O2 + H2O | ⇔ | 2 Fe(OH)3 |
| Aging of Fe(OH)3 | Fe(OH)3 | ⇔ | FeOOH; Fe2O3, Fe3O4 |
| Fe2+ complexation | Fe2+ + EDTA | ⇔ | Fe(EDTA)2+ |
| Method | Fe0 Dissolution in H2SO4 | Fe0 Dissolution in H2O | Fe0 Dissolution in Complexing Solutions (EDTA, Phen) |
|---|---|---|---|
| Test conditions | 2.0 ≤ pH ≤ 3.1 | Neutral pH | Neutral pH |
| Monitored parameter | H2 volume | H2 volume | Fe concentration |
| Test duration (hours) | 24 | 200 ≤ t ≤ 3840 | 24 ≤ t ≤ 96 |
| Used Fe0 mass (g) | 2.0 | 75 ≤ m ≤ 600 | 0.01 ≤ m ≤ 0.10 |
| Number of used chemicals | 1 | 0 | 1 to 4 |
| Corrosion mechanism | H2 evolution | O2 adsorption | O2 adsorption |
| Advantages | Uses only lab accessories and H2SO4, no scientific instrument; Enables the exhaustion of Fe0 dissolution within a reasonable time. | Uses no scientific instrument. Fe0 corrosion is not artificially accelerated. | Controls Fe0 corrosion to make it a linear function of time; Simulates natural conditions; Uses only a spectrophotometer. |
| Disadvantages | H2 evolution is not the mechanism occurring in natural systems and water filters. | Measured H2 volume represents an excess and cannot be strictly related to the stoichiometry of Equation (1); The experiments last for longer time and needs larger Fe0 amounts | Only the initial dissolution kinetics are recorded; In the EDTA test, Fe dissolved from corrosion products (hydroxides and oxides) are also recorded. |
| References | Noubactep [32], This study | Reardon [31,41] | Lufingo et al. [22], Noubactep et al. [38] |
| Fe0 | Element (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C | Si | Mn | P | S | Cr | Mo | Ni | Fe | |
| ST1 | 0.49 | 2.80 | 0.40 | 0.011 | 0.003 | 8.60 | 0.03 | 0.15 | 87.52 |
| ST2 | 0.15 | 0.05 | 1.34 | 0.068 | 0.287 | 0.12 | 0.03 | 0.08 | 97.88 |
| C15 | 0.12 | 0.26 | 0.62 | 0.019 | 0.023 | 0.084 | n.d. * | 0.055 | bal. ** |
| C45 | 0.48 | 0.22 | 0.70 | 0.012 | 0.038 | 0.12 | n.d. | 0.13 | bal. |
| C60 | 0.59 | 0.29 | 0.65 | 0.007 | 0.027 | 0.19 | n.d. | 0.093 | bal. |
| C100 | 0.99 | 0.25 | 0.22 | 0.002 | 0.008 | 0.078 | n.d. | 0.051 | bal. |
| DRI | 1.96 | 0.12 | 0.09 | 0.027 | 0.14 | 0.003 | n.d. | <0.001 | bal. |
| GGG | 3.13 | 2.17 | 0.36 | 0.022 | 0.029 | 0.077 | n.d. | 0.056 | bal. |
| HGG | 3.13 | 0.17 | 0.42 | 0.053 | 0.065 | 0.16 | n.d. | 0.23 | bal. |
| HGM | 3.39 | 0.41 | 1.10 | n.d. | 0.105 | 0.34 | n.d. | 0.088 | bal. |
| S69 | 3.52 | 2.12 | 0.93 | n.d. | n.d. | 0.66 | n.d. | n.d. | bal. |
| S15 | 0.45 | 0.28 | 0.78 | n.d. | n.d. | 2.67 | n.d. | 1.34 | bal. |
| Fe0 | (VH2)24 (mL) | vmax (mL/min) | tvmax (h) | [Fe] (mM) | E (%) | kEDTA (M h−1) |
|---|---|---|---|---|---|---|
| ST1 ** | 152 | 0.24 | 2.7 | 22.1 | 76 | 1.35 |
| ST2 ** | 192 | 0.31 | 3.8 | 25.2 | 80 | 1.95 |
| C15 | 131 | 0.20 | 5.0 | n.d. * | 84 | 1.92 |
| C45 | 122 | 0.28 | 1.2 | n.d. | 82 | 1.95 |
| C60 | 104 | 0.22 | 5.2 | n.d. | 86 | 1.94 |
| C100 | 109 | 0.23 | 2.5 | n.d. | 87 | 1.83 |
| DRI | 149 | 1.05 | 0.1 | 22.4 | 91 | 1.86 |
| GGG | 137 | 0.22 | 1.7 | 21.7 | 88 | 1.82 |
| HGG | 109 | 0.20 | 5.9 | 21.8 | 78 | 1.54 |
| HGM | 40 | 0.05 | 1.4 | 12.8 | 82 | 1.55 |
| S15 | 108 | 0.30 | 3.2 | 19.8 | 89 | 1.98 |
| S69 | 118 | 0.24 | 2.5 | 22.3 | 93 | 2.06 |
| Characterization Test | Monitored Parameter | Used Chemical | Compounds Used for Validation | Reference |
|---|---|---|---|---|
| Iodine method | I3− reduction rate constant | Iodine | CAHs and PCP | Kim et al. [18] |
| Ferric method | Fe concentration | Ferric iron | Cu(II) and TCE | Li et al. [19] |
| H2 evolution | H2 volume | None | CAHs | Velimirovic et al. [54] |
| EDTA test | Fe concentration | EDTA | U(VI) | Noubactep et al. [38] |
| Phen test | Fe concentration | 1,10 Phenanthroline | EDTA test | Lufingo et al. [22] |
| H2 evolution | H2 volume | H2SO4 | Not validated | This study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ndé-Tchoupé, A.I.; Hu, R.; Gwenzi, W.; Nassi, A.; Noubactep, C. Characterizing the Reactivity of Metallic Iron for Water Treatment: H2 Evolution in H2SO4 and Uranium Removal Efficiency. Water 2020, 12, 1523. https://doi.org/10.3390/w12061523
Ndé-Tchoupé AI, Hu R, Gwenzi W, Nassi A, Noubactep C. Characterizing the Reactivity of Metallic Iron for Water Treatment: H2 Evolution in H2SO4 and Uranium Removal Efficiency. Water. 2020; 12(6):1523. https://doi.org/10.3390/w12061523
Chicago/Turabian StyleNdé-Tchoupé, Arnaud Igor, Rui Hu, Willis Gwenzi, Achille Nassi, and Chicgoua Noubactep. 2020. "Characterizing the Reactivity of Metallic Iron for Water Treatment: H2 Evolution in H2SO4 and Uranium Removal Efficiency" Water 12, no. 6: 1523. https://doi.org/10.3390/w12061523
APA StyleNdé-Tchoupé, A. I., Hu, R., Gwenzi, W., Nassi, A., & Noubactep, C. (2020). Characterizing the Reactivity of Metallic Iron for Water Treatment: H2 Evolution in H2SO4 and Uranium Removal Efficiency. Water, 12(6), 1523. https://doi.org/10.3390/w12061523







