Macroinvertebrate Communities in a Lake of an Inter-Basin Water Transfer Project and Its Implications for Sustainable Management
Abstract
:1. Introduction
2. Materials and Methods
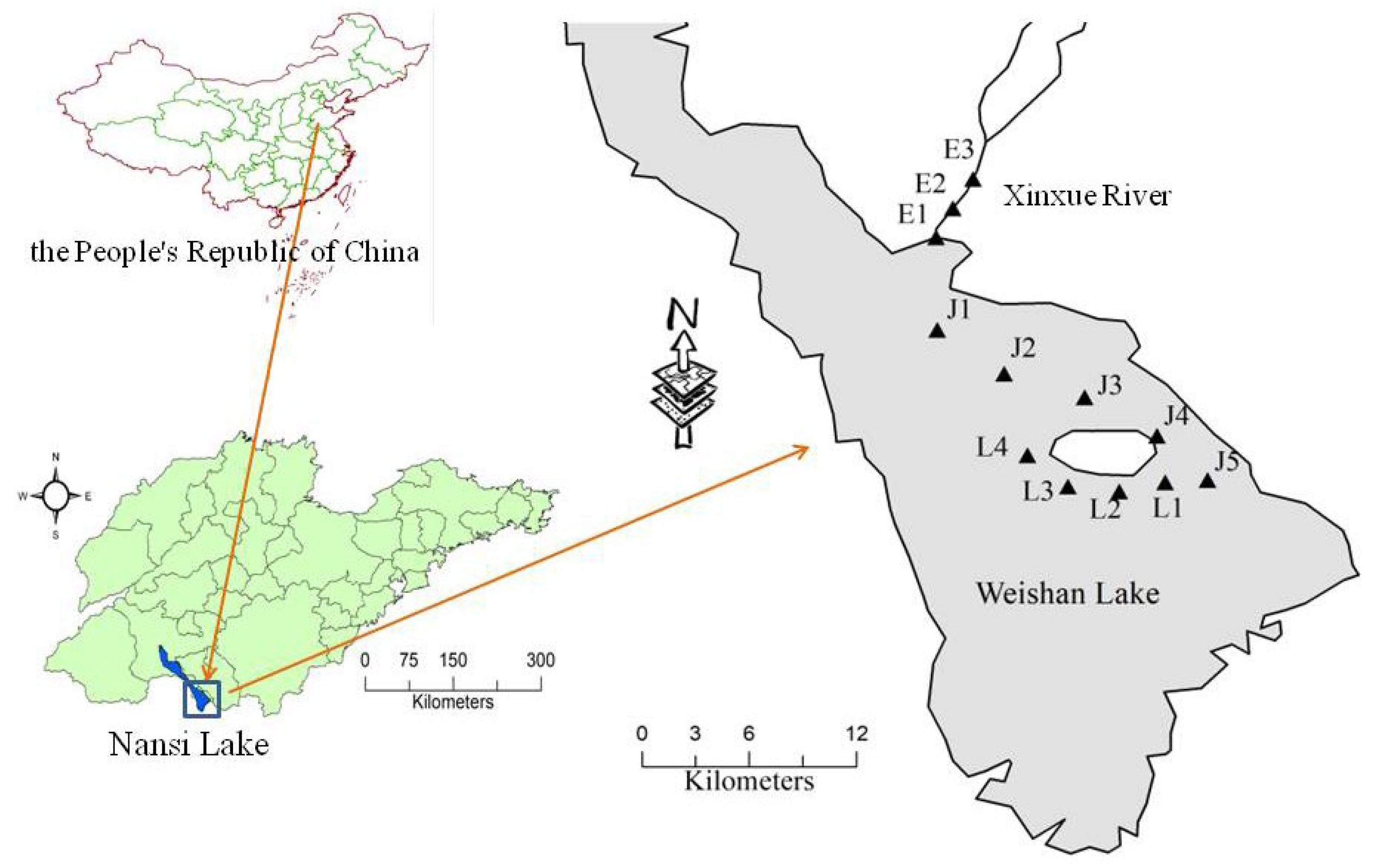
2.1. Study Area and Sites Selection
2.2. Macroinvertebrate Collection and Identification
2.3. Environmental Variables
2.4. Biodiversity Indices and Date Analysis
3. Results
3.1. Physicochemical Parameters
3.2. Species Composition
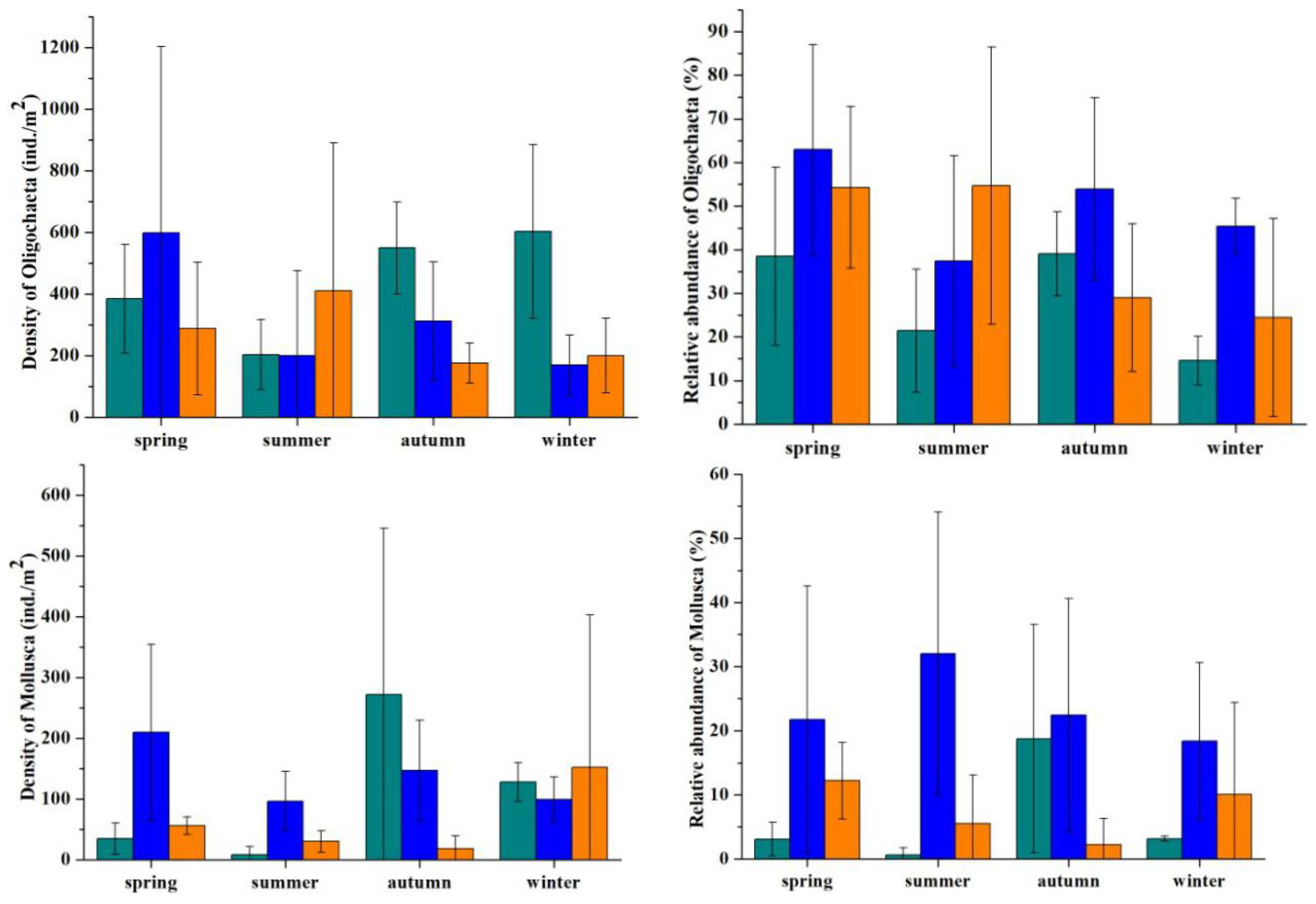
3.3. Density, Biomass, Biodiversity Indices and Main Taxa Groups
3.4. Community Structure
3.5. Assemblage-Environmental Relationships
4. Discussion
4.1. Variation of Macroinvertebrate Assemblages
4.2. Relationship between Assemblages and Environmental Variables
4.3. Implications for Aquatic Ecosystem Assessment and Management under Impact of SNWD
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Factors | Spring | Summer | Autumn | Winter | River Mouth | Canal | Lake |
|---|---|---|---|---|---|---|---|
| WD (cm) | 218 ± 111 a | 104 ± 68 b | 196 ± 31 a | 205 ± 29 a | 163 ± 53 a | 210 ± 100 a | 159 ± 61 a |
| WT (°C) | 15.8 ± 2.2 a | 29.1 ± 1.2 b | 18.6 ± 0.2 c | 6.5 ± 0.9 d | 19.0 ± 8.5 a | 17.0 ± 8.4 a | 17.0 ± 8.3 a |
| ST (°C) | 13.0 ± 0.5 a | 28.4 ± 0.8 b | 17.7 ± 0.3 c | 6.6 ± 0.9 d | 16.9 ± 8.2 a | 16.5 ± 8.2 a | 15.9 ± 8.3 a |
| SD (cm) | 82.1 ± 50.9 a | 29.4 ± 9.5 b | 46.5 ± 11.5 bc | 62.9 ± 6.4 ac | 71.1 ± 36.7 a | 39.1 ± 12.2 b | 63.6 ± 38.9 a |
| Chl-a (mg/L) | 0.10 ± 0.06 a | 0.12 ± 0.06 a | 0.09 ± 0.05 a | 0.07 ± 0.04 a | 0.04 ± 0.01 a | 0.12 ± 0.05 b | 0.10 ± 0.05 b |
| TN (mg/L) | 0.93 ± 0.20 a | 0.83 ± 0.08 a | 1.10 ± 0.16 b | 0.81 ± 0.08 a | 1.12 ± 0.19 a | 0.82 ± 0.09 b | 0.90 ± 0.13 b |
| NO3-N (mg/L) | 0.38 ± 0.06 ab | 0.37 ± 0.06 a | 0.45 ± 0.08 b | 0.35 ± 0.06 a | 0.47 ± 0.06 a | 0.34 ± 0.05 b | 0.39 ± 0.04 c |
| NH4-N (mg/L) | 0.45 ± 0.11 ab | 0.38 ± 0.09 bc | 0.55 ± 0.08 a | 0.35 ± 0.09 c | 0.57 ± 0.09 a | 0.35 ± 0.09 b | 0.42 ± 0.07 b |
| TP (mg/L) | 0.05 ± 0.02 a | 0.04 ± 0.01 a | 0.05± 0.02 a | 0.04 ± 0.01 a | 0.069 ± 0.01 a | 0.033 ± 0.01 b | 0.044 ± 0.01 c |
| PO4-P (mg/L) | 0.032 ± 0.01 a | 0.023 ± 0.01 a | 0.027 ± 0.01 a | 0.026 ± 0.01 a | 0.041 ± 0.01 a | 0.017 ± 0.00 b | 0.030 ± 0.01 c |
| TDS (mg/L) | 616 ± 172 a | 580 ± 71 a | 677 ± 132 a | 707 ± 145 a | 777 ± 125 a | 665 ± 123 b | 521 ± 20 c |
| DO (mg/L) | 9.44 ± 0.8 a | 8.42 ± 0.7 b | 9.72 ± 1.27 a | 9.28 ± 0.5 ab | 8.97 ± 0.92 a | 8.74 ± 0.70 a | 9.99 ± 0.83 b |
| Cond (ms/cm) | 1.16 ± 0.21 a | 1.18 ± 0.17 a | 1.04 ± 0.20 a | 1.03 ± 0.25 a | 1.23 ± 0.15 a | 1.21 ± 0.14 a | 0.88 ± 0.15 b |
| pH | 8.51 ± 0.66 a | 8.69 ± 0.69 a | 7.90 ± 0.26 b | 7.79 ± 0.29 b | 8.02 ± 0.33 a | 7.86 ± 0.28 a | 8.22 ± 0.63 b |
| Groups | Taxa | Groups | Taxa |
|---|---|---|---|
| Arthropoda | Culicoides sp. | Arthropoda | Goera sp. |
| Hydrobaenus kondio | Coleoptera spp. | ||
| Hydrobaenus pilipes | Ophiogomphus spinicornis | ||
| Orthocaldius obumbratus | Macrobrachium | ||
| Propsilocerus akamusi | Gammarus sp. | ||
| Cricotopus trifasciatus | Lebertia sp. | ||
| Cryptochironomus sp. | Alocinma longicornis | ||
| Cryptochironomus rostratus | Mollusca | Cipangopaludina cahayensis | |
| Cryptotendipes sp.A | Parafossarulus eximius | ||
| Cryptotendipes defectus | Stenothyra glabra | ||
| Chironomus riparius | Bellamya purificata | ||
| Chironomus ochreatus | Bellamya aeruginosa | ||
| Chironomus flaviplumus | Bellamya limnophila | ||
| Chironomus acerbiphilus | Semisulcospira cancellata | ||
| Chironomus decorus | Hippeutis cantori | ||
| Chironomus plumosus | Corbicula fluminea | ||
| Microchironomus tener | Lamprotula leai | ||
| Parachironomus chaetoalus | Anodonate woodiana | ||
| Parachironomus arcuatus | Unio douglasiae | ||
| Einfeldia dissidens | Lanceolaria gladiola | ||
| Dicrotendipes lobifer | Novaculina chinensis | ||
| Dicrotendipes tritomus | Limnoperna lacustris | ||
| Glyptotendipes barbipes | Annelida | Hemiclepsis sp. | |
| Glyptotendipes paripes | Stephanodrilus sp. | ||
| Glyptotendipes amplus | Allonais pectinata | ||
| Gillotia alboviridis | Aulodrius paucichaeta | ||
| Polypedilum sp. | Aulodrilus pluriseta | ||
| Polypedilum halterale | Aulodrius limnobius | ||
| Parochlus sp. | Aulodrius americanus | ||
| Rheopelopia paramaculipennis | Branchiura sowerbyi | ||
| Tanypus concavus | Limnodrilus hoffmeisteri | ||
| Procladius sp.A | Limnodrilus claparedianus | ||
| Clinotanypus sp.A | Limnodrilus grandisetosus | ||
| Chironomidae pupa | Tubifex tubifex | ||
| Caenis sp. | Bothrioneurum vejdovskyanum | ||
| Cheumatopsyche sp. | Laonome sp. |
| Groups | t | p (perm) | Unique Perms | |
|---|---|---|---|---|
| Spring | River mouth, Canal | 2.5953 | 0.019 | 56 |
| River mouth, Lake | 2.4773 | 0.0289 | 35 | |
| Canal, Lake | 1.9814 | 0.0087 | 126 | |
| Summer | River mouth, Canal | 2.2534 | 0.0187 | 56 |
| River mouth, Lake | 2.3062 | 0.0258 | 35 | |
| Canal, Lake | 1.5762 | 0.0176 | 126 | |
| Autumn | River mouth, Canal | 2.43 | 0.0175 | 56 |
| River mouth, Lake | 2.3215 | 0.0279 | 35 | |
| Canal, Lake | 2.2203 | 0.0143 | 126 | |
| Winter | River mouth, Canal | 1.8639 | 0.0156 | 56 |
| River mouth, Lake | 2.2427 | 0.0283 | 35 | |
| Canal, Lake | 1.6193 | 0.0089 | 126 | |
| River mouth | Spring, Summer | 3.6132 | 0.1014 | 10 |
| Spring, Autumn | 1.7681 | 0.0996 | 10 | |
| Spring, Winter | 3.1107 | 0.1062 | 10 | |
| Summer, Autumn | 2.6029 | 0.105 | 10 | |
| Summer, Winter | 4.1638 | 0.1022 | 10 | |
| Autumn, Winter | 1.6168 | 0.0943 | 10 | |
| Canal | Spring, Summer | 1.8514 | 0.0087 | 126 |
| Spring, Autumn | 1.5248 | 0.0149 | 126 | |
| Spring, Winter | 1.5846 | 0.0075 | 126 | |
| Summer, Autumn | 1.2885 | 0.0896 | 126 | |
| Summer, Winter | 1.194 | 0.1482 | 126 | |
| Autumn, Winter | 1.2743 | 0.1006 | 126 | |
| Lake | Spring, Summer | 1.6654 | 0.0291 | 35 |
| Spring, Autumn | 1.9187 | 0.0274 | 35 | |
| Spring, Winter | 1.4698 | 0.0265 | 35 | |
| Summer, Autumn | 1.1913 | 0.2209 | 35 | |
| Summer, Winter | 1.5605 | 0.0297 | 35 | |
| Autumn, Winter | 1.3257 | 0.0878 | 35 | |
| All the year | River mouth, Canal | 3.6323 | 0.0001 | 9937 |
| River mouth, Lake | 3.7194 | 0.0001 | 9941 | |
| Canal, Lake | 2.8747 | 0.0001 | 9935 | |
| All the regions | Spring, Summer | 2.429 | 0.0001 | 9910 |
| Spring, Autumn | 2.0167 | 0.0003 | 9927 | |
| Spring, Winter | 1.8519 | 0.0002 | 9925 | |
| Summer, Autumn | 1.5949 | 0.0106 | 9940 | |
| Summer, Winter | 1.789 | 0.0007 | 9924 | |
| Autumn, Winter | 1.3542 | 0.066 | 9926 |
| IV from Randomized Groups | |||||
|---|---|---|---|---|---|
| Indicator Species | Maxgrp | Observed IV | Mean | S.Dev | p |
| Culicoides sp. | River mouth | 98.7 | 22.7 | 7.62 | 0.0002 |
| Propsilocerus akamusi | River mouth | 93.7 | 31.8 | 9.36 | 0.0002 |
| Limnodrilus grandisetosus | River mouth | 43.2 | 17.6 | 5.89 | 0.0022 |
| Gillotia alboviridis | River mouth | 41.7 | 10.4 | 5.21 | 0.0012 |
| Procladius sp.A | River mouth | 41.7 | 10.3 | 4.91 | 0.0006 |
| Orthocaldius obumbratus | River mouth | 25.0 | 8.4 | 4.48 | 0.0092 |
| Parachironomus chaetoalus | River mouth | 25.5 | 8.4 | 4.54 | 0.0124 |
| Hemiclepsis sp. | Canal | 58.9 | 23.3 | 7.82 | 0.001 |
| Bellamya purificata | Canal | 57.4 | 33.4 | 6.07 | 0.0022 |
| Gammarus sp. | Canal | 46.7 | 19.5 | 6.51 | 0.0012 |
| Corbicula fluminea | Canal | 35.0 | 12.5 | 5.52 | 0.0054 |
| Laonome sp. | Canal | 35.0 | 12.6 | 5.53 | 0.0052 |
| Semisulcospira cancellata | Canal | 30.0 | 11.9 | 5.52 | 0.0096 |
| Tanypus concavus | Lake | 68.5 | 35.1 | 8.29 | 0.0022 |
| Einfeldia dissidens | Lake | 37.5 | 13.7 | 6.09 | 0.0024 |
| Glyptotendipes amplus | Lake | 23.3 | 10.7 | 5.22 | 0.0274 |
| Cryptotendipes sp.A | Lake | 18.7 | 8.1 | 4.47 | 0.0452 |
| Parachironomus chaetoalus | Spring | 25.0 | 9.2 | 5.1 | 0.0462 |
| Microchironomus tener | Summer | 44.8 | 20.4 | 8.4 | 0.0154 |
| Cryptochironomus rostratus | Summer | 33.3 | 9.7 | 5.2 | 0.0114 |
| Chironomus riparius | Summer | 25.5 | 10.9 | 5.7 | 0.0356 |
| Propsilocerus akamusi | Winter | 66.1 | 29 | 9.3 | 0.002 |
| Dicrotendipes tritomus | Winter | 33.3 | 10.4 | 5.6 | 0.0094 |
References
- Wang, Y.; Zhang, W.; Zhao, Y.; Peng, H.; Shi, Y. Modelling water quality and quantity with the influence of inter-basin water diversion projects and cascade reservoirs in the middle-lower hanjiang river. J. Hydrol. 2016, 541, 1348–1362. [Google Scholar] [CrossRef]
- Biswas, A.K. Integrated water resources management: A reassessment: A water forum contribution. Water Int. 2004, 29, 248–256. [Google Scholar] [CrossRef]
- Zeng, Q.; Liu, Y.; Zhao, H.; Sun, M.; Li, X. Comparison of models for predicting the changes in phytoplankton community composition in the receiving water system of an inter-basin water transfer project. Environ. Pollut. 2017, 223, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Belinda, G.; Aldridge, D.C. Inter-basin water transfers and the expansion of aquatic invasive species. Water Res. 2018, 143, 282–291. [Google Scholar]
- Pohlner, H. Institutional change and the political economy of water megaprojects: China’s south-north water transfer. Glob. Environ. Chang. 2016, 38, 205–216. [Google Scholar] [CrossRef]
- Dong, Z.; Yan, Y.; Duan, J.; Fu, X.; Zhou, Q.; Huang, X.; Zhao, J. Computing payment for ecosystem services in watersheds: An analysis of the Middle Route Project of South-to-North Water Diversion in China. J. Environ. Sci. 2011, 23, 2005–2012. [Google Scholar] [CrossRef]
- Burger, J. Bioindicators: A review of their use in the environmental literature 1970–2005. Environ. Bioindic. 2006, 1, 136–144. [Google Scholar] [CrossRef]
- Odountan, H.; Abou, Y. Structure and Composition of Macroinvertebrates during Flood Period of the Nokoue Lake, Benin. Open J. Ecol. 2016, 6, 62–73. [Google Scholar] [CrossRef] [Green Version]
- Belanger, D. Utilisation de la Faune Macrobenthique Comme Bio-indicateur de la Qualité de I’environnement côtier. Master’s Thesis, Université de Sherbrooke, Sherbrooke, QC, Canada, 2009. [Google Scholar]
- Prygiel, J.; Rosso-Darmet, A.; Lafont, M.; Lesniak, C.; Durbec, A.; Ouddane, B. Use of oligochaete communities for assessment of ecotoxicological risk in fine sediment of rivers and canals of the artois-picardie water basin (france). Hydrobiologia 1999, 410, 25–37. [Google Scholar] [CrossRef]
- Vivien, R.; Tixier, G.; Lafont, M. Use of oligochaete communities for assessing the quality of sediments in watercourses of the Geneva area (Switzerland) and Artois-Picardie basin (France): Proposition of heavy metal toxicity thresholds. Ecohydrol. Hydrobiol. 2014, 14, 142–151. [Google Scholar] [CrossRef]
- Gerami, M.H.; Patimar, R.; Negarestan, H.; Jafarian, H.; Mortazavi, M.S. Temporal variability in macroinvertebrates diversity patterns and their relation with environmental factors. Biodiversitas J. Biol. Divers. 2016, 17, 36–43. [Google Scholar] [CrossRef]
- Xue, L.; Yuan, Z.; Guo, F.; Xin, G.; Wang, Y. Predicting the effect of land use and climate change on stream macroinvertebrates based on the linkage between structural equation modeling and bayesian network. Ecol. Indic. 2018, 85, 820–831. [Google Scholar]
- Jonsson, M.; Burrows, R.M.; Lidman, J.; Fältström, E.; Laudon, H.; Sponseller, R.A. Land use influences macroinvertebrate community composition in boreal headwaters through altered stream conditions. Ambio 2017, 46, 311–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durance, I.; Ormerod, S.J. Climate change effects on upland stream macroinvertebrates over a 25-year period. Glob. Chang. Biol. 2010, 13, 942–957. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, X.; Li, Z.; Meng, X.; Heino, J.; Xie, Z.; Wang, X.M.; Yu, J. Changes in multiple facets of macroinvertebrate alpha diversity are linked to afforestation in a subtropical riverine natural reserve. Environ. Sci. Pollut. Res. 2018, 25, 36124–36135. [Google Scholar] [CrossRef] [PubMed]
- Miserendino, M.L.; Archangelsky, M.; Brand, C.; Epele, L.B. Environmental changes and macroinvertebrate responses in patagonian streams (argentina) to ashfall from the chaitén volcano (may 2008). Sci. Total Environ. 2012, 424, 202–212. [Google Scholar] [CrossRef]
- Boda, P.; Móra, A.; Várbíró, G.; Csabai, Z. Livin’ on the edge: The importance of adjacent intermittent habitats in maintaining macroinvertebrate diversity of permanent freshwater marsh systems. Inland Waters 2018, 8, 312–321. [Google Scholar] [CrossRef]
- Marin, J.R.; Miller, J.A. Spatial variability of the surf zone fish and macroinvertebrate community within dissipative sandy beaches in Oregon, USA. Mar. Ecol. 2016, 37, 1027–1035. [Google Scholar] [CrossRef]
- Sullivan, S.M.; Manning, D.W. Seasonally distinct taxonomic and functional shifts in macroinvertebrate communities following dam removal. PeerJ 2017, 5, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Chi, S.; Li, S.; Chen, S.; Chen, M.; Zheng, J.; Hu, J. Temporal variations in macroinvertebrate communities from the tributaries in the three gorges reservoir catchment, China. Rev. Chil. De Hist. Nat. 2017, 90, 6–13. [Google Scholar] [CrossRef]
- Jiang, X.; Xiong, J.; Xie, Z. Longitudinal and seasonal patterns of macroinvertebrate communities in a large undammed river system in southwest china. Quat. Int. 2017, 440, 1–12. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Wang, M.; Qin, B. Compositional differences of chromophoric dissolved organic matter derived from phytoplankton and macrophytes. Org. Geochem. 2013, 55, 26–37. [Google Scholar] [CrossRef]
- Meng, X.; Jiang, X.; Xiong, X.; Wu, C.; Xie, Z. Mediated spatio-temporal patterns of macroinvertebrate assemblage associated with key environmental factors in the qinghai lake area, China. Limnologica 2016, 56, 14–22. [Google Scholar] [CrossRef]
- Yang, Y.F.; Zhou, X.D.; Yi, Y.J.; Xu, M.Z.; Yang, Z.F. Influence of debris flows on macroinvertebrate diversity and assemblage structure. Ecol. Indic. 2018, 85, 781–790. [Google Scholar] [CrossRef]
- Yang, L.; Liu, E. The human pollution evaluation of phosphorus in surface sediments of Nansihu Lake. Procedia Environ. Sci. 2011, 10, 918–921. [Google Scholar]
- Wu, Z.; Jian, Z.; Jie, Z.; Jie, R.; Shan, C. A monitoring project planning technique of the water quality spatial distribution in Nansi Lake. Procedia Environ. Sci. 2011, 10, 2320–2328. [Google Scholar]
- Lu, M.S.; Kong, F.S.; Zhuang, X.H. Comprehensive environmental-geological survey of the Nansi Lake drainage area, southwestern Shandong. Geol. China 2003, 30, 424–428. [Google Scholar]
- Ma, Z.D.; Gao, H.; Yang, J.; Xi, J.C.; Li, X.M.; Ge, Q.S. Valuation of Nansihu Lake wetland ecosystem services based on multi-sources data fusion. Resour. Sci. 2014, 6, 840–847. [Google Scholar]
- Zhuang, W.; Wang, Q.; Tang, L.; Liu, J.; Yue, W.; Liu, Y.; Zhou, F.; Chen, Q.; Wang, M. A new ecological risk assessment index for metal elements in sediments based on receptor model, speciation, and toxicity coefficient by taking the nansihu lake as an example. Ecol. Indic. 2018, 89, 725–737. [Google Scholar] [CrossRef]
- Brinkhurst, R.O. Guide to the Freshwater Aquatic Microdrile Oligochaetes of North America; Canadian Special Publication of Fisheries and Aquatic Sciences; Department of Fisheries and Oceans: Ottawa, ON, Canada, 1986. [Google Scholar]
- Morse, J.C.; Yang, L.; Tian, L. Aquatic Insects of China Useful for Monitoring Water Quality; Hohai University Press: Nanjing, China, 1994. [Google Scholar]
- Epler, J.H. Identification Manual for the Larval Chironomidae (Diptera) of North and South Carolina; EPA: Florida, FL, USA, 2001.
- Liu, Y.; Zhang, W.; Wang, Y.; Wang, E. Economic Fauna of China (Freshwater Mollusk); Science Press: Beijing, China, 1979. [Google Scholar]
- Wang, H.Z. Studies on Taxonomy, Distribution and Ecology of Microdrile Oligochaetes of China, with Descriptions of Two New Species from the Vicinity of the Great Wall Station of China, Antarctica; Higher Education Press: Beijing, China, 2002. [Google Scholar]
- Wang, J.C.; Wang, X.H. Chironomidae Larvae in Northern China; Yanshi Press: Beijing, China, 2011. [Google Scholar]
- Wei, F.S.; Kou, H.R.; Hong, S.J. Methods for the Examination of Water and Wastewater; China Environmental Science Press: Beijing, China, 1989. [Google Scholar]
- Huang, X.F.; Chen, W.; Cai, Q.H. Standard Methods for Observation and Analysis in Chinese Ecosystem Research Networke Survey, Observation and Analysis of Lake Ecology; Standards Press of China: Beijing, China, 1999. [Google Scholar]
- McCune, B.; Mefford, M.J. PC-ORD. Multivariate Analysis of Ecological Data; Version 5; MjM Software: Gleneden Beach, OR, USA, 2006. [Google Scholar]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Anderson, M.J. PERMANOVA: A FORTRAN Computer Program for Permutational Multivariate Analysis of Variance; Department of Statistics, University of Auckland: Auckland, New Zealand, 2005. [Google Scholar]
- Legendre, P.; Anderson, M.J. Distance-based redundancy analysis: Testing multispecies responses in multifactorial ecological experiments. Ecol. Monogr. 1999, 69, 1–24. [Google Scholar] [CrossRef]
- Bunn, S.E.; Edward, D.H.; Loneragan, N.R. Spatial and temporal variation in the macroinvertebrate fauna of streams of the northern jarrah forest, western australia: Community structure. Freshw. Biol. 2010, 16, 67–91. [Google Scholar] [CrossRef]
- Tonkin, J.D.; Death, R.G.; Collier, K.J. Do productivity and disturbance interact to modulate macroinvertebrate diversity in streams? Hydrobiologia 2012, 701, 159–172. [Google Scholar] [CrossRef]
- Hu, Z.; Jia, X.; Chen, X.; Zhang, Y.; Liu, Q. Spatial and seasonal pattern of macrozoobenthic assemblages and the congruence in water quality bioassessment using different taxa in artificial mingzhu lake in shanghai. Chin. J. Oceanol. Limnol. 2016, 34, 928–936. [Google Scholar] [CrossRef]
- Hirabayashi, K.; Yoshizawa, K.; Yoshida, N.; Kazama, F. Progress of eutrophication and change of chironomid fauna in Lake Yamanakako, Japan. Limnology 2004, 5, 47–53. [Google Scholar] [CrossRef]
- Blair, N.E.; Leithold, E.L.; Aller, R.C. From bedrock to burial: The evolution of particulate organic carbon across coupled watershed-continental margin systems. Mar. Chem. 2004, 92, 141–156. [Google Scholar] [CrossRef]
- Sor, R.; Boets, P.; Chea, R.; Goethals, P.L.M.; Lek, S. Spatial organization of macroinvertebrate assemblages in the lower mekong basin. Limnol.-Ecol. Manag. Inland Waters 2017, 64, 20–30. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, L.; Tolonen, K.E.; Yin, H.; Gao, J.; Zhang, Z.; Li, K.; Cai, Y. Substrate degradation and nutrient enrichment structuring macroinvertebrate assemblages in agriculturally dominated lake chaohu basins, China. Sci. Total Environ. 2018, 627, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.Y.; Xu, X.H.; Gao, S.X. Study on Process of Nutrition Release during the Decay of Submerged Macrophytes. Res. Environ. Sci. 2008, 21, 64–68. [Google Scholar]
- Kim, P.J.; Lee, J.H.; Huh, I.A.; Kong, D. Development of benthic macroinvertebrates sediment index (BSI) for bioassessment of freshwater sediment. Int. J. Sediment. Res. 2019, 34, 368–378. [Google Scholar] [CrossRef]
- Johnson, R.C.; Carreiro, M.M.; Jin, H.S.; Jack, J.D. Within-year temporal variation and life-cycle seasonality affect stream macroinvertebrate community structure and biotic metrics. Ecol. Indic. 2012, 13, 206–214. [Google Scholar] [CrossRef]
- Yamagishi, H.; Fukuhara, H. Vertical migration of Spaniotoma akamusi larvae (Diptera: Chironomidae) through the bottom deposits of Lake Suwa. Jpn. J. Ecol. 1972, 22, 226–227. [Google Scholar]
- Gong, Z. Studies on Ecology of Macrozoobenthos in Shallow Lakes along the Middle Reaches of the Changjiang River. Ph.D. Thesis, Institute of Hydrobiology, Chinese Academy of Sciences, Beijing, China, 2002. [Google Scholar]
- Deng, S. Macroinvertebrate Secondary Productivity and Nutritional Basis Analysis in the Shengli River. Master’s Thesis, Huazhong University of Science and Technology, Wuhan, China, 2011. [Google Scholar]
- Brendonck, L.; Riddoch, B.J.; Van de Weghe, V.; Van Dooren, T. The maintenance of egg banks in very short-lived pools-a case study with anostracans (Branchiopoda). Arch. Hydrobiol. 1998, 52, 141–161. [Google Scholar]
- Stubbington, R.; Greenwood, A.M.; Wood, P.J.; Armitage, P.D.; Gunn, J.; Robertson, A.L. The response of perennial and temporary headwater stream invertebrate communities to hydrological extremes. Hydrobiologia 2009, 630, 299–312. [Google Scholar] [CrossRef]
- Boulton, A.J.; Lake, P.S. The ecology of two intermittent streams in victoria, australia: Ii. comparisons of faunal composition between habitats, rivers and years. Freshw. Biol. 1992, 27, 99–121. [Google Scholar] [CrossRef]
- Yekta, F.A.; Kiabi, B.; Ardalan, A.A.; Shokri, M. Temporal Variation in Rocky Intertidal Gastropods of the Qeshm Island in the Persian Gulf. J. Persian Gulf 2013, 4, 9–18. [Google Scholar]
- Soucek, D.J.; Dickinson, A. Acute toxicity of nitrate and nitrite to sensitive freshwater insects, mollusks, and a crustacean. Arch. Environ. Contam. Toxicol. 2012, 62, 233–242. [Google Scholar] [CrossRef]
- Camargo, J.A.; Alonso, A. Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: A global assessment. Environ. Int. 2006, 32, 831–849. [Google Scholar] [CrossRef]
- Beermann, A.J.; Elbrecht, V.; Karnatz, S.; Ma, L.; Matthaei, C.D.; Piggott, J.J.; Leese, F. Multiple-stressor effects on stream macroinvertebrate communities: A mesocosm experiment manipulating salinity, fine sediment and flow velocity. Sci. Total Environ. 2017, 610–611, 961–971. [Google Scholar] [CrossRef]
- Dube, T.; Denecker, L.; Vuren, J.H.J.V.; Wepener, V.; Smit, N.J.; Brendonck, L. Spatial and temporal variation of invertebrate community structure in flood-controlled tropical floodplain wetlands. J. Freshw. Ecol. 2017, 32, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.; Xu, H.; Vilmi, A.; Tolonen, K.T.; Tang, X.; Qin, B.; Gong, Z.; Heino, J. Relative roles of spatial processes, natural factors and anthropogenic stressors in structuring a lake macroinvertebrate metacommunity. Sci. Total Environ. 2017, 601–602, 1702–1711. [Google Scholar] [CrossRef] [PubMed]
- Reyjol, Y.; Argillier, C.; Bonne, W.; Borja, A.; Buijse, A.D.; Cardoso, A.C.; Daufresne, M.; Kernan, M.; Ferreira, M.T.; Poikane, S.; et al. Assessing the ecological status in the context of the european water framework directive: Where do we go now? Sci. Total Environ. 2014, 497–498, 332–344. [Google Scholar] [CrossRef]
- Heino, J.; Melo, A.S.; Siqueira, T.; Soininen, J.; Valanko, S.; Bini, L.M. Metacommunity organization, spatial extent and dispersal in aquatic system: Patterns, processes and prospects. Freshw. Biol. 2015, 60, 845–869. [Google Scholar] [CrossRef]
- Snaddon, C.; Davies, B.; Wishart, M. A Global Overview of Inter-Basin Water Transfer Schemes with an Appraisal of Their Ecological, Socio-Economic and Socio-Political Implications and Recommendations for Their Management; WRC Publication: Wiltshire, UK, 1999; TT 120/00. [Google Scholar]
- Qin, B.Q.; Zhou, J.; Elser, J.J.; Gardner, W.S.; Deng, J.M.; Brookes, J.D. Water Depth Underpins the Relative Role and Fates of Nitrogen and Phosphorus in Lakes. Environ. Sci. Technol. 2020, 54, 3191–3198. [Google Scholar] [CrossRef] [PubMed]
- Bazzantia, M.; Mastrantuonoa, L.; Pilottob, F. Depth-related response of macroinvertebrates to the reversal of eutrophication in a Mediterranean lake: Implications for ecological assessment. Sci. Total Environ. 2017, 579, 456–465. [Google Scholar] [CrossRef]
- Li, S. China’s huge investment on water facilities: An effective adaptation to climate change, natural disasters, and food security. Nat. Hazards 2012, 61, 1473–1475. [Google Scholar] [CrossRef]
- Guo, C.B.; Chen, Y.S.; Liu, H.; Lu, Y.; Qu, X.; Yuan, H.; Sovan, L.; Xie, S.G. Modelling fish communities in relation to water quality in the impoundedlakes of China’s South-to-North Water Diversion Project. Ecol. Model. 2019, 397, 25–35. [Google Scholar] [CrossRef]
- Zhuang, W. Eco-environmental impact of inter-basin water transfer projects: A review. Environ. Sci. Pollut. Res. 2016, 23, 12867–12879. [Google Scholar] [CrossRef] [PubMed]
- Dou, X. China’s inter-basin water management in the context of regional water shortage. Sustain. Water Resour. Manag. 2018, 4, 519–526. [Google Scholar] [CrossRef]




| Factors | Seasons | Regions | ||||
|---|---|---|---|---|---|---|
| X2 | df | p | X2 | df | p | |
| water depth (WD) | 18.99 | 3 | <0.001 | 2.07 | 2 | 0.355 |
| water temperature (WT) | 42.11 | 3 | <0.001 | 1.73 | 2 | 0.42 |
| sediment temperature (ST) | 44.45 | 3 | <0.001 | 0.68 | 2 | 0.712 |
| water transparency (SD) | 22.78 | 3 | <0.001 | 12.64 | 2 | 0.002 |
| Chlorophyll a (Chl-a) | 5.24 | 3 | 0.155 | 21.79 | 2 | <0.001 |
| total nitrogen (TN) | 21.56 | 3 | <0.001 | 19.39 | 2 | <0.001 |
| nitrate (NO3-N) | 8.85 | 3 | 0.031 | 25.85 | 2 | <0.001 |
| ammonium (NH4-N) | 21.33 | 3 | <0.001 | 22.08 | 2 | <0.001 |
| total phosphorus (TP) | 4.56 | 3 | 0.207 | 32.93 | 2 | <0.001 |
| orthophosphate (PO4-P) | 4.51 | 3 | 0.211 | 37.5 | 2 | <0.001 |
| total dissolved solids (TDS) | 4.8 | 3 | 0.187 | 31.97 | 2 | <0.001 |
| dissolved oxygen (DO) | 10.85 | 3 | 0.013 | 16.66 | 2 | <0.001 |
| electrical conductivity (Cond) | 3.89 | 3 | 0.274 | 23.87 | 2 | <0.001 |
| pH | 21.16 | 3 | <0.001 | 20.19 | 2 | <0.001 |
| Dominate Taxa | Annual | River Mouth | Canal | Lake | Spring | Summer | Autumn | Winter |
|---|---|---|---|---|---|---|---|---|
| Limnodrilus hoffmeisteri | 27.9 | 20.1 | 43.5 | 33.1 | 53.5 | 27.4 | 31.0 | 28.8 |
| Propsilocerus akamusi | 20.9 | 39.4 | 17.8 | 5.2 | ||||
| Tanypus concavus | 15.6 | 5.9 | 39.7 | 14.9 | 33.8 | 42.1 | ||
| Microchironomus tener | 7.7 | 22.6 | ||||||
| Culicoides sp. | 7.1 | 14.3 | 6.6 | |||||
| Limnodilus claparedianus | 8.5 | 9.8 | ||||||
| Bellamya purificata | 10.7 | |||||||
| Hemiclepsis sp. | 5.5 | |||||||
| Alocinma longicornis | 6.4 |
| F | p-Value | Ranking (Post Hoc Test or Contrasts) | |
|---|---|---|---|
| Biomass | |||
| Between-subjects (regions) | 3.41 | 0.079 | |
| Within-subjects (seasons) | 1.22 | 0.321 | |
| Season × region | 2.21 | 0.073 | |
| Density | |||
| Between-subjects (regions) | 55.18 | <0.001 | River Mouth > Lake > Canal |
| Within-subjects (seasons) | 14.17 | <0.001 | Spring vs. summer vs. autumn vs. winter |
| Season × region | 5.86 | <0.001 | Spring vs. summer vs. autumn vs. winter |
| Evenness index | |||
| Between-subjects (regions) | 2.62 | 0.127 | |
| Within-subjects (seasons) | 0.36 | 0.779 | |
| Season × region | 1.94 | 0.110 | |
| Margalef index | |||
| Between-subjects (regions) | 10.04 | 0.005 | Canal > Lake |
| Within-subjects (seasons) | 2.28 | 0.102 | |
| season×region | 1.20 | 0.335 | |
| Species richness | |||
| Between-subjects (regions) | 8.16 | 0.010 | River Mouth, Canal > Lake |
| Within-subjects (seasons) | 2.84 | 0.057 | Autumn vs. winter (F = 13.48, p = 0.005) |
| Season × region | 1.08 | 0.402 | |
| Shannon-Weiner index | |||
| Between-subjects (regions) | 4.67 | 0.041 | Canal > Lake |
| Within-subjects (seasons) | 1.63 | 0.206 | |
| Season × region | 2.50 | 0.047 | Spring vs. all others |
| Density of Chironomidae | |||
| Between-subjects (regions) | 41.04 | <0.001 | River Mouth > Lake > Canal |
| Within-subjects (seasons) | 19.81 | <0.001 | Spring vs. summer vs. autumn vs. winter |
| Season × region | 11.10 | 0.015 | Spring vs. all others, autumn vs. winter |
| % Chironomidae | |||
| Between-subjects (regions) | 101.99 | <0.001 | River Mouth, Lake > Canal |
| Within-subjects (seasons) | 3.85 | 0.021 | Spring vs. all others, autumn vs. winter |
| Season × region | 3.62 | 0.009 | |
| Density of Oligochaeta | |||
| Between-subjects (regions) | 2.17 | 0.171 | |
| Within-subjects (seasons) | 0.46 | 0.716 | |
| Season × region | 1.30 | 0.291 | |
| % Oligochaeta | |||
| Between-subjects (regions) | 6.41 | 0.019 | Canal > River Mouth |
| Within-subjects (seasons) | 2.47 | 0.084 | Spring vs. all others |
| Season × region | 1.11 | 0.382 | |
| Density of Mollusca | |||
| Between-subjects (regions) | 1.49 | 0.277 | |
| Within-subjects (seasons) | 1.86 | 0.161 | |
| Season × region | 3.55 | 0.073 | |
| % Mollusca | |||
| Between-subjects (regions) | 6.14 | 0.021 | Canal > River Mouth, Lake |
| Within-subjects (seasons) | 0.17 | 0.916 | |
| Season × region | 1.20 | 0.339 |
| Source | df | SS | MS | Pseudo-F | p (perm) |
|---|---|---|---|---|---|
| Region | 2 | 27,346 | 13673 | 11.065 | 0.0001 |
| Season | 3 | 12,860 | 4286.7 | 3.4692 | 0.0001 |
| Region × Season | 6 | 15,762 | 2627 | 2.126 | 0.0001 |
| Residual | 36 | 44,484 | 1235.7 | ||
| Total | 47 | 100,540 |
| Seasons | Variable | Adj.R2 | Pseudo-F | p | Seasons | Variable | Adj.R2 | Pseudo-F | p |
|---|---|---|---|---|---|---|---|---|---|
| Spring | ST | 0.27 | 5.10 | 0.001 | Autumn | PO4-P | 0.30 | 5.76 | 0.001 |
| pH | 0.45 | 4.28 | 0.002 | Cond | 0.52 | 5.63 | 0.001 | ||
| TDS | 0.51 | 2.04 | 0.043 | TP | 0.60 | 2.77 | 0.014 | ||
| WD | 0.54 | 1.57 | 0.073 | pH | 0.68 | 2.48 | 0.049 | ||
| Summer | NH4-N | 0.22 | 4.17 | 0.001 | Winter | PO4-P | 0.16 | 3.13 | 0.002 |
| pH | 0.35 | 2.97 | 0.004 | ST | 0.30 | 3.01 | 0.004 | ||
| SD | 0.44 | 2.52 | 0.010 | TDS | 0.40 | 2.42 | 0.026 | ||
| WD | 0.51 | 2.08 | 0.066 | pH | 0.52 | 2.30 | 0.039 |
| Investigation Time | Species Composition | Dominant Taxa |
|---|---|---|
| 1959 | Total 25 species. Annelida 2, Mollusca 17, Arthropoda 6 species | Dominant species were Bellamya quadrata, Limnoperna lacustris, Lymnaeidae spp., Unio douglasiae, Unio douglasiae, Semisulcospira cancellata, Corbicula fluminea |
| 1983–1984 | Total 68 species. Annelida 8, Mollusca36, Arthropoda 24 species (Insecta 15 families) | Dominant Mollusca species were Alocinma longicorris, Parafossarula siratulus, Bellamya lapidea, Corbicula fluminea; dominant Chironomidae were Chironomus plumosus and Chironomus attenuatus |
| 2010 | Total 37 species. Annelida 10, Mollusca 10, Arthropoda 16, Nomatoda 1species | Dominant species were Limnodrilus hoffmeisteri, Propsilocerus akamusi |
| 2012 | Total 72 species. Annelida 14, Mollusca15, Arthropoda 43 species | Dominant species were Limnodrilus hoffmeisteri, Propsilocerus akamusi, Tanypus concavus, Culicoides sp. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, W.; Pan, B.; Chen, J.; Jiang, X.; Shen, H.; Zhu, T. Macroinvertebrate Communities in a Lake of an Inter-Basin Water Transfer Project and Its Implications for Sustainable Management. Water 2020, 12, 1900. https://doi.org/10.3390/w12071900
Jiang W, Pan B, Chen J, Jiang X, Shen H, Zhu T. Macroinvertebrate Communities in a Lake of an Inter-Basin Water Transfer Project and Its Implications for Sustainable Management. Water. 2020; 12(7):1900. https://doi.org/10.3390/w12071900
Chicago/Turabian StyleJiang, Wanxiang, Baozhu Pan, Jing Chen, Xiaoming Jiang, Henglun Shen, and Tianshun Zhu. 2020. "Macroinvertebrate Communities in a Lake of an Inter-Basin Water Transfer Project and Its Implications for Sustainable Management" Water 12, no. 7: 1900. https://doi.org/10.3390/w12071900





