Effectiveness and Energy Requirements of Pasteurisation for the Treatment of Unfiltered Secondary Effluent from a Municipal Wastewater Treatment Plant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Laboratory Scale Testing
2.1.1. Male Specific Coliphage (MS2) Tests
2.1.2. Escherichia coli Tests
2.1.3. Enterococci Tests
2.1.4. Coxsackievirus and Adenovirus Tests
2.1.5. Cryptosporidium Infectivity Test
2.1.6. Helminths Tests
2.1.7. Effect of Turbidity Test
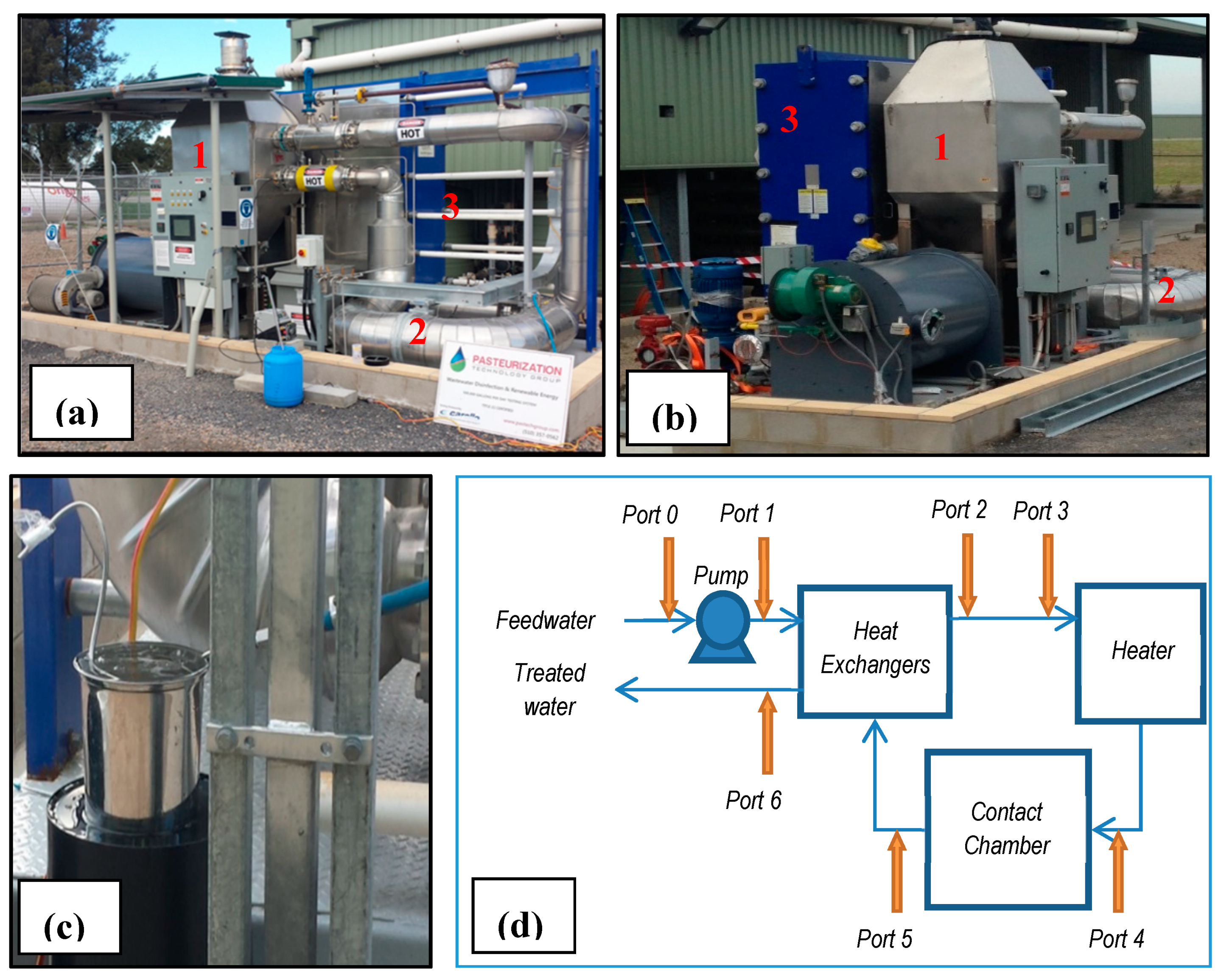
2.2. Pilot Plant Testing
2.2.1. Contact Chamber Testing
2.2.2. Whole Plant Testing
2.2.3. Clean in Place
3. Results
3.1. Water Quality Data
3.2. Laboratory-Scale Testing
3.3. Pilot Scale Testing
3.3.1. Contact Chamber Tests
3.3.2. Entire Plant Tests
3.3.3. Energy Efficiency Changes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Natural Resource Management Ministerial Council. Environment Protection and Heritage Council, Australian Health Minister’s Conference, Australian Government, Australian Guidelines for Water Recycling: Managing Health and Environmental Risks (Phase 1); Environment Protection and Heritage Council: Adelaide, South Australia, Australia, 2006.
- The Water Research Foundation, Leaders Innovation Forum for Technology. Available online: https://www.waterrf.org/news/pasteurization-technology-group (accessed on 1 June 2020).
- Salveson, A. Use of Pasteurization for Pathogen Inactivation for Ventura Water. In 2012 Guidelines for Water Reuse; EPA/600/R-12/618; US EPA Office of Research and Development: Washington, DC, USA, 2012; p. D-55. [Google Scholar]
- Da Costa, J.B.; Rodgher, S.; Daniel, L.A.; Espíndola, E.L. Toxicity on aquatic organisms exposed to secondary effluent disinfected with chlorine, peracetic acid, ozone and UV radiation. Ecotoxicology 2014, 23, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Abou-Elela, S.I.; El-Sayed, M.M.H.; El-Gendy, A.S.; Abou-Taleb, E.M. Comparative Study of Disinfection of Secondary Treated Wastewater Using Chlorine, UV and Ozone. J. Appl. Sci. Res. 2012, 8, 5190–5197. [Google Scholar]
- Blatchley, E.R.; Hunt, B.A.; Duggirala, R.; Thompson, J.E.; Zhao, J.; Halaby, T.; Cowger, R.L.; Straub, C.M.; Alleman, J.E. Effects of disinfectants on wastewater effluent toxicity. Water Res. 1997, 31, 1581–1588. [Google Scholar] [CrossRef]
- Gray, N.F. Ozone Disinfection in Microbiology of Waterborne Disease, Microbial Aspects of Risks, 2nd ed.; Percival, S.L., Yates, P.V., Williams, D.W., Chalmers, R.M., Gray, N.F., Eds.; Academic Press: Cambridge, MA, USA, 2014; Chapter 33; pp. 599–615. [Google Scholar]
- Gray, N.F. Ultraviolet Disinfection in Microbiology of Waterborne Diseases, Microbial Aspects of Risks, 2nd ed.; Percival, S.L., Yates, P.V., Williams, D.W., Chalmers, R.M., Gray, N.F., Eds.; Academic Press: Cambridge, MA, USA, 2014; Chapter 34; pp. 617–630. [Google Scholar]
- Den Besten, H.M.W.; van der Mark, E.J.; Hensen, L.; Abee, T.; Zwietering, M.H. Quantification of the Effect of Culturing Temperature on Salt-Induced Heat Resistance of Bacillus Species. Appl. Environ. Microbiol. 2010, 76, 4286–4292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juneja, V.K.; Altuntaş, E.G.; Ayhan, K.; Hwang, C.A.; Sheen, S. Mendel Friedman predictive model for the reduction of heat resistance of Listeria monocytogenes in ground beef by the combined effect of sodium chloride and apple polyphenols. Int. J. Food Microbiol. 2013, 164, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Volkin, D.B.; Burke, C.J.; Marfia, K.E.; Oswald, C.B.; Wolanski., B.; Middaugh, C.R. Size and conformational stability of the hepatitis A virus used to prepare VAQTA, a highly purified inactivated vaccine. J. Pharm. Sci. 1997, 86, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Institute of Food Technologists for the Food and Drug Administration of the U.S. Department of Health and Human Services, Kinetics of Microbial Inactivation for Alternative Food Processing Technologies. J. Food Sci. 2000, 65 (Suppl. S8), 16–31.
- Kaur, J.; Ledward, D.A.; Park, R.W.; Robson, R.L. Factors affecting the heat resistance of Escherichia coli O157:H7. Lett. Appl. Microbiol. 1998, 26, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Geornaras, I.; Belk, K.E.; Smith, G.C.; Sofos, J.N. Thermal Inactivation of Acid, Cold, Heat, Starvation, and Desiccation Stress–Adapted Escherichia coli O157:H7 in Moisture-Enhanced Nonintact Beef. J. Food Prot. 2011, 74, 531–538. [Google Scholar] [CrossRef] [PubMed]
- US EPA Office of Research and Development. 2012 Guidelines for Water Reuse; EPA/600/R-12/618; US EPA Office of Research and Development: Washington, DC, USA, 2012.
- Lau, M.; Monis, P.; Ryan, G.; Salveson, A.; Blackbeard, J.; Gray, S.; Sanciolo, P. Selection of surrogate pathogens and process indicator organisms for pasteurisation of municipal wastewater—A survey of literature data on heat inactivation of pathogens. Process. Saf. Environ. Prot. 2020, 133, 301–314. [Google Scholar] [CrossRef]
- Kahler, A.M.; Cromeans, T.L.; Roberts, J.M.; Hill, V.R. Source water quality effects on monochloramine inactivation of adenovirus, coxsackievirus, echovirus, and murine norovirus. Water Res. 2011, 45, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- King, B.J.; Keegan, A.R.; Robinson, B.S.; Monis, P.T. Cryptosporidium cell culture infectivity assay design. Parasitology 2011, 138, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Jeska, E.L.; Caruso, J.P.; Donohue, M.J. Collection of fertile Ascaris suum eggs. J. Parasitol. 1986, 72, 964–965. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Office of Water, Guidance Manual for Disinfection Profiling and Benchmarking; EPA 815-R-99-013; USEPA: Washington, DC, USA, 1999.
- Department of Health, Government of State of Victoria. Guidelines for Validating Treatment Processes for Pathogen Reduction-Supporting Class a Recycled Water Schemes in Victoria; Department of Health, Government of State of Victoria: Melbourne, Victoria, Australia, 2013.
- Cunault, C.; Burton, C.H.; Pourcher, A.M. The impact of fouling on the process performance of the thermal treatment of pig slurry using tubular heat exchangers. J. Environ. Manag. 2013, 117, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Australian Energy Regulator. Report 14–20; Australian Energy Regulator: Melbourne, Australia, 2018.
- Moce-Llivina, L.; Muniesa, M.; Pimenta-Vale, H.; Lucena, F.; Jofre, J. Survival of Bacterial Indicator Species and Bacteriophages after Thermal Treatment of Sludge and Sewage. Appl. Environ. Microbiol. 2003, 69, 1452–1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, N.L.; Smith, S.R. Time and temperature inactivation kinetics of enteric bacteria relevant to sewage sludge treatment processes for agricultural use. Water Res. 2008, 42, 2229–2241. [Google Scholar] [CrossRef] [PubMed]
- Sahlstrom, L.; Bagge, E.; Emmoth, E.; Holmqvist, A.; Danielsson-Tham, M.L.; Albihn, A. A laboratory study of survival of selected microorganisms after heat treatment of biowaste used in biogas plants. Bioresour. Technol. 2008, 99, 7859–7865. [Google Scholar] [CrossRef] [PubMed]
- Pasteurization Technology Group. Available online: https://www.ptgwe.com/wp-content/uploads/2017/08/Pasteurization_Melbourne-Water-and-WaterRF-Updates-WEFTEC-2015.pdf (accessed on 1 June 2020).
- USEPA. Office of Water, Membrane Filtration Guidance Manual; EPA 815-R-06-009; USEPA: Washington, DC, USA, 2005.
- Melbourne Water. 2018. Available online: https://medium.com/mark-and-focus/melbourne-water-harnessing-renewable-energy-b6aab6809b71 (accessed on 1 June 2020).
- GE—Jenbacker Gas Engines Technical Description. 2007. Available online: http://www.provincia.livorno.it/new/spawdocs/ambiente/Technical%20Description_AB%20Energy%20320.pdf (accessed on 1 June 2020).
- Hallet, C.K. Energy intensity of water: Literature suggests increasing interest despite limited and inconsistent data. In Proceedings of the ASME 2011 International Mechanical Engineering Congress & Exposition IMECE2011, Denver, CO, USA, 11–17 November 2011. [Google Scholar]






| Month of Sampling | April | April | June | June |
|---|---|---|---|---|
| Pond ID * | P2 | P10 | P2 | P10 |
| Escherichia coli (cfu/100 mL) | 71,000 | 7 | 520,000 | 6 |
| Faecal coliforms (cfu/100 mL) | 71,000 | 29 | 520,000 | 15 |
| Enterococci (cfu/100 mL) | 48,000 | 9 | 23,000 | 0 |
| FRNA (pfu/mL) | 30 | 680 | - | - |
| pH | 7.3 | 8.217 | - | - |
| Turbidity (NTU) | 8.5 | 1.879 | - | - |
| Ammonia (mg/L) | 67.8 | <0.5 | 63.0 | 0.2 |
| Nitrate+nitrite (mg/L) | 0.007 | 21.7 | 0.007 | 20.9 |
| Phosphate (mg/L) | 10.9 | 8.988 | 10.9 | 8.4 |
| TKN (mg/L) | 63.8 | 1.659 | 65.5 | 1.7 |
| DOC (mg/L) | 15.3 | 8.9 | 17.1 | 8.4 |
| TOC (mg/L) | 21.2 | 10.7 | 29.5 | 9.5 |
| BOD (mg/L) | 14 | <2 | 24 | <2 |
| COD (mg/L) | 104 | 223 | 127 | 84 |
| Conductivity (EC) (µScm) | 2150 | 1860 | 2170 | 1700 |
| TDS (mg/L) | 1200 | 1000 | 1200 | 940 |
| SS (mg/L) | 12 | 4 | ||
| VSS (mg/L) | 11 | 2 | - | - |
| Test Temperature (°C) | Alkalinity (mg/L as CaCO3) | pH | Ca (mg/L) | Turbidity (NTU) | SS (mg/L) | VSS (mg/L) | COD (mg/L) | TOC (mg/L) | EC (uS/cm) | UVT (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 75, 72, 69, 66 | 60 | 6.6 | 16 | 3.5 | 16 | 6 | 65 | 14 | 850 | 44 |
| 68, 64, 60, 57 | 42 | 6.6 | 16 | 6.8 | 24 | 6 | 60 | 15 | 830 | 41 |
| 75, 72, 69 | 59 | 6.9 | 18 | 4.6 | 24 | 4 | 70 | 15 | 890 | 35 |
| 75, 72, 69 | 43 | 6.6 | 16 | 2.3 | 16 | 2 | 50 | 13 | 730 | 43 |
| 75, 72, 69 | 53 | 6.7 | 17 | 9 | 23 | 33 | 44 | 16 | 830 | 40 |
| Infectious Agent | Temperature (°C) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 55 | 65 | 75 | |||||||
| Contact Time (Seconds) | Contact Time (Seconds) | Contact Time (Seconds) | |||||||
| 5 | 30 | 60 | 5 | 30 | 60 | 5 | 30 | 60 | |
| MS2 | 0 | 0.28 ± 0.10 * | 1.09 ± 0.03 * | 0.59 ± 0.43 * | >6.24, >6.24 **, 5.94 | >6.24, >6.24, 5.94 | |||
| FRNA—Wild | 0 | 0.10 ± 0.15 * | 0.37 ± 0.12 * | 0.50 ± 0.20 * | 1.63, 1.47 | >8.01, 7.72, >7.96 | |||
| Enterococci (wild) | 0.63 ± 0.14 * | 2.09 ± 0.40 * | 1.78 ± 1.06 * | >5.90 | >5.90 | ||||
| E. coli—Lab | 2.47 ± 0.71 * | >6.08, 6.91, >4.49 | |||||||
| E. coli—Wild | 1.37 ± 0.51 * | 6.17 ± 0.95 * | |||||||
| Ascaris | 0.01 | 0.04 | 0.90 | >1.79 | >1.68 | >1.68 | >1.83 | >1.74 | >1.99 |
| Coxsackievirus | 5.0, 5.2 | 5.6, 6.0 | >7.0 | >7.0 | >7.0 | >7.0 | |||
| Adenovirus | 2.16 ± 0.19 * | >7.76 | >7.62 | >7.62 | >7.62 | >7.62 | |||
| Cryptosporidium | >2.98 | >2.98 | |||||||
| Infectious Agent | Pond Water | Temperature (°C) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 55 | 65 | 75 | ||||||||
| Contact Time (Seconds) | Contact Time (Seconds) | Contact Time (Seconds) | ||||||||
| 5 | 30 | 60 | 5 | 30 | 60 | 5 | 30 | 60 | ||
| MS2 | P2 | −0.05 | 0.09 | 0.82 | 1.05 | 5.56 | >7.05 ** | |||
| P10 | −0.03 | 0.16 | 0.66 | 1.04 | 5.66 | >6.98 | ||||
| E. coli | P2 | 0.31 | 0.47 | 1.04 | >6.61 | >6.61 | >6.61 | |||
| P10 | 0.14 | 0.25 | 1.02 | >6.40 | >6.40 | >6.40 | ||||
| Cryptosporidium | P2 | >2.58 | ||||||||
| P10 | >2.46 | |||||||||
| Turbidity (NTU) | Time (Minutes) | |
|---|---|---|
| 30 | 60 | |
| 2.08 | 1.90 | 6.65 |
| 1.90 | 6.54 | |
| 1.15 | 6.97 | |
| 14.6 | 2.14 | 6.91 |
| 1.58 | 6.86 | |
| 1.08 | 6.82 | |
| t-test P | 0.90 | 0.35 |
| Advanced Treatment Technology | Energy Intensity kWh/kL |
|---|---|
| Ultraviolet (UV) | 0.026 |
| Ozone | 0.16 |
| Ultrafiltration (UF) | 0.13 |
| Reverse osmosis (RO) | 2.6 |
| Membrane bioreactor (MBR) | 6.6 |
| Electrodialysis reversal (EDR) | 2.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanciolo, P.; Monis, P.; Lewis, J.; Ryan, G.; Salveson, A.; Fontaine, N.; Blackbeard, J.; Gray, S. Effectiveness and Energy Requirements of Pasteurisation for the Treatment of Unfiltered Secondary Effluent from a Municipal Wastewater Treatment Plant. Water 2020, 12, 2100. https://doi.org/10.3390/w12082100
Sanciolo P, Monis P, Lewis J, Ryan G, Salveson A, Fontaine N, Blackbeard J, Gray S. Effectiveness and Energy Requirements of Pasteurisation for the Treatment of Unfiltered Secondary Effluent from a Municipal Wastewater Treatment Plant. Water. 2020; 12(8):2100. https://doi.org/10.3390/w12082100
Chicago/Turabian StyleSanciolo, Peter, Paul Monis, Justin Lewis, Greg Ryan, Andrew Salveson, Nicola Fontaine, Judy Blackbeard, and Stephen Gray. 2020. "Effectiveness and Energy Requirements of Pasteurisation for the Treatment of Unfiltered Secondary Effluent from a Municipal Wastewater Treatment Plant" Water 12, no. 8: 2100. https://doi.org/10.3390/w12082100
APA StyleSanciolo, P., Monis, P., Lewis, J., Ryan, G., Salveson, A., Fontaine, N., Blackbeard, J., & Gray, S. (2020). Effectiveness and Energy Requirements of Pasteurisation for the Treatment of Unfiltered Secondary Effluent from a Municipal Wastewater Treatment Plant. Water, 12(8), 2100. https://doi.org/10.3390/w12082100







