Selective Inhibition on Growth and Photosynthesis of Harmful Cyanobacteria (Microcystis aeruginosa) by Water Soluble Substances of Dendranthema indicum Flowers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture Condition
2.2. Experimental Design for Selective Effects of Aqueous Extract from D. indicum Flowers
2.3. Characterization of Its Selective Inhibition Substances
2.4. Determination of Growth Parameters
2.5. Measurement of Photosynthesis Pigments
2.6. Measurement of Chlorophyll Fluorescence Parameters
2.7. Data Analysis
3. Results
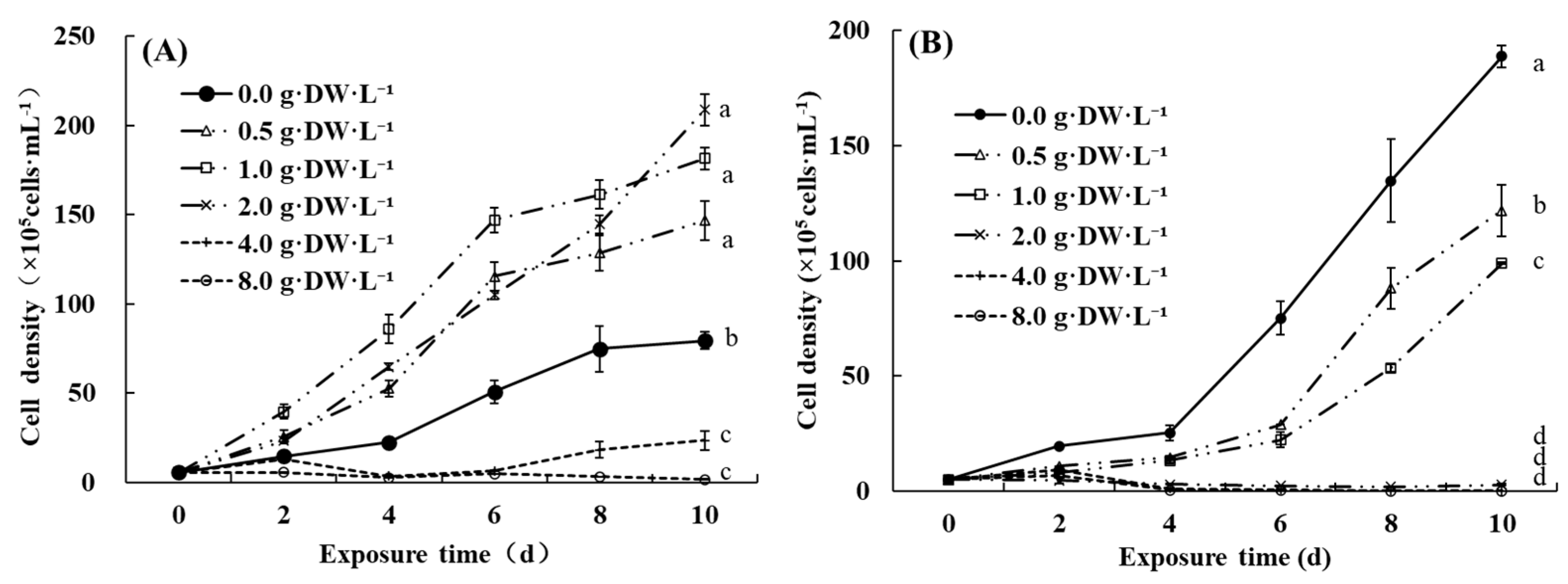
3.1. Concentration-Dependent Effects of Aqueous Extracts from D. indicum Flowers
3.2. Species-Specific Effects of Aqueous Extracts from D. indicum Flowers
3.3. Effects of Water Extraction Temperature on Cyanoabcterial Inhibition by Aqueous Extracts of D. indicum Flowers
3.4. Effects of Eluting Solvents on Cyanoabcterial Inhibition by SPE-Enriched Aqueous Extracts of D. indicum Flowers
4. Discussion
4.1. Selective Growth Inhibition of Cyanobacteria and the Promotion of Green Algae by Aqueous Extracts of D. indicum Flowers
4.2. Chemical Characteristics of the Selective Inhibition Substances of D. indicum Flowers
4.3. Photosynthetic Activity of M. aeruginosa Treated with Water-Soluble Substances of D. indicum Flowers
4.4. The Application of Water-Soluble Substances of D. indicum Flowers in Aquaculture Ponds
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Paerl, H.W.; Otten, T.G. Harmful Cyanobacterial Blooms: Causes, Consequences, and Controls. Microb. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Li, M.; Reynolds, C.S. Colony formation in the cyanobacterium Microcystis. Biol. Rev. 2018, 93, 1399–1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinden, A.; Sinang, S.C. Cyanobacteria in aquaculture systems: Linking the occurrence, abundance and toxicity with rising temperatures. Int. J. Environ. Sci. Technol. 2016, 13, 2855–2862. [Google Scholar] [CrossRef]
- Rimando, A.M.; Schrader, K.K. Off-Flavors in Aquaculture; American Chemical Society: Washington, DC, USA, 2003. [Google Scholar]
- Dai, R.; Wang, P.; Jia, P.; Zhang, Y.; Chu, X.; Wang, Y. A review on factors affecting microcystins production by algae in aquatic environments. World J. Microbiol. Biotechnol. 2016, 32, 1–7. [Google Scholar] [CrossRef]
- Gobler, C.J.; Burkholder, J.A.M.; Davis, T.W.; Harke, M.J.; Johengen, T.; Stow, C.A.; Van de Waal, D.B. The dual role of nitrogen supply in controlling the growth and toxicity of cyanobacterial blooms. Harmful Algae 2016, 54, 87–97. [Google Scholar] [CrossRef]
- Paerl, H.W.; Xu, H.; McCarthy, M.J.; Zhu, G.; Qin, B.; Li, Y.; Gardner, W.S. Controlling harmful cyanobacterial blooms in a hyper-eutrophic lake (Lake Taihu, China): The need for a dual nutrient (N & P) management strategy. Water Res. 2011, 45, 1973–1983. [Google Scholar]
- Mitrovic, S.M.; Hardwick, L.; Dorani, F. Use of flow management to mitigate cyanobacterial blooms in the Lower Darling River, Australia. J. Plankton Res. 2011, 33, 229–241. [Google Scholar] [CrossRef]
- Visser, P.M.; Ibelings, B.W.; Bormans, M.; Huisman, J. Artificial mixing to control cyanobacterial blooms: A review. Aquat. Ecol. 2016, 50, 423–441. [Google Scholar] [CrossRef] [Green Version]
- Matthijs, H.C.P.; Visser, P.M.; Reeze, B.; Meeuse, J.; Slot, P.C.; Wijn, G.; Talens, R.; Huisman, J. Selective suppression of harmful cyanobacteria in an entire lake with hydrogen peroxide. Water Res. 2012, 46, 1460–1472. [Google Scholar] [CrossRef]
- Matthijs, H.C.P.; Jančula, D.; Visser, P.M.; Maršálek, B. Existing and emerging cyanocidal compounds: New perspectives for cyanobacterial bloom mitigation. Aquat. Ecol. 2016, 50, 443–460. [Google Scholar] [CrossRef] [Green Version]
- Van Wichelen, J.; Vanormelingen, P.; Codd, G.A.; Vyverman, W. The common bloom-forming cyanobacterium Microcystis is prone to a wide array of microbial antagonists. Harmful Algae 2016, 55, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Liu, J. Practical success of biomanipulation using filter-feeding Fish to control cyanobacteria blooms: A synthesis of decades of research and application in a subtropical hypereutrophic lake. Sci. World J. 2001, 1, 337–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, F.; Gao, Y.; Yu, T.; Zhang, Y.; Xu, D.; Xiao, E.; He, F.; Zhou, Q.; Wu, Z. The management of undesirable cyanobacteria blooms in channel catfish ponds using a constructed wetland: Contribution to the control of off-flavor occurrences. Water Res. 2011, 45, 6479–6488. [Google Scholar] [CrossRef]
- Blanchard, J.L.; Watson, R.A.; Fulton, E.A.; Cottrell, R.S.; Nash, K.L.; Bryndum-Buchholz, A.; Büchner, M.; Carozza, D.A.; Cheung, W.W.L.; Elliott, J.; et al. Linked sustainability challenges and trade-offs among fisheries, aquaculture and agriculture. Nat. Ecol. Evol. 2017, 1, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Liu, B.; Wang, J.; Gao, Y.; Wu, Z. Study on the mechanism of allelopathic influence on cyanobacteria and chlorophytes by submerged macrophyte (Myriophyllum spicatum) and its secretion. Aquat. Toxicol. 2010, 98, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Švanys, A.; Paškauskas, R.; Hilt, S. Effects of the allelopathically active macrophyte Myriophyllum spicatum on a natural phytoplankton community: A mesocosm study. Hydrobiologia 2013, 737, 57–66. [Google Scholar] [CrossRef]
- Le Rouzic, B.; Thiébaut, G.; Brient, L. Selective growth inhibition of cyanobacteria species (Planktothrix agardhii) by a riparian tree leaf extract. Ecol. Eng. 2016, 97, 74–78. [Google Scholar] [CrossRef]
- Tanuja, P.; Venugopal, N.; Sashidhar, R.B. Development and evaluation of thin-layer chromatography-digital image-based analysis for the quantitation of the botanical pesticide azadirachtin in agricultural matrixes and commercial formulations: Comparison with ELISA. J. AOAC Int. 2007, 90, 857–863. [Google Scholar] [CrossRef] [Green Version]
- Tu, Y.Y. Artemisia Annua and Artemisinin-Based Drugs; Chemical Industry Press: Beijing, China, 2009. [Google Scholar]
- Van Hai, N. The use of medicinal plants as immunostimulants in aquaculture: A review. Aquaculture 2015, 446, 88–96. [Google Scholar] [CrossRef]
- Zhou, K.; Guo, W.; Xu, Y. Advances of research on allelopathic potential in Compositae. Acta Ecol. Sin. 2004, 24, 1776–1784. [Google Scholar]
- Rasdi, N.H.M.; Samah, O.A.; Sule, A.; Ahmed, Q.U. Antimicrobial studies of Cosmos caudatus Kunth. (Compositae). J. Med. Plants Res. 2010, 4, 669–673. [Google Scholar]
- Bukhari, I.A. The central analgesic and anti-inflammatory activities of the methanolic extract of Carthamus oxycantha. J. Physiol. Pharmacol. 2013, 64, 369–375. [Google Scholar] [PubMed]
- Gao, Y.N.; Liu, B.Y.; Ge, F.J.; He, Y.; Lu, Z.Y.; Zhou, Q.H.; Zhang, Y.Y.; Wu, Z.B. Joint effects of allelochemical nonanoic acid, n-phenyl-1-naphtylamine and caffeic acid on the growth of Microcystis aeruginosa. Allelopath. J. 2015, 35, 249–258. [Google Scholar]
- Xu, F.; He, W.; Zheng, X.; Zhang, W.; Cai, W.; Tang, Q. Inhibitive effects on Microcystis aeruginosa by Artemisia lavandulaefoli and its three organic solvents extracts. Acta Ecol. Sin. 2010, 30, 745–750. [Google Scholar]
- Fang, H.; Guo, Q.; Shen, H. Study on antioxidation ability of essential oil from Dendranthema indicum dry flower in five edible oils. J. Plant Resour. Environ. 2010, 19, 54–59. [Google Scholar]
- Liu, X.Q.; Zhang, X.F.; Lee, K.S. Antimicrobial activity of the extracts of Forsythia suspensa and Dendranthema indicum. J. Appl. Biol. Chem. 2005, 48, 29–31. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Ni, L.X.; Acharya, K.; Hao, X.Y.; Li, S.Y.; Li, Y.; Li, Y.P. Effects of artemisinin on photosystem II performance of Microcystis aeruginosa by in vivo chlorophyll fluorescence. Bull. Environ. Contam. Toxicol. 2012, 89, 1165–1169. [Google Scholar] [CrossRef]
- Fan, L.; Xu, P.; Wu, W.; Qu, J.; Qiu, L.; Chen, J. Regulation of micro-ecological environment in freshwater aquaculture pond: A review. Chin. J. Ecol. 2013, 32, 3094–3100. [Google Scholar]
- Daume, S. The roles of bacteria and micro and macro algae in abalone aquaculture: A review. J. Shellfish Res. 2006, 25, 151–157. [Google Scholar] [CrossRef]
- Cong, H.; Sun, F.; Wu, J.; Zhou, Y.; Yan, Q.; Ren, A.; Xu, H. Study on method and mechanism of deep well circulation for the growth control of Microcystis in aquaculture pond. Water Sci. Technol. 2017, 75, 2692–2701. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.L.; Boyer, G.L.; Zimba, P.V. A review of cyanobacterial odorous and bioactive metabolites: Impacts and management alternatives in aquaculture. Aquaculture 2008, 280, 5–20. [Google Scholar] [CrossRef]
- Qin, J.; Culver, D.A.; Yu, N. Effect of organic fertilizer on heterotrophs and autotrophs: Implications for water quality management. Aquac. Res. 1995, 26, 911–920. [Google Scholar] [CrossRef]
- Terziyski, D.; Grozev, G.; Kalchev, R.; Stoeva, A. Effect of organic fertilizer on plankton primary productivity in fish ponds. Aquac. Int. 2007, 15, 181–190. [Google Scholar] [CrossRef]
- Lee, D.Y.; Choi, G.; Yoon, T.; Cheon, M.S.; Choo, B.K.; Kim, H.K. Anti-inflammatory activity of Chrysanthemum indicum extract in acute and chronic cutaneous inflammation. J. Ethnopharmacol. 2009, 123, 149–154. [Google Scholar] [CrossRef]
- Shunying, Z.; Yang, Y.; Huaidong, Y.; Yue, Y.; Guolin, Z. Chemical composition and antimicrobial activity of the essential oils of Chrysanthemum indicum. J. Ethnopharmacol. 2005, 96, 151–158. [Google Scholar] [CrossRef]
- Chen, X.; Huang, G.; Fu, H.; An, C.; Yao, Y.; Cheng, G.; Suo, M. Allelopathy inhibitory effects of Hydrodictyon reticulatum on Chlorella pyrenoidosa under co-culture and liquor-cultured conditions. Water 2017, 9, 416. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Dai, Q. Determination of active ingredients in flos Chrysanthemi Indici. Guangdong Chem. Ind. 2019, 46, 195–196. [Google Scholar]
- He, D.; Zhang, W.; Qin, M. Content analysis of flavonoids in Dendranthema indicum flower from different locations by HPLC. J. Plant Resour. Environ. 2009, 18, 91–93. [Google Scholar]
- Huang, H.; Xiao, X.; Ghadouani, A.; Wu, J.; Nie, Z.; Peng, C.; Xu, X.; Shi, J. Effects of natural flavonoids on photosynthetic activity and cell integrity in Microcystis aeruginosa. Toxins 2015, 7, 66–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Peng, X.; Liu, B.; Zhang, Y.; Zhou, Q.; Wu, Z. Effects of the decomposing liquid of Cladophora oligoclona on Hydrilla verticillata turion germination and seedling growth. Ecotoxicol. Environ. Saf. 2018, 157, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Appenroth, K.J.; Stöckel, J.; Srivastava, A.; Strasser, R.J. Multiple effects of chromate on the photosynthetic apparatus of Spirodela polyrhiza as probed by OJIP chlorophyll a fluorescence measurements. Environ. Pollut. 2001, 115, 49–64. [Google Scholar] [CrossRef]
- Vaseeharan, B.; Thaya, R. Medicinal plant derivatives as immunostimulants: An alternative to chemotherapeutics and antibiotics in aquaculture. Aquac. Int. 2014, 22, 1079–1091. [Google Scholar] [CrossRef]





| Parameters | Formulae and Terms | Illustrations |
|---|---|---|
| Vj | Vj = (Fj − Fo)/(Fm − Fo) | Relative variable fluorescence intensity at the J-step |
| Mo | Mo = 4(F300μs − Fo)/(Fm − Fo) | Approximated initial slope of the fluorescence transient |
| Quantum efficiencies or flux ratios | ||
| Fv/Fm | Fv/Fm = TRo/ABS= 1 − Fo/Fm = φPo | Maximum quantum yield for primary photochemistry |
| Ψo | Ψo = ETo/TRo = (Fm − Fj)/(Fm − Fo) | Probability that a trapped exciton moves an electron into the electron transport chain beyond QA (at t = 0) |
| φEo | φEo = ETo/ABS= (Fm − Fj)/Fm | Quantum yield for electron transport (at t = 0) |
| Specific activities or specific fluxes | ||
| ABS/RC | ABS/RC = Mo·(1/Vj)·(1/φPo) | Absorption flux per reaction center |
| TRo/RC | TRo/RC = Mo·(1/Vj) | Trapped energy flux per reaction center (at t = 0) |
| ETo/RC | ETo/RC = Mo·(1/Vj)·Ψo | Electron transport flux per reaction center (at t = 0) |
| DIo/RC | DIo/RC = (ABS/RC) − (TRo/RC) | Dissipated energy flux per reaction center (at t = 0) |
| Performance indexes | ||
| PIABS | PIABS = (RC/ABS)·[φPo/(1 − φPo)]·[Ψo/(1 − Ψo)] | Performance index on absorption basis |
| Parameters | Time | Treatments | ||
|---|---|---|---|---|
| Control | 25 °C | 100 °C | ||
| Fv/Fm | day 3 | 0.44 ± 0.01 a | 0.36 ± 0.01 b | 0.37 ± 0.01 b |
| day 6 | 0.55 ± 0.01 a | 0.36 ± 0.08 b | 0.41 ± 0.04 b | |
| day 9 | 0.54 ± 0.01 a | 0.41 ± 0.03 b | 0.41 ± 0.04 b | |
| ψo | day 3 | 0.61 ± 0.01 a | 0.55 ± 0.03 b | 0.55 ± 0.02 b |
| day 6 | 0.67 ± 0.01 a | 0.49 ± 0.03 b | 0.47 ± 0.02 b | |
| day 9 | 0.66 ± 0.02 a | 0.58 ± 0.01 ab | 0.53 ± 0.07 b | |
| φEo | day 3 | 0.27 ± 0.01 a | 0.19 ± 0.01 b | 0.21 ± 0.01 b |
| day 6 | 0.37 ± 0.01 a | 0.18 ± 0.03 b | 0.19 ± 0.02 b | |
| day 9 | 0.35 ± 0.02 a | 0.24 ± 0.02 b | 0.21 ± 0.02 b | |
| ABS/RC | day 3 | 2.30 ± 0.17 a | 2.86 ± 0.32 a | 2.65 ± 0.15 a |
| day 6 | 1.72 ± 0.18 a | 2.72 ± 0.52 b | 2.47 ± 0.25 ab | |
| day 9 | 1.69 ± 0.06 a | 2.26 ± 0.30 b | 2.10 ± 0.19 ab | |
| TRo/RC | day 3 | 1.02 ± 0.09 a | 1.02 ± 0.10 a | 0.99 ± 0.05 a |
| day 6 | 0.94 ± 0.09 a | 0.95 ± 0.07 a | 1.00 ± 0.03 a | |
| day 9 | 0.91 ± 0.02 a | 0.92 ± 0.07 a | 0.85 ± 0.11 a | |
| ETo/RC | day 3 | 0.62 ±0.05 a | 0.55 ± 0.06 a | 0.55 ± 0.04 a |
| day 6 | 0.63 ± 0.06 a | 0.47 ± 0.02 b | 0.47 ± 0.01 b | |
| day 9 | 0.59 ± 0.01 a | 0.53 ± 0.03 b | 0.45 ± 0.00 c | |
| DIo/RC | day 3 | 1.28 ±0.08 a | 1.85 ± 0.22 b | 1.66 ± 0.12 ab |
| day 6 | 0.78 ± 0.09 a | 1.77 ± 0.56 b | 1.47 ± 0.24 b | |
| day 9 | 0.78 ± 0.04 a | 1.34 ± 0.23 b | 1.25 ± 0.14 b | |
| PIABS | day 3 | 0.54 ± 0.05 a | 0.23 ± 0.04 b | 0.28 ± 0.03 b |
| day 6 | 1.43 ± 0.22 a | 0.21 ± 0.09 b | 0.25 ± 0.06 b | |
| day 9 | 1.32 ± 0.20 a | 0.44 ± 0.13 b | 0.38 ± 0.10 b | |
| Parameters | Time | Treatments | |||||
|---|---|---|---|---|---|---|---|
| Control | Hexane | Dichloromethane | Ethyl Acetate | Acetone | Methanol | ||
| Fv/Fm | day 3 | 0.53 ± 0.01 a | 0.52 ± 0.01 a | 0.38 ± 0.02 b | 0.37 ± 0.01 b | 0.40 ± 0.01 b | 0.38 ± 0.01 b |
| day 6 | 0.53 ± 0.01 a | 0.51 ± 0.01 a | 0.44 ± 0.00 b | 0.42 ± 0.01 b | 0.38 ± 0.01 c | 0.37 ± 0.01 c | |
| day 9 | 0.53 ± 0.02 a | 0.51 ± 0.01 a | 0.50 ± 0.00 ab | 0.48 ± 0.01 b | 0.44 ± 0.01 c | 0.36 ± 0.01 d | |
| ψo | day 3 | 0.79 ± 0.01 a | 0.77 ± 0.03 a | 0.61 ± 0.01 c | 0.66 ± 0.01 b | 0.70 ± 0.01 b | 0.68 ± 0.02 b |
| day 6 | 0.80 ±0.01 a | 0.81 ± 0.00 a | 0.69 ± 0.01 cd | 0.74 ± 0.01 b | 0.71 ± 0.01 bc | 0.66 ± 0.03 d | |
| day 9 | 0.79 ± 0.03 a | 0.81 ± 0.01 a | 0.71 ± 0.01 b | 0.75 ± 0.02 b | 0.74 ± 0.00 b | 0.74 ± 0.01 b | |
| φEo | day 3 | 0.42 ± 0.01 a | 0.40 ± 0.02 a | 0.23 ± 0.02 b | 0.24 ± 0.01 b | 0.28 ± 0.01 b | 0.25 ± 0.00 b |
| day 6 | 0.42 ± 0.01 a | 0.41 ± 0.01 a | 0.31 ± 0.01 b | 0.31 ± 0.01 b | 0.27 ± 0.01 c | 0.25 ± 0.01 d | |
| day 9 | 0.37 ± 0.03 a | 0.36 ± 0.01 a | 0.36 ± 0.01 a | 0.36 ± 0.01 a | 0.32 ± 0.01 b | 0.27 ± 0.00 c | |
| ABS/RC | day 3 | 2.43 ± 0.23 a | 2.51 ± 0.25 a | 3.03 ± 0.44 a | 3.69 ± 0.74 a | 2.88 ± 0.20 a | 3.16 ± 0.21 a |
| day 6 | 2.36 ± 0.07 b | 2.51 ± 0.09 b | 2.40 ± 0.06 b | 2.96 ± 0.04 ab | 2.88 ± 0.34 ab | 3.44 ± 0.54 a | |
| day 9 | 2.43 ± 0.06 b | 2.93 ± 0.12 b | 2.16 ± 0.12 c | 2.26 ±0.07 c | 2.71 ± 0.20 b | 3.31 ± 0.08 a | |
| TRo/RC | day 3 | 1.28 ± 0.11 a | 1.30 ± 0.13 a | 1.16 ± 0.11 a | 1.37 ± 0.28 a | 1.16 ± 0.08 a | 1.19 ± 0.10 a |
| day 6 | 1.24 ± 0.03 a | 1.29 ± 0.04 a | 1.06 ± 0.02 b | 1.24 ± 0.05 a | 1.10 ± 0.17 b | 1.28 ± 0.22 a | |
| day 9 | 1.24 ± 0.06 a | 1.30 ± 0.02 a | 1.08 ± 0.06 b | 1.09 ± 0.03 b | 1.19 ± 0.06 ab | 1.19 ± 0.03 ab | |
| ETo/RC | day 3 | 1.01 ± 0.09 a | 1.00 ± 0.13 a | 0.71 ± 0.06 b | 0.90 ± 0.18 a | 0.81 ± 0.06 ab | 0.80 ± 0.05 ab |
| day 6 | 0.99 ± 0.0 a | 1.04 ± 0.03 a | 0.73 ± 0.03 b | 0.92 ± 0.04 ab | 0.78 ± 0.11 b | 0.84 ± 0.11 a | |
| day 9 | 0.98 ± 0.08 a | 1.05 ± 0.01 a | 0.77 ± 0.04 b | 0.82 ± 0.04 b | 0.88 ± 0.04 ab | 0.88 ± 0.02 ab | |
| DIo/RC | day 3 | 1.15 ± 0.12 a | 1.21 ± 0.12 ab | 1.87 ± 0.33 b | 2.33 ± 0.46 b | 1.71 ± 0.12 b | 1.97 ± 0.11 b |
| day 6 | 1.12 ± 0.06 a | 1.22 ± 0.07 a | 1.34 ± 0.04 ab | 1.72 ± 0.03 b | 1.79 ± 0.17 bc | 2.15 ± 0.32 c | |
| day 9 | 1.42 ± 0.05 b | 1.63 ± 0.09 b | 1.08 ± 0.07 c | 1.16 ± 0.04 c | 1.52 ± 0.14 b | 2.12 ± 0.06 a | |
| PIABS | day 3 | 1.73 ± 0.23 a | 1.43 ± 0.24 a | 0.33 ± 0.09 b | 0.32 ± 0.08 b | 0.55 ± 0.06 b | 0.40 ± 0.04 b |
| day 6 | 1.86 ± 0.14 a | 1.75 ± 0.12 a | 0.74 ± 0.04 b | 0.70 ± 0.05 b | 0.54 ± 0.06 b | 0.35 ± 0.10 c | |
| day 9 | 1.73 ± 0.29 a | 1.13 ± 0.13 a | 1.13 ± 0.09 a | 1.08 ± 0.00 a | 0.82 ± 0.10 ab | 0.48 ± 0.01 b | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Zhang, F.; Wu, J.; Yang, H.; Dong, J.; Zhang, M.; Li, X. Selective Inhibition on Growth and Photosynthesis of Harmful Cyanobacteria (Microcystis aeruginosa) by Water Soluble Substances of Dendranthema indicum Flowers. Water 2020, 12, 2014. https://doi.org/10.3390/w12072014
Gao Y, Zhang F, Wu J, Yang H, Dong J, Zhang M, Li X. Selective Inhibition on Growth and Photosynthesis of Harmful Cyanobacteria (Microcystis aeruginosa) by Water Soluble Substances of Dendranthema indicum Flowers. Water. 2020; 12(7):2014. https://doi.org/10.3390/w12072014
Chicago/Turabian StyleGao, Yunni, Fang Zhang, Jing Wu, Hui Yang, Jing Dong, Man Zhang, and Xuejun Li. 2020. "Selective Inhibition on Growth and Photosynthesis of Harmful Cyanobacteria (Microcystis aeruginosa) by Water Soluble Substances of Dendranthema indicum Flowers" Water 12, no. 7: 2014. https://doi.org/10.3390/w12072014





