Optimization of Process Parameters for Obtaining Polyethersulfone/Additives Membranes
Abstract
:1. Introduction
- the composition (concentration, solvent, additives) of the polymer solution;
- the support material (glass, polymer, metal, non-wovens);
- the thickness of the cast polymer film, which is adjustable by smoothing out the viscous solution on the support among wires of the desired diameter;
- the non-solvent or the mixture of non-solvents;
- the temperature of the polymer solution, the coagulation bath or the environment;
- the duration of a dry phase inversion before the wet phase inversion (combination of processes);
- air moisture.
2. Materials and Methods
2.1. Materials
2.2. Design of Experiments
2.3. Wiring Solution Preparation and Hollow Fiber Membranes Production
2.4. Permeation Module Production
2.5. Characterizations
3. Results and Discussion
3.1. Design of Experiments
3.2. X-ray Diffraction (XRD)
3.3. Scanning Electron Microscopy (SEM)
3.4. Flow Measurement Tests
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liao, Y.; Loh, C.H.; Tian, M.; Wang, R.; Fane, A.G. Progress in electrospun polymeric nanofibrous membranes for water treatment. Prog. Polym. Sci. 2018, 77, 69–94. [Google Scholar] [CrossRef]
- Kamali, M.; Suhas, D.P.; Costa, M.E.; Capela, I.; Aminabhavi, T.M. Sustainability considerations in membrane-based technologies for industrial effluents treatment. Chem. Eng. J. 2019, 368, 474–494. [Google Scholar] [CrossRef]
- Pendergast, M.M.; Hoek, E.M.V. A review of water treatment membrane nanotechnologies. Energy Environ. Sci. 2011, 4, 1946–1971. [Google Scholar] [CrossRef] [Green Version]
- Rather, L.J.; Jameel, S.; Dar, O.A.; Ganie, S.A.; Bhat, K.A.; Mohammad, F. Advances in the sustainable technologies for water conservation in textile industries. Water Text. Fash. 2019, 175–194. [Google Scholar] [CrossRef]
- Kim, J.; Van der Bruggen, B. The use of nanoparticles in polymeric and ceramic membrane structures. Environ. Pollut. 2010, 158, 2335–2349. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Rehman, S.A.U.; Luan, H.Y.; Farid, M.U.; Huang, H. Challenges and opportunities in functional carbono nanotubes for membrane-based water treatment and desalination. Sci. Total Environ. 2019, 646, 1126–1139. [Google Scholar] [CrossRef]
- Farahani, M.H.D.A.; Rabiee, H.; Vatanpour, V. Comparing the effect of incorporation of various nanoparticulate on the performance and antifouling properties of polyethersulfone nanocomposite membranes. J. Water Process. Eng. 2019, 27, 47–57. [Google Scholar] [CrossRef]
- Wang, K.; Abdalla, A.A.; Khaleel, M.A.; Hilal, N.; Khraisheh, M.K. Mechanical properties of water desalination and wastewater treatment membranes. Desalination 2017, 401, 190–205. [Google Scholar] [CrossRef] [Green Version]
- Nath, K. Membrane Separation Processes, 2nd ed.; PHI Learning Pvt. Ltd.: Gujarat, India, 2017. [Google Scholar]
- Ng, L.Y.; Mohammad, A.W.; Leo, C.P.; Hilal, N. Polymeric membranes incorporated with metal/metal oxide nanoparticles: A comprehensive review. Desalination 2013, 308, 15–33. [Google Scholar] [CrossRef]
- Bet-moushoul, E.; Mansourpanah, Y.; Farhadi, K.; Tabatabaei, M. TiO2 nanocomposite based polymeric membranes: A review on performance improvement for various applications in chemical engineering processes. Chem. Eng. J. 2016, 283, 29–46. [Google Scholar] [CrossRef]
- Saranya, R.; Arthanareeswaren, G.; Dionysiou, D. Treatment of paper mill effluent using Polyethersulfone/functionalized multiwalled carbon nanotubes based nanocomposite membranes. Chem. Eng. J. 2014, 236, 369–377. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, J.; Dong, G.; Zhang, Y.; Guo, N.; Liu, J. Sulfonated halloysite nanotubes/polyethersulfone nanocomposite membrane for efficient dye purification. Sep. Purif. Technol. 2015, 150, 243–251. [Google Scholar] [CrossRef]
- Mierzwa, J.C.; Arieta, V.; Verlage, M.; Carvalho, J.; Vecitis, C.D. Effect of Clay nanoparticles on the structure and performance of polyethersulfone ultrafiltration membranes. Desalination 2013, 314, 147–158. [Google Scholar] [CrossRef]
- Abdel-Karim, A.; Gad-Allah, T.A.; El-Kalliny, A.S.; Ahmed, S.I.A.; Souaya, E.R.; Badawy, M.I.; Ulbricht, M. Fabrication of modified polyethersulfone membranes for wastewater treatment by submerged membrane bioreactor. Sep. Purif. Technol. 2017, 175, 36–46. [Google Scholar] [CrossRef]
- Mansur, S.; Othman, M.H.D.; Ismail, A.F.; Abidin, M.N.Z.; Said, N.; Goh, P.S.; Hasbullah, H.; Kadir, S.H.S.A. Study on the effect of PVP additive on the performance of PSf/PVP ultrafiltration hollow fiber membrane. Malays. J. Fund. Appl. Sci. 2018, 14, 343–347. [Google Scholar] [CrossRef]
- Kesting, R.E. Synthetic Polymeric Membranes; McGraw-Hill: New York, NY, USA, 1971. [Google Scholar]
- Koros, W.J.; Pinnau, I. Membrane formation for gas separation processes. In Polymeric Gas Separation Membranes; Paul, D.R., Yampol’skii, Y., Eds.; CRC Press: Boca Raton, FL, USA, 1994; pp. 209–271. [Google Scholar]
- Barth, C.; Gonçalves, M.C.; Pires, A.T.N.; Roeder, J.; Wolf, B.A. Asymmetric polysulfone and polyethersulfone membranes: Effects of thermodynamic conditions during formation on their performance. J. Membr. Sci. 2000, 169, 287–299. [Google Scholar] [CrossRef]
- Qasim, A.; Nisar, S.; Shah, A.; Khalid, M.S.; Sheik, M.A. Optimization of process parameters for machining of AISI-1045 steel using Taguchi design and ANOVA. Simul. Model. Pract. Theory 2015, 59, 36–51. [Google Scholar] [CrossRef]
- Moldovan, M.L.; Iurian, S.; Puscas, C.; Silaghi-Dumitrescu, R.; Hanganu, D.; Bodgan, C.; Vlase, L.; Oniga, I.; Benedec, D. A design of experiments strategy to enhance the recovery of polyphenolic compounds from vitis vinifera by-products through heat reflux extraction. Biomolecules 2019, 9, 529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matos, V.E.D.; Peres, A.E.C.; Pereira, C.A.; Nogueira, S.D.C.S. Analysis of quartz floatability using design of experiments. REM Int. Eng. J. 2019, 72, 501–506. [Google Scholar] [CrossRef]
- Nardi, J.V.; Acchar, W.; Hotza, D. Enhancing the properties of ceramic products through mixture design and response surface analysis. J. Eur. Ceram. Soc. 2004, 24, 375–379. [Google Scholar] [CrossRef]
- Jacyna, J.; Kordalewska, M.; Markuszewski, M.J. Design of Experiments in metabolomics-related studies: An overview. J. Pharm. Biomed. Anal. 2018, 164, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, D.C. Design and Analysis of Experiments, 5th ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2001. [Google Scholar]
- Xiangli, F.; Wei, W.; Chen, Y.; Jin, W.; Xu, N. Optimization of preparation conditions for polydimethylsiloxane (PDMS)/ceramic composite pervaporation membranes using response surface methodology. J. Membr. Sci. 2008, 311, 23–33. [Google Scholar] [CrossRef]
- Ferreira, R.D.S.B.; Oliveira, S.S.L.; Salviano, A.F.; Araújo, E.M.; Leite, A.M.D.; Lira, H.D.L. Polyethersulfone hollow fiber membranes developed for oily emulsion treatment. Mater. Res. 2019, 22, e20180854. [Google Scholar] [CrossRef]
- Khan, A.S.; Biggs, T.C.; Faoury, M. Improving the allocation of junior doctor resources using Pareto analysis of pager activity. Int. J. Otorhinolaryngol. Head Neck Surg. 2019, 5, 1–4. [Google Scholar] [CrossRef]
- Barzin, J.; Sadatnia, B. Theoretical phase diagram calculation and membrane morphology evaluation for water/solvent/polyethersulfone systems. Polymer 2007, 48, 1620–1631. [Google Scholar] [CrossRef]
- Anadão, P.; Sato, L.F.; Wiebeck, H.; Valenzuela-Díaz, F.R. Montmorillonite as a component of polysulfone nanocomposite membranes. Appl. Clay Sci. 2010, 48, 127–132. [Google Scholar] [CrossRef]
- Ge, L.; Zhu, Z.; Rudolph, V. Enhanced gas permeability by fabricating functionalized multi-walled carbon nanotubes and polyethersulfone nanocomposite membrane. Sep. Purif. Technol. 2011, 78, 76–82. [Google Scholar] [CrossRef]
- Salahi, A.; Mohammadi, T.; Behbahani, R.M.; Hemmati, M. Asymmetric polyethersulfone ultrafiltration membranes for oily wastewater treatment: Synthesis, characterization, ANFIS modeling and performance. J. Environ. Chem. Eng. 2015, 3, 170–178. [Google Scholar] [CrossRef]
- Sun, D.; Yang, Q.C.; Sun, H.L.; Liu, J.M.; Xing, Z.L.; Li, B.B. Effects of PES support layer structure on pervaporation performances of PDMS/PES hollow fiber composite membranes. Desalin. Water Treat. 2016, 57, 9123–9135. [Google Scholar] [CrossRef]
- Yuan, Z.; Dan-Li, X. Porous PVDF/TPU blends asymmetric hollow fiber membranes prepared with the use of hydrophilic additive PVP (K30). Desalination 2008, 223, 438–447. [Google Scholar] [CrossRef]
- Singha, N.R.; Karmakar, M.; Chattopadhyay, P.K.; Roy, S.; Deb, M.; Mondal, H.; Mahapatra, M.; Dutta, A.; Mitra, M.; Roy, J.S.D. Structures, properties and performances-relationships of polymeric membranes for pervaporative desalination. Membranes 2019, 9, 58. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, R.D.S.B.; Salviano, A.F.; Oliveira, S.S.L.; Araújo, E.M.; Medeiros, V.D.N.; Lira, H.D.L. Treatment of effluents from the textile industry through polyethersulfone membranes. Water 2019, 11, 2540. [Google Scholar] [CrossRef] [Green Version]
- Chu, Z.; Chen, K.; Xiao, C.; Ji, D.; Ling, H.; Li, M.; Liu, H. Improving pressure durability and fractionation property via reinforced PES loose nanofiltration hollow fiber membranes for textile wastewater treatment. J. Taiwan Inst. Chem. Eng. 2020, 108, 71–81. [Google Scholar] [CrossRef]
- Xu, Z.L.; Qusay, F.A. Polyethersulfone (PES) hollow fiber ultrafiltration membranes prepared by PES/non-solvent/NMP solution. J. Membr Sci. 2004, 233, 101–111. [Google Scholar] [CrossRef]
- Alsalhy, Q.F.; Salih, H.A.; Simone, S.; Zablouk, M.; Drioli, E.; Figoli, A. Poly(ether sul-fone) (PES) hollow-fiber membranes prepared from various spinning parame-ters. Desalination 2014, 345, 21–35. [Google Scholar] [CrossRef]
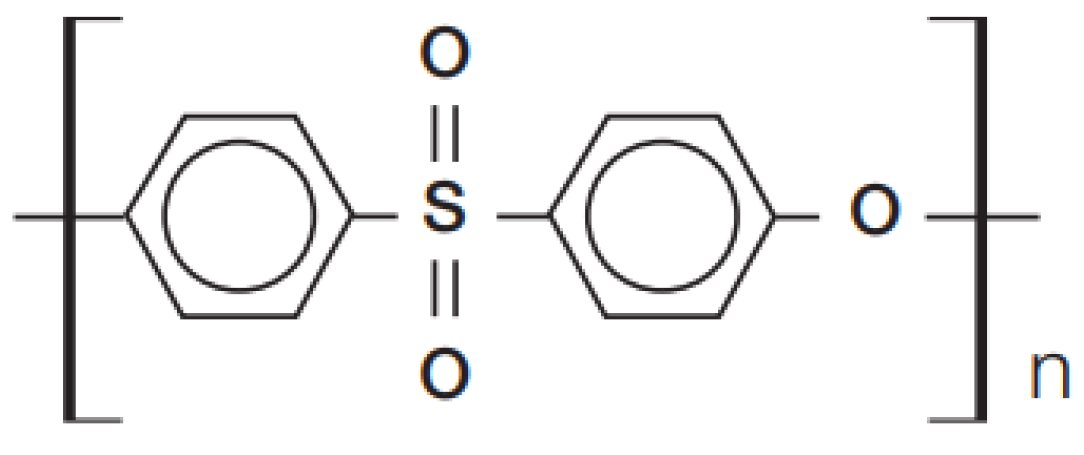



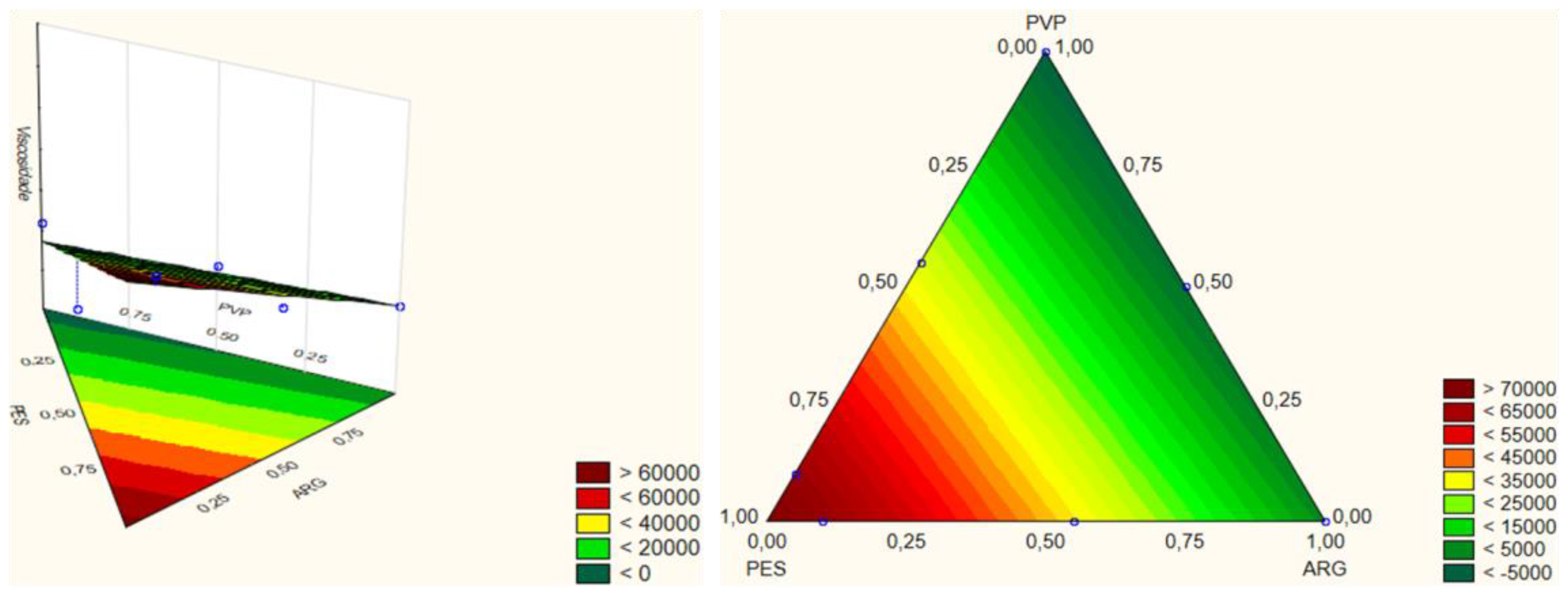




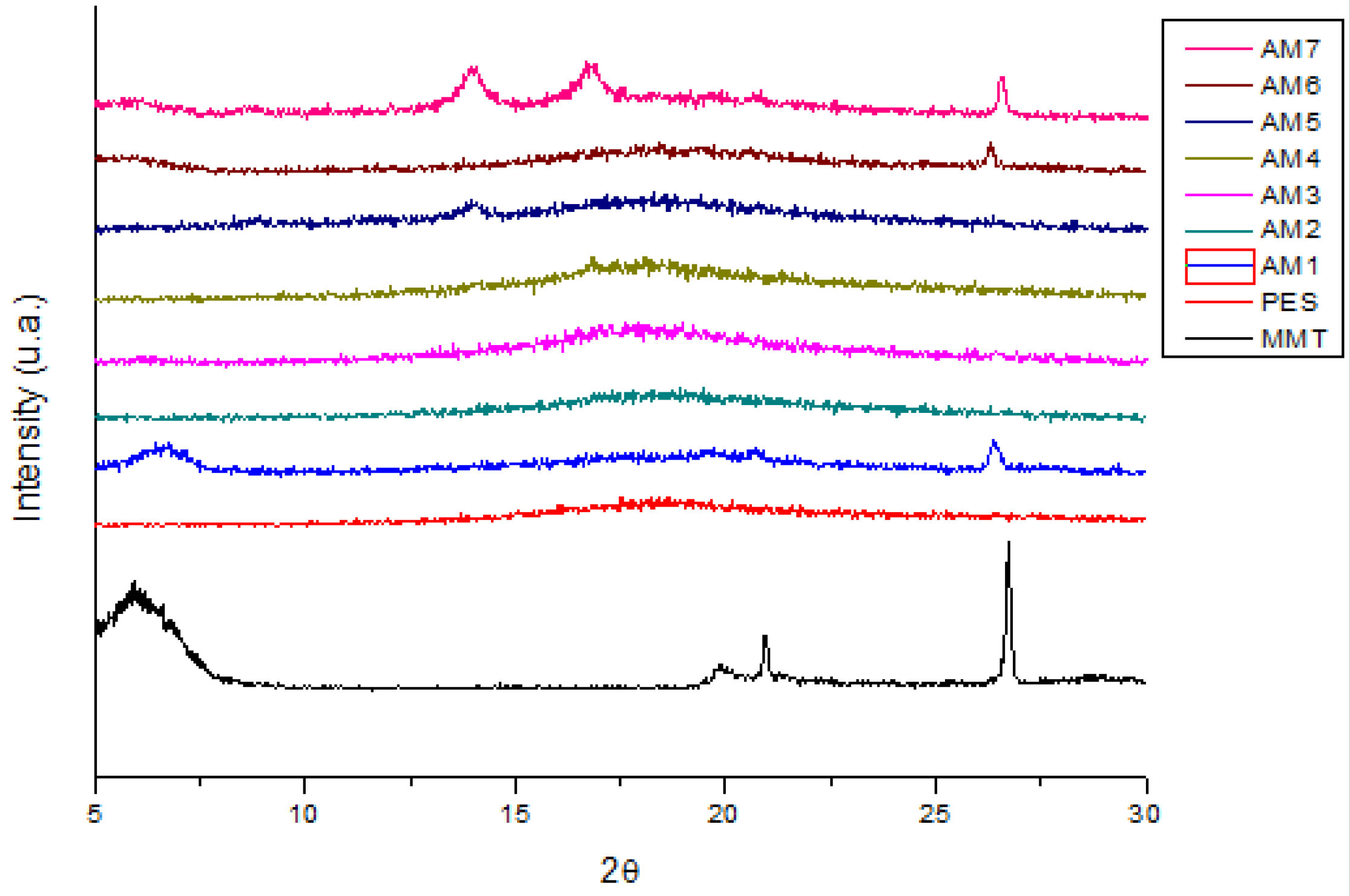


| PARAMETERS | VALUES |
|---|---|
| Solution temperature | ≈26 °C |
| GAP | 5 cm |
| Flow of internal liquid (distilled water) | 3 g/min |
| Solution flow | 6 g/min |
| Extruder outer diameter | 2.5 mm |
| Extruder inner diameter | 2.0 mm |
| Samples | PES (%) | Clay (%) | PVP (%) | Viscosity (mPa·s) | Contact Angle (°) |
| AM1 | 70 | 30 | 0 | 3603.5 | 39.0 |
| AM2 | 70 | 0 | 30 | 3655.8 | 46.6 |
| AM3 | 97 | 3 | 0 | 71,092.0 | 59.0 |
| AM4 | 97 | 0 | 3 | 77,581.0 | 56.8 |
| AM5 | 84 | 0 | 16 | 5899.5 | 54.9 |
| AM6 | 84 | 16 | 0 | 30,108.0 | 54.1 |
| AM7 | 70 | 15 | 15 | 2501.6 | 40.0 |
| AM8 | 70 | 30 | 0 | 3352.7 | 40.6 |
| AM9 | 70 | 15 | 15 | 2586.0 | 38.3 |
| Model | R2 | RA2 | F | p |
|---|---|---|---|---|
| Linear | 0.884425 | 0.845900 | 22.95718 | 0.001544 |
| Quadratic | 0.986417 | 0.963924 | 7.54302 | 0.065548 |
| Model | Coded Mathematical Model |
|---|---|
| Linear | V = 74341.13XPES + 3834.03XCLAY − 5409.50XPVP |
| Quadratic | V = 92522XPES + 3814XCLAY + 2984XPVP − 64916XPESXCLAY − 141148XPESXPVP − 3421XCLAYXPVP |
| Model | R2 | RA2 | F | p |
|---|---|---|---|---|
| Linear | 0.892845 | 0.857127 | 24.99689 | 0.001230 |
| Quadratic | 0.992880 | 0.981014 | 14.05013 | 0.028490 |
| Model | Coded Mathematical Model |
|---|---|
| Linear | CA = 61.64XPES + 39.56XCLAY + 44.72XPVP |
| Quadratic | CA = 57.59XPES + 39.76XCLAY + 46.68XPVP + 26.70XPESXCLAY + 12.22XPESXPVP − 16.22XCLAYXPVP |
| Samples | Average Thickness * (mm) |
|---|---|
| AM1 | 0.271 ± 0.075 |
| AM2 | 0.391 ± 0.175 |
| AM3 | 0.222 ± 0.035 |
| AM4 | 0.228 ± 0.045 |
| AM5 | 0.426 ± 0.175 |
| AM6 | 0.171 ± 0.039 |
| AM7 | 0.352 ± 0.160 |
| AM8 | 0.301 ± 0.047 |
| AM9 | 0.326 ± 0.054 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medeiros, V.d.N.; Silva, B.I.A.; Ferreira, R.d.S.B.; Oliveira, S.S.L.; Dias, R.A.; Araújo, E.M. Optimization of Process Parameters for Obtaining Polyethersulfone/Additives Membranes. Water 2020, 12, 2180. https://doi.org/10.3390/w12082180
Medeiros VdN, Silva BIA, Ferreira RdSB, Oliveira SSL, Dias RA, Araújo EM. Optimization of Process Parameters for Obtaining Polyethersulfone/Additives Membranes. Water. 2020; 12(8):2180. https://doi.org/10.3390/w12082180
Chicago/Turabian StyleMedeiros, Vanessa da Nóbrega, Bárbara Ianny Arruda Silva, Rodholfo da Silva Barbosa Ferreira, Sandriely Sonaly Lima Oliveira, Rafael Agra Dias, and Edcleide Maria Araújo. 2020. "Optimization of Process Parameters for Obtaining Polyethersulfone/Additives Membranes" Water 12, no. 8: 2180. https://doi.org/10.3390/w12082180





