Nitrogen Removal for Liquid-Ammonia Mercerization Wastewater via Partial Nitritation/Anammox Based on Zeolite Sequencing Batch Reactor
Abstract
:1. Introduction
2. Materials and Methods
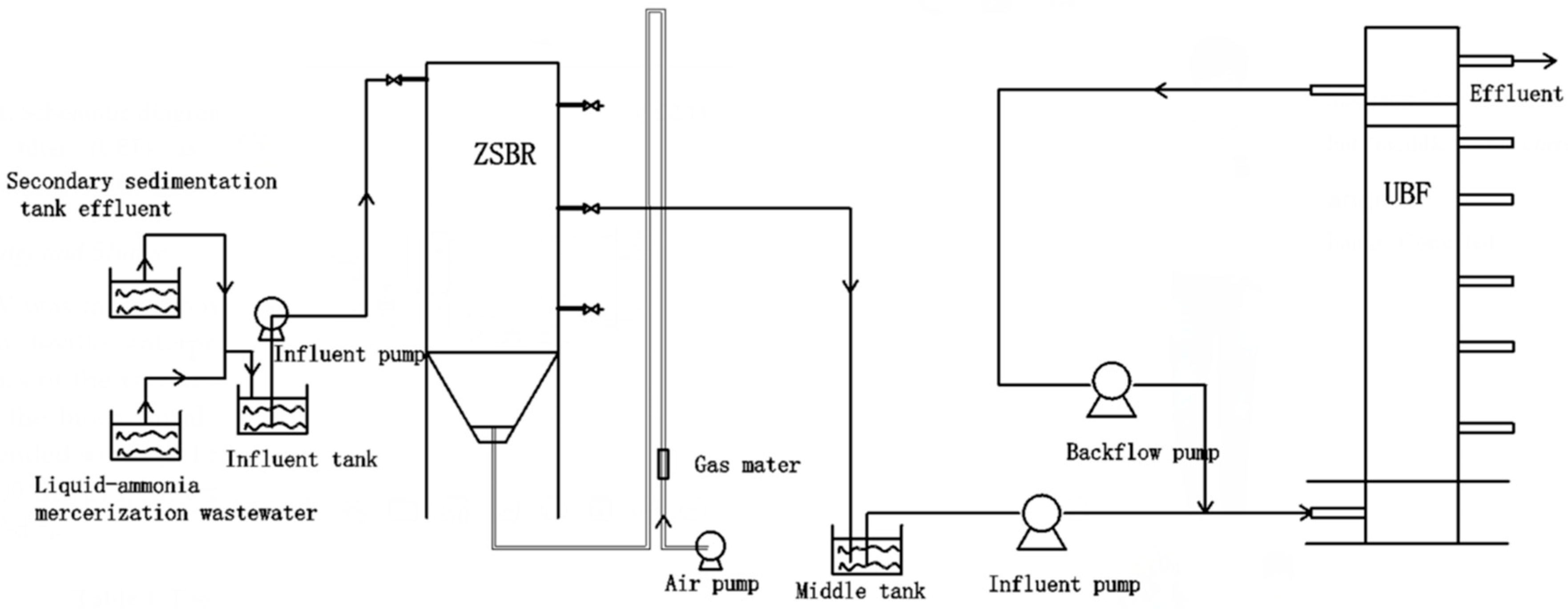
2.1. Reactor Set Up and Operation
2.2. Wastewater and Sludge
2.3. Experimental Procedure
2.4. Analytical Methods
2.5. DNA Extraction, PCR Amplification, and High-Throughput Sequencing Analysis
3. Results and Discussion
3.1. Performance of ZSBR
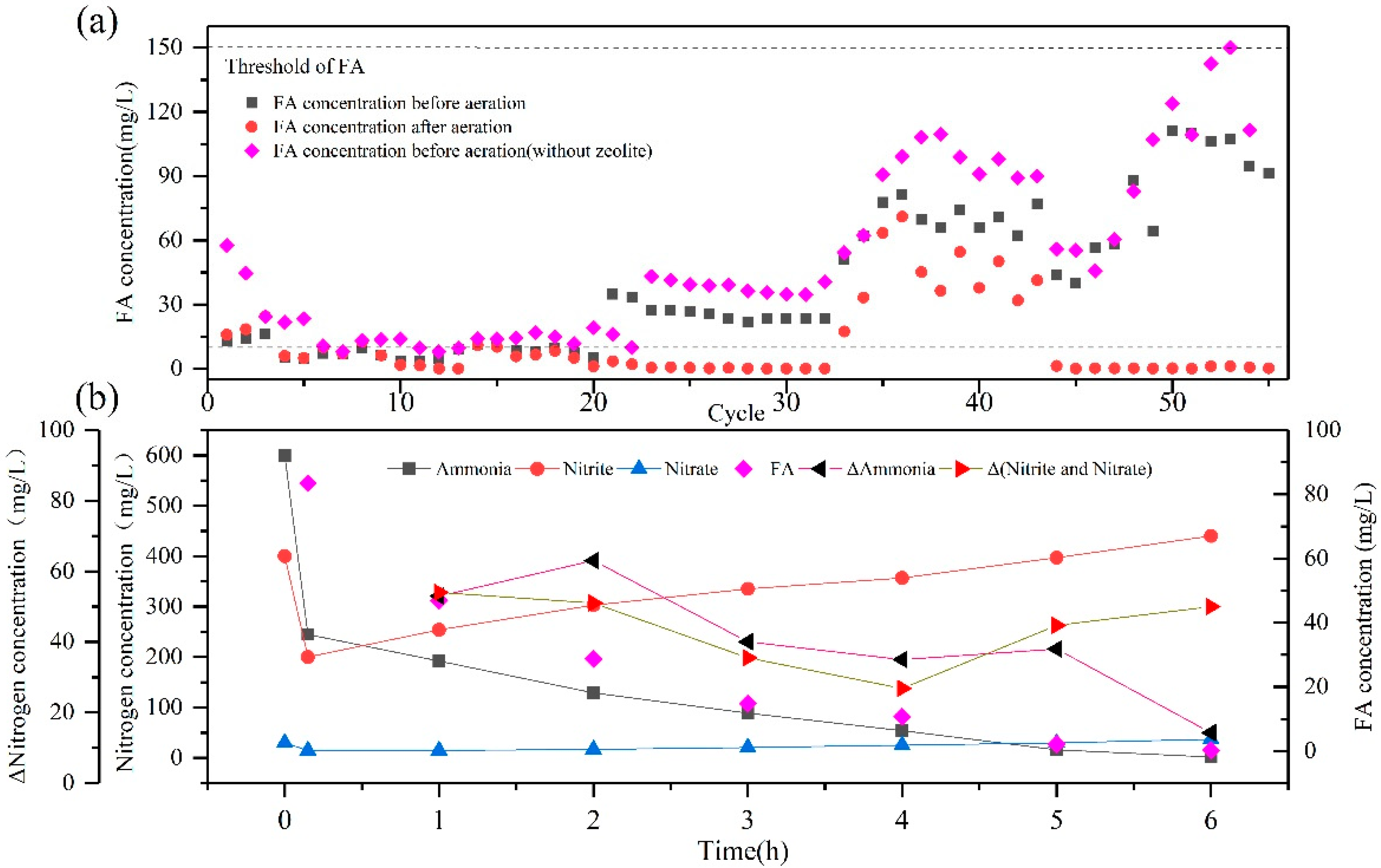
3.1.1. Start-Up Period
3.1.2. Mechanism of the Rapid Nitritation in ZSBR
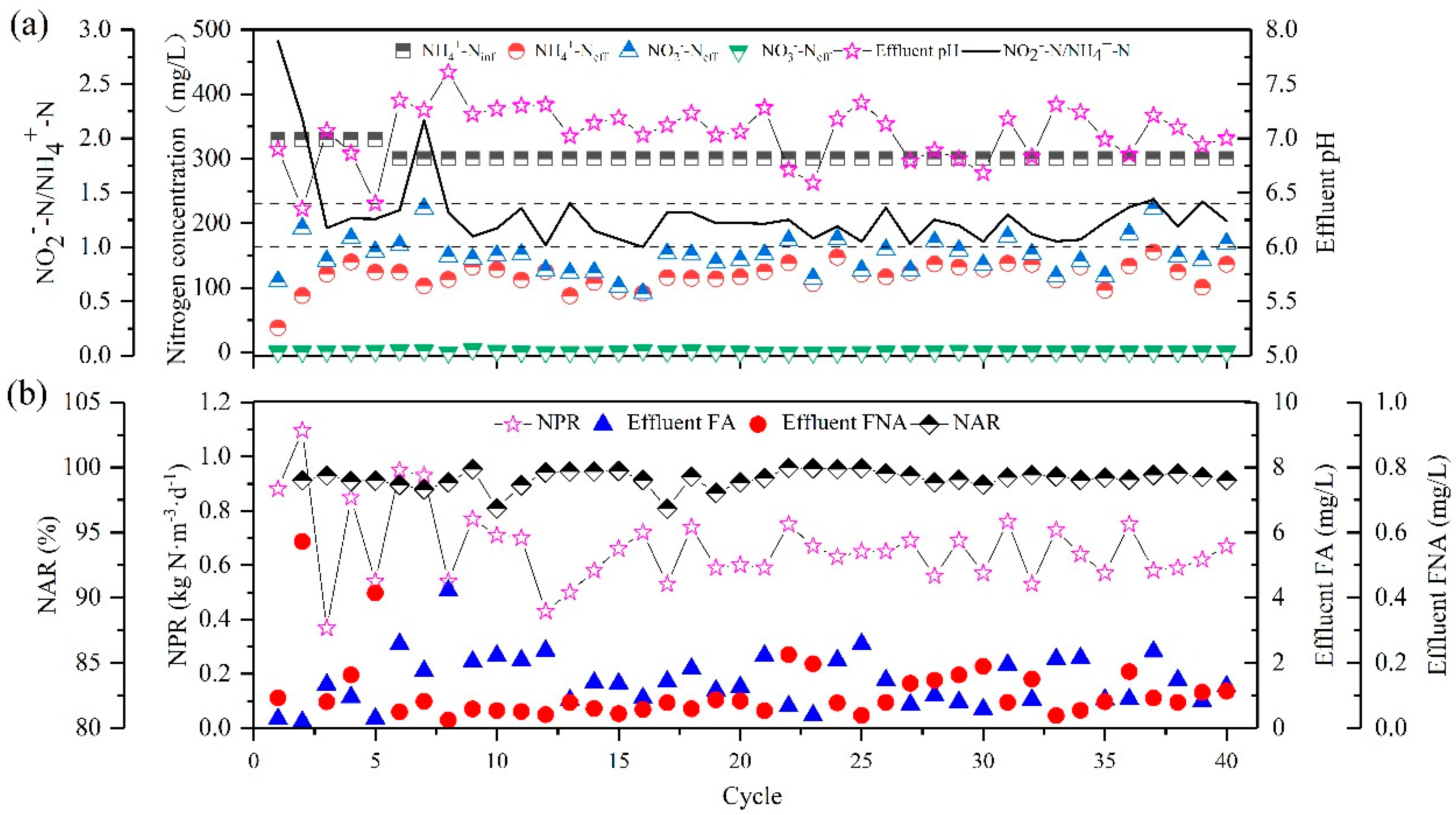
3.1.3. Partial Nitritation of ZSBR
3.2. Performance of UBF
3.2.1. Start-Up Period
3.2.2. The Influence of Influent Substrate Ratio
3.3. Microbial Community Analysis
3.4. Significance of ZSBR Coupling with Anammox for LMWW
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AnAOB | anaerobic ammonia oxidizing bacteria |
| AOB | ammonia oxidizing bacteria |
| ARR | ammonia removal rate |
| DO | dissolved oxygen |
| FA | free ammonia |
| FNA | free nitrous acid |
| LMWW | liquid-ammonia mercerization wastewater |
| MLSS | mixed liquid suspended solids |
| NAR | nitrite accumulation ratio |
| NLR | nitrogen loading rate |
| NOB | nitrite oxidizing bacteria |
| NPR | nitrite production rate |
| NRE | nitrogen removal efficiency |
| NRR | nitrogen removal rate |
| OTU | operational taxonomic unit |
| PN | partial nitritation |
| PN/A | partial nitritation/anammox |
| TN | total nitrogen |
| UBF | up-flow blanket filter |
| WW | wastewater |
| ZBAF | zeolite biological aerated filter |
| ZSBR | zeolite sequencing batch reactor |
References
- Wakida, T.; Kida, K.; Lee, M. Dyeing and mechanical properties of cotton fabrics treated with sodium hydroxide/liquid ammonia and liquid ammonia/sodium hydroxide. Text. Res. J. 2000, 70, 328–332. [Google Scholar] [CrossRef]
- Bertoniere, N.; King, W. Effect of scouring/bleaching, caustic mercerization, and liquid ammonia treatment on the pore structure of cotton textile fibers. Text. Res. J. 1989, 59, 114–121. [Google Scholar] [CrossRef]
- Gabarro, J.; Ganigue, R.; Gich, F.; Ruscalleda, M.; Balaguer, M.D.; Colprim, J. Effect of temperature on AOB activity of a partial nitritation SBR treating landfill leachate with extremely high nitrogen concentration. Bioresour. Technol. 2012, 126, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Siegrist, H.; Salzgeber, D.; Eugster, J.; Joss, A. Anammox brings WWTP closer to energy autarky due to increased biogas production and reduced aeration energy for N-removal. Water Sci. Technol. 2008, 57, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Strous, M.; Heijnen, J.J.; Kuenen, J.G.; Jetten, M.S.M. The sequencing batch reactor as a powerful tool for the study of slowly growing anaerobic ammonium-oxidizing microorganisms. Appl. Microbiol. Biotechnol. 1998, 50, 589–596. [Google Scholar] [CrossRef]
- Veuillet, F.; Lacroix, S.; Bausseron, A.; Gonidec, E.; Ochoa, J.; Christensson, M.; Lemaire, R. Integrated fixed-film activated sludge ANAMMOX process-a new perspective for advanced nitrogen removal. Water Sci. Technol. 2014, 69, 915–922. [Google Scholar] [CrossRef]
- Hellinga, C.; Schellen, A.A.J.C.; Mulder, J.W.; Van Loosdrecht, M.C.M.; Heijnen, J.J. The Sharon process: An innovative method for nitrogen removal from ammonium-rich waste water. Water Sci. Technol. 1998, 37, 135–142. [Google Scholar] [CrossRef]
- Jubany, I.; Lafuente, J.; Baeza, J.A.; Carrera, J. Total and stable washout of nitrite oxidizing bacteria from a nitrifying continuous activated sludge system using automatic control based on Oxygen Uptake Rate measurements. Water Res. 2009, 43, 2761–2772. [Google Scholar] [CrossRef]
- Shijian, G.; Peng, Y.; Shuang, Q.; Zhu, A.; Nanqi, R. Complete nitrogen removal from municipal wastewater via partial nitrification by appropriately alternating anoxic/aerobic conditions in a continuous plug-flow step feed process. Water Res. 2014, 55, 95–105. [Google Scholar]
- Ge, S.; Wang, S.; Yang, X.; Qiu, S.; Li, B.; Peng, Y. Detection of nitrifiers and evaluation of partial nitrification for wastewater treatment: A review. Chemosphere 2015, 140, 85–98. [Google Scholar] [CrossRef]
- Jung, J.Y.; Chung, Y.C.; Shin, H.S.; Son, D.H. Enhanced ammonia nitrogen removal using consistent biological regeneration and ammonium exchange of zeolite in modified SBR process. Water Res. 2004, 38, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, Z.; Wang, X.; Zheng, L.; Gu, X. Partial nitrification performance and mechanism of zeolite biological aerated filter for ammonium wastewater treatment. Bioresour. Technol. 2017, 241, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, X.; Chen, Z.; Feng, X.; Chen, X. Application of synthetic zeolite as a storage medium in SBR to achieve stable partial nitrification of ammonium. Environ. Sci. Water Res. Technol. 2019, 5, 287–295. [Google Scholar] [CrossRef]
- Jetten, M.; Strous, M.; van de Pas-Schoonen, K.; Schalk, J.; van Dongen, U.; van de Graaf, A.; Logemann, S.; Muyzer, G.; van Loosdrecht, M.; Kuenen, J. The anaerobic oxidation of ammonium. Fems Microbiol. Rev. 1998, 22, 421–437. [Google Scholar] [CrossRef]
- EPA. Standard Methods of Examination of Water and Wastewater; EPA: Beijing, China, 2004. [Google Scholar]
- Magoc, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Cole, J.R.; Wang, Q.; Cardenas, E.; Fish, J.; Chai, B.; Farris, R.J.; Kulam-Syed-Mohideen, A.S.; McGarrell, D.M.; Marsh, T.; Garrity, G.M.; et al. The Ribosomal Database Project: Improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009, 37, D141–D145. [Google Scholar] [CrossRef] [Green Version]
- Gu, S.; Shuying, W.; Qing, Y.; Yang, P.; Peng, Y. Start up partial nitrification at low temperature with a real-time control strategy based on blower frequency and pH. Bioresour. Technol. 2012, 112, 34–41. [Google Scholar] [CrossRef]
- Zhang, D.; Su, H.; Antwi, P.; Xiao, L.; Liu, Z.; Li, J. High-rate partial-nitritation and efficient nitrifying bacteria enrichment/out-selection via pH-DO controls: Efficiency, kinetics, and microbial community dynamics. Sci. Total Environ. 2019, 692, 741–755. [Google Scholar] [CrossRef]
- Jiang, C.; Xu, S.; Wang, R.; Zhou, S.; Wu, S.; Zeng, X.; Bai, Z. Comprehensive assessment of free nitrous acid based technology to establish partial nitrification. Environ. Sci. Water Res. Technol. 2018, 4, 2113–2124. [Google Scholar] [CrossRef]
- Wei, D.; Du, B.; Xue, X.; Dai, P.; Zhang, J. Analysis of factors affecting the performance of partial nitrification in a sequencing batch reactor. Environ. Biotechnol. 2014, 98, 1863–1870. [Google Scholar] [CrossRef] [PubMed]
- Pacek, L.; Svehla, P.; Hrncirova, H.; Radechovsky, J. Rapid achievement of nitrification in CSTR and SBR treating reject water at high ammonia levels. Desalin. Water Treat. 2016, 57, 15958–15969. [Google Scholar] [CrossRef]
- Park, S.; Bae, W. Modeling kinetics of ammonium oxidation and nitrite oxidation under simultaneous inhibition by free ammonia and free nitrous acid. Process Biochem. 2009, 44, 631–640. [Google Scholar] [CrossRef]
- Wang, G.; Xu, X.C.; Zhou, L.; Wang, C.; Yang, F.L. A pilot-scale study on the start-up of partial nitrification-anammox for anaerobic sludge digester liquor treatment. Bioresour. Technol. 2017, 241, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Vadivelu, V.M.; Yuan, Z.G.; Fux, C.; Keller, J. The Inhibitory Effects of Free Nitrous Acid on the Energy Generation and Growth Processes of an Enriched Nitrobacter Culture. Environ. Sci. Technol. 2006, 40, 4442–4448. [Google Scholar] [CrossRef] [PubMed]
- Fux, C.; Boehler, M.; Huber, P.; Brunner, I.; Siegrist, H. Biological treatment of ammonium-rich wastewater by partial nitritation and subsequent anaerobic ammonium oxidation (anammox) in a pilot plant. J. Biotechnol. 2002, 99, 295–306. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, G.; Yang, Z.; Luo, L.; Xu, H.; Huang, J. Operation of partial nitrification to nitrite of landfill leachate and its performance with respect to different oxygen conditions. Biochem. Eng. J. 2014, 87, 62–68. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Q.; Fu, J.; Zhang, J.; Zhao, Y.; Jin, L.; Fan, N.; Huang, B.; Jin, R. Deciphering the microbial community and functional genes response of anammox sludge to sulfide stress. Bioresour. Technol. 2020, 302, 122885. [Google Scholar] [CrossRef]
- Carvajal-Arroyo, J.M.; Sun, W.; Sierra-Alvarez, R.; Field, J.A. Inhibition of anaerobic ammonium oxidizing (anammox) enrichment cultures by substrates, metabolites and common wastewater constituents. Chemosphere 2013, 91, 22–27. [Google Scholar] [CrossRef]
- Chen, Z.; Zheng, X.; Chen, Y.; Wang, X. Nitrite accumulation stability evaluation for low-strength ammonium wastewater by adsorption and biological desorption of zeolite under different operational temperature. Sci. Total Environ. 2020, 704, 135260. [Google Scholar] [CrossRef]



| Items | NH4+-N (mg/L) | pH | Alkalinity (as CaCO3 mg/L) | CODCr (mg/L) |
|---|---|---|---|---|
| Raw LMWW | 1500~2500 | 8.90~9.15 | 1300~2000 | 150~250 |
| Diluted LMWW | 300~330 | 7.35~7.55 | 750~1000 | 50~100 |
| Phase | Cycle | Airflow (L/min) | Influent Ammonium (mg NH4+-N/L) | HRT(h) | Alkalinity Ratio (Alkalinity: NH4+-N) | NaHCO3 (g/L) |
|---|---|---|---|---|---|---|
| Ⅰ | 1~55 | 0.25~1.50 | 100~600 | 6 | 7:1 | 1.17–7.0 |
| Ⅱ | 56~85 | 1.20~1.50 | 1490 | 6 | 7:1 | 17.4 |
| Ⅲ | 86~125 | 1.00~1.50 | 300~330 | 3 | 3:1 | 1.5–1.65 |
| Influent Substrate Ratio (NO2−-N/NH4+-N) | NLR (kg N/m3/day) | NREammonia (%) | NREnitrite (%) | NRETN (%) |
|---|---|---|---|---|
| 1.1 | 0.851 | 78.19 | 81.55 | 69.04 |
| 1.2 | 0.852 | 77.69 | 86.24 | 71.30 |
| 1.3 | 0.815 | 87.19 | 73.61 | 69.84 |
| Process | ZSBR–UBF | Complete Nitrification–Denitrification | |||
|---|---|---|---|---|---|
| ZSBR | UBF | Complete Nitrification 3 | Denitrification 4 | ||
| Dosage (kg/kg N) | Alkalinity 1 | 1.50 | 0 | 1.90 | 0 |
| Glucose 2 | 0 | 0 | 0 | 0.90 | |
| Cost (dollars/kg N) | Alkalinity | 0.543 | 0 | 0.689 | 0 |
| Glucose | 0 | 0 | 0 | 0.522 | |
| Total (dollars/kg N) | 0.543 | 1.211 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, L.; Chen, Y.; Zhou, S.; Chen, Y.; Wang, X.; Wang, X.; Zhang, L.; Chen, Z. Nitrogen Removal for Liquid-Ammonia Mercerization Wastewater via Partial Nitritation/Anammox Based on Zeolite Sequencing Batch Reactor. Water 2020, 12, 2234. https://doi.org/10.3390/w12082234
Zheng L, Chen Y, Zhou S, Chen Y, Wang X, Wang X, Zhang L, Chen Z. Nitrogen Removal for Liquid-Ammonia Mercerization Wastewater via Partial Nitritation/Anammox Based on Zeolite Sequencing Batch Reactor. Water. 2020; 12(8):2234. https://doi.org/10.3390/w12082234
Chicago/Turabian StyleZheng, Lei, Yongxing Chen, Songwei Zhou, Yuchen Chen, Xingxing Wang, Xiaojun Wang, Lijuan Zhang, and Zhenguo Chen. 2020. "Nitrogen Removal for Liquid-Ammonia Mercerization Wastewater via Partial Nitritation/Anammox Based on Zeolite Sequencing Batch Reactor" Water 12, no. 8: 2234. https://doi.org/10.3390/w12082234




