Enhanced Sulfamerazine Removal via Adsorption–Photocatalysis Using Bi2O3–TiO2/PAC Ternary Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of Bi2O3–TiO2/PAC Composites
2.3. Characterization of Bi2O3–TiO2/PAC Composites
2.4. Experimental Procedures
2.4.1. Adsorption Experiments
2.4.2. Photocatalytic Experiments
2.5. Analytic Methods
3. Results
3.1. Adsorption Characteristics
3.1.1. Adsorption Kinetics
3.1.2. Adsorption Isotherm
3.2. Photocatalytic Characteristics
3.2.1. Photocatalytic Performance
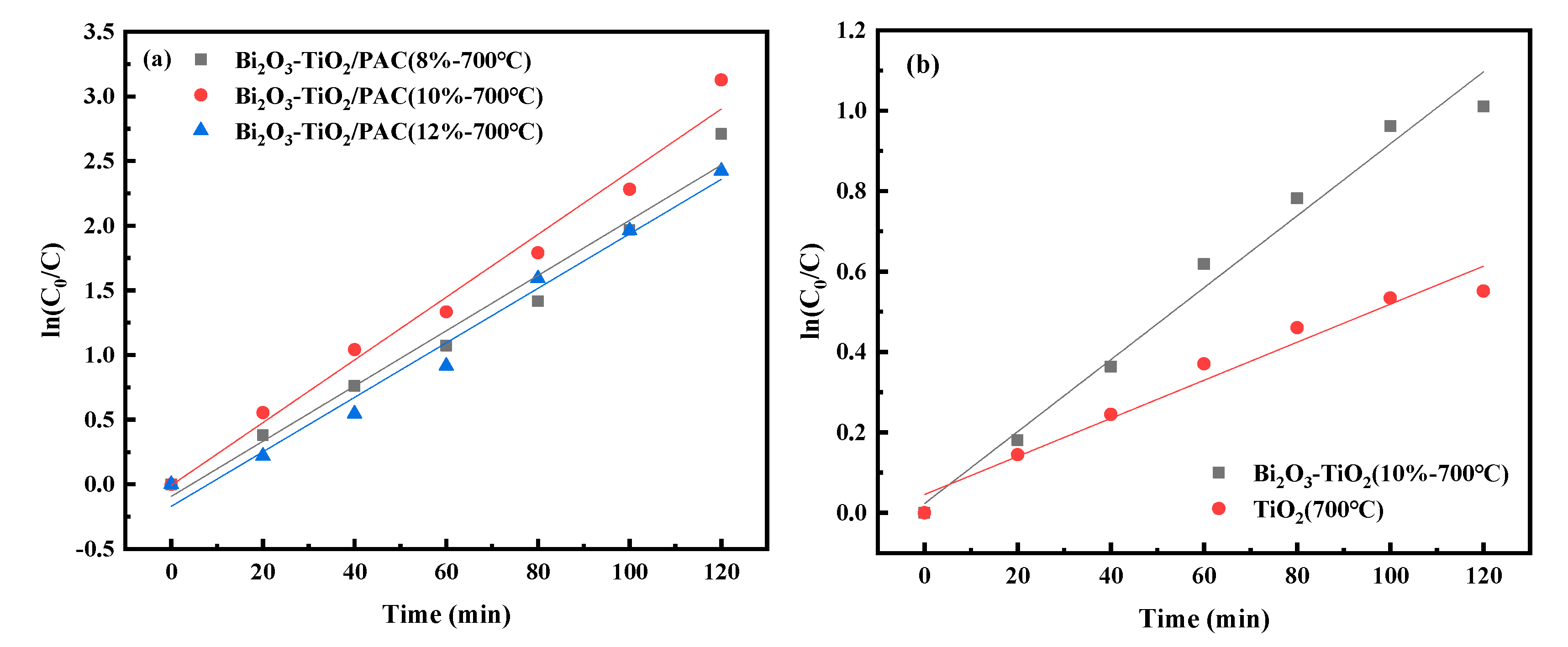
3.2.2. Photocatalytic Kinetics
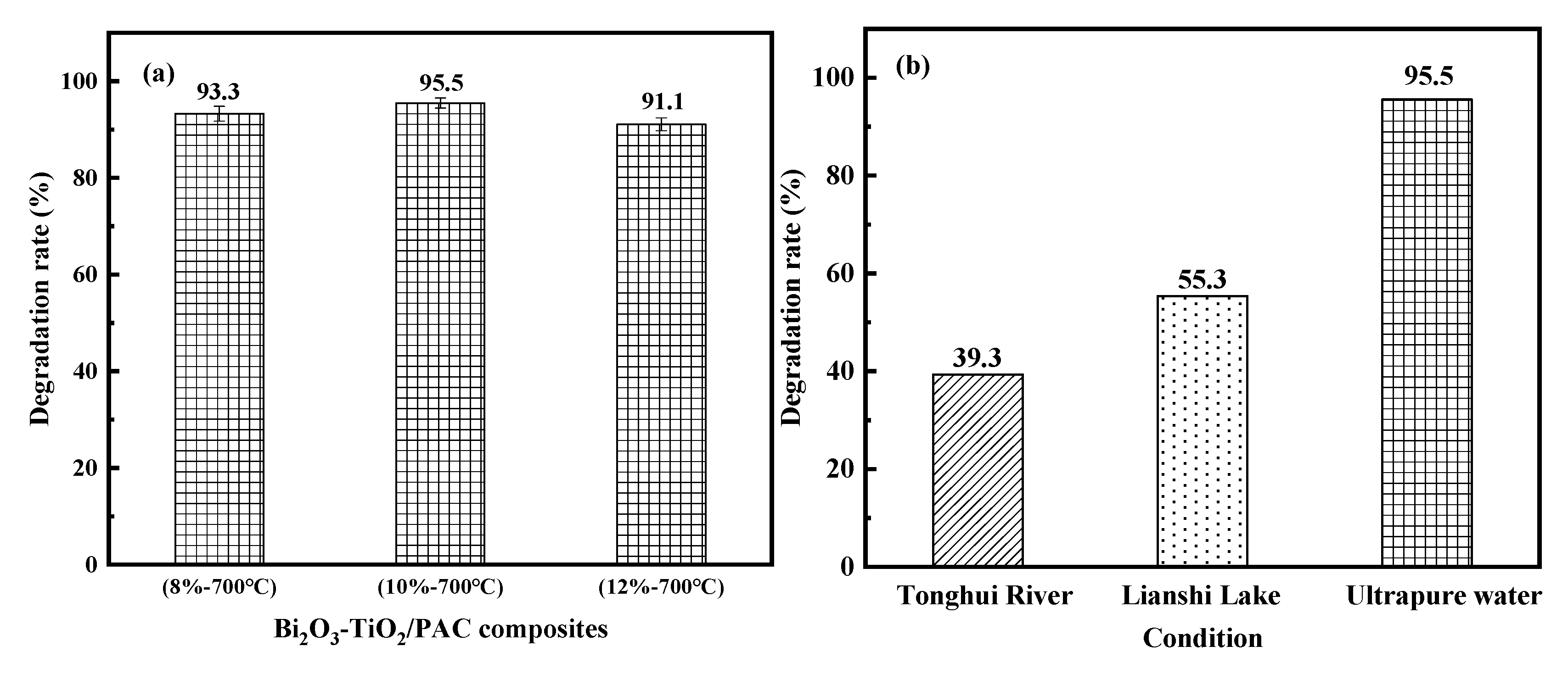
3.3. Overall Adsorption–Photocatalysis Performance
3.4. Effect of Water Quality Parameters
3.4.1. Effect of Initial pH
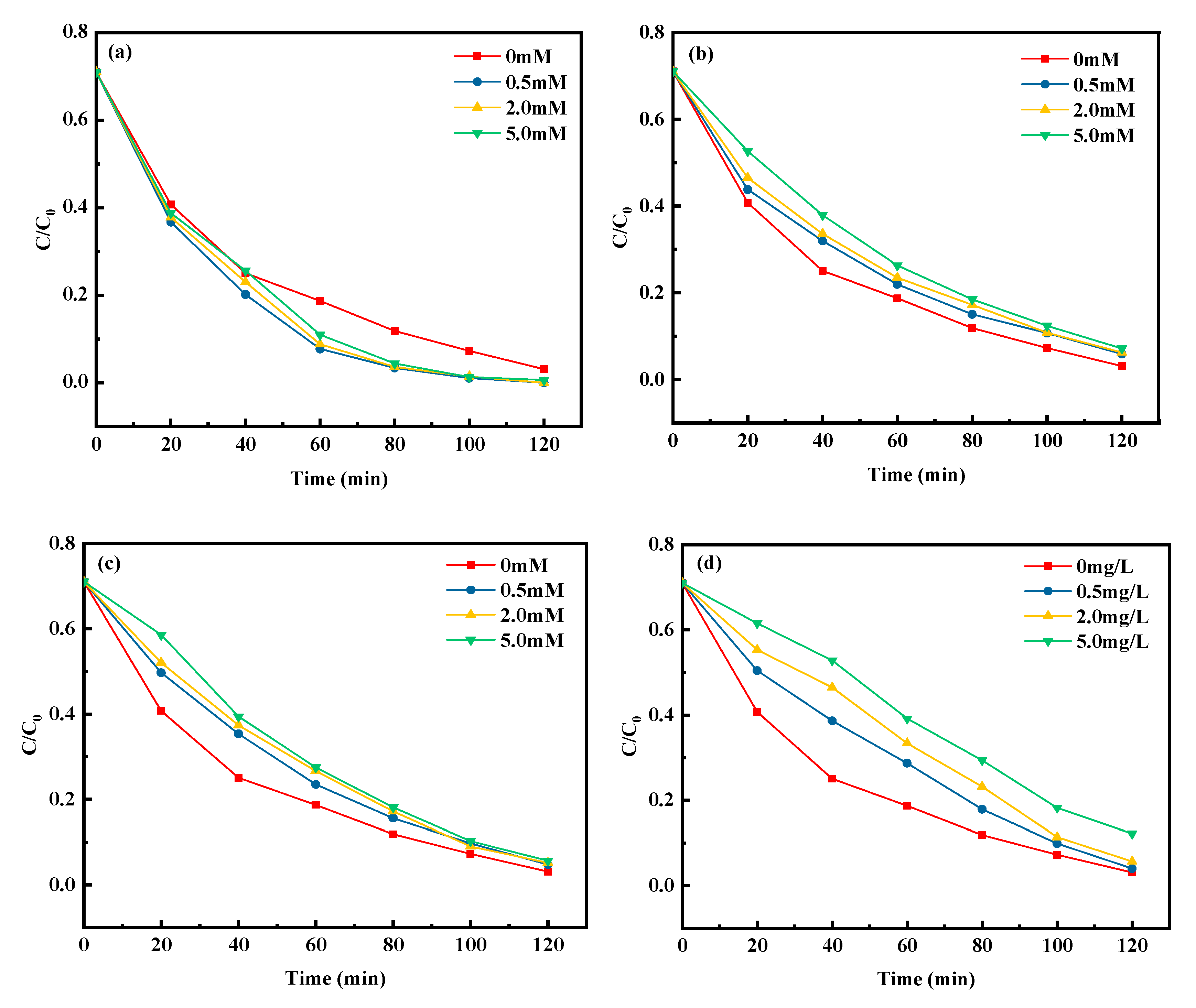
3.4.2. Effect of Inorganic Anions
3.4.3. Effect of HA
3.5. Characterization of Bi2O3–TiO2/PAC Composite
3.5.1. XRD Analysis
3.5.2. BET Surface Area and Pore Size Distribution Analysis
3.5.3. UV-Vis Diffuse Reflectance Spectrum Analysis
4. Discussions
4.1. Adsorption Mechanism
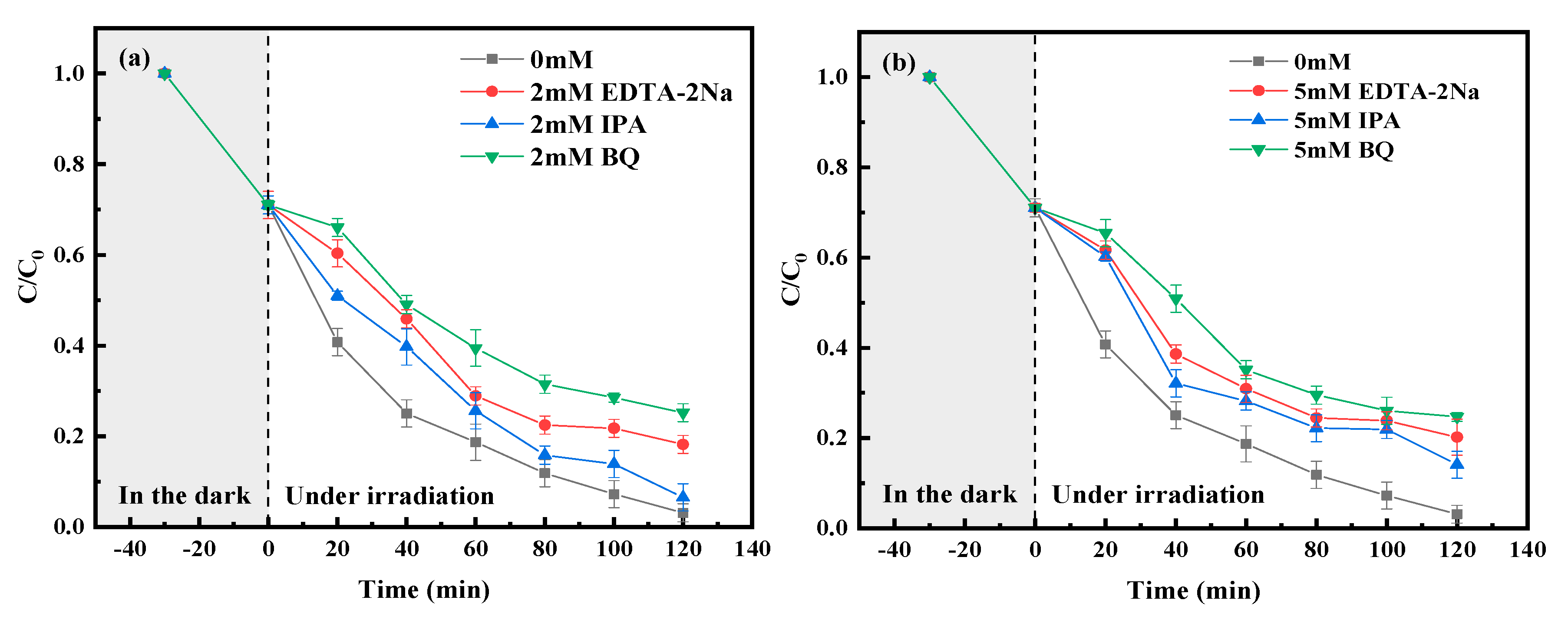
4.2. Photocatalytic Mechanism
4.3. Adsorption–Photocatalysis Mechanism
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cheng, D.M.; Feng, Y.; Liu, Y.W.; Xue, J.M.; Li, Z.J. Dynamics of oxytetracycline, sulfamerazine, and ciprofloxacin and related antibiotic resistance genes during swine manure composting. J. Environ. Manag. 2019, 230, 102–109. [Google Scholar] [CrossRef]
- Blanco-Márquez, J.H.; Ortiz, C.P.; Cerquera, N.E.; Martínez, F.; Jouyban, A.; Delgado, D.R. Thermodynamic analysis of the solubility and preferential solvation of sulfamerazine in (acetonitrile + water) cosolvent mixtures at different temperatures. J. Mol. Liq. 2019, 293, 111507. [Google Scholar] [CrossRef]
- Boreen, A.L.; Arnold, W.A.; McNeill, K. Degradation of pharmaceuticals in the aquatic environment: A review. Aquat. Sci. Res. Across Bound. 2003, 65, 320–341. [Google Scholar] [CrossRef]
- Pei, R.; Kim, S.C.; Carlson, K.H.; Pruden, A. Effect of river landscape on the sediment concentrations of antibiotics and corresponding antibiotic resistance genes (ARG). Water Res. 2006, 40, 2427–2435. [Google Scholar] [PubMed]
- Tian, S.H.; Zhang, C.; Huang, D.L.; Wang, R.Z.; Zeng, G.M.; Yan, M.; Xiong, W.P.; Zhou, C.Y.; Cheng, M.; Xue, W.J.; et al. Recent progress in sustainable technologies for adsorptive and reactive removal of sulfonamides. Chem. Eng. J. 2020, 389, 123423. [Google Scholar]
- Wang, J.L.; Wang, S.Z. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Zhang, J.L.; Zhai, J.R.; Zheng, H.; Li, X.Y.; Wang, Y.R.; Li, X.P.; Xing, B.S. Adsorption, desorption and coadsorption behaviors of sulfamerazine, Pb (II) and benzoic acid on carbon nanotubes and nano-silica. Sci. Total Environ. 2020, 73810, 139685. [Google Scholar] [CrossRef]
- Yoon, Y.; Westerhoff, P.; Snyder, S.A.; Wert, E.C.; Yoon, J. Removal of endocrine disrupting compounds and pharmaceuticals by nanofiltration and ultrafiltration membranes. Desalination 2007, 202, 16–23. [Google Scholar] [CrossRef]
- Zhao, S.S.; Yao, Y.X.; Ba, C.Y.; Zheng, W.H.; Economy, J.; Wang, P. Enhancing the performance of polyethylenimine modified nanofiltration membrane by coating a layer of sulfonated poly (ether ether ketone) for removing sulfamerazine. J. Membr. Sci. 2015, 492, 620–629. [Google Scholar] [CrossRef]
- Fabiańska, A.; Białk-Bielińska, A.; Stepnowski, P.; Stolte, S.; Siedlecka, E.M. Electrochemical degradation of sulfonamides at BDD electrode: Kinetics, reaction pathway and eco-toxicity evaluation. J. Hazard. Mater. 2014, 280, 579–587. [Google Scholar] [CrossRef]
- Oliveira, G.H.; Santos-Neto, A.J.; Zaiat, M. Evaluation of sulfamethazine sorption and biodegradation by anaerobic granular sludge using batch experiments. Bioprocess Biosyst. Eng. 2016, 39, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Li, P.S.; Guo, M.; Wang, Q.; Li, Z.; Wang, C.Z.; Chen, N.; Wang, C.C.; Wan, C.Q.; Chen, S.W. Controllable synthesis of cerium zirconium oxide nanocomposites and their application for photocatalytic degradation of sulfonamides. Appl. Catal. B Environ. 2019, 259, 118107. [Google Scholar]
- Lai, C.; Zhang, M.; Li, B.; Huang, D.; Zeng, G.; Qin, L.; Liu, X.; Yi, H.; Cheng, M.; Li, L.; et al. Fabrication of CuS/BiVO4 (040) binary heterojunction photocatalysts with enhanced photocatalytic activity for Ciprofloxacin degradation and mechanism insight. Chem. Eng. J. 2019, 358, 891–902. [Google Scholar] [CrossRef]
- Gobel, A.; McArdell, C.S.; Joss, A.; Siegrist, H.; Giger, W. Fate of sulfonamides, macrolides, and trimethoprim in different wastewater treatment technologies. Sci. Total Environ. 2007, 372, 361–371. [Google Scholar] [CrossRef]
- Dong, H.R.; Zeng, G.M.; Tang, L.; Fan, C.Z.; Zhang, C.; He, X.X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar]
- Kalnaowakul, P.; Phairatana, T.; Ubolchollakhet, K.; Sangchay, W.; Rodchanarowan, A. Synthesis of Bi2O3-doped and TiO2-doped porous Lava for photocatalytic studies. Mater. Today Proc. 2018, 5, 9312–9318. [Google Scholar] [CrossRef]
- Rachel, A.; Lavedrine, B.; Subrahmanyam, M.; Boule, P. Use of porous lavas as supports of photocatalysts. Catal. Commun. 2002, 3, 165–171. [Google Scholar] [CrossRef]
- Li, J.; Mao, N.; Li, X.; Chen, F.; Li, Y.; Jiang, K.; Hu, Z.; Chu, J. Controllable fabrication of Bi2O3 nanoparticles by atomic layer deposition on TiO2 films and application in photodegradation. Sol. Energy Mater. Sol. Cells 2020, 204, 110218. [Google Scholar] [CrossRef]
- Zou, Q.; Li, H.; Yang, Y.P.; Miao, Y.C.; Huo, Y.N. Bi2O3/TiO2 photocatalytic film coated on floated glass balls for efficient removal of organic pollutant. Appl. Surf. Sci. 2019, 467, 354–360. [Google Scholar] [CrossRef]
- Reddy, N.L.; Reddy, G.K.; Basha, K.M.; Mounika, P.K.; Shankar, M.V. Highly efficient hydrogen production using Bi2O3/TiO2 nanostructured photocatalysts under Led light irradiation. Mater. Today Proc. 2016, 3, 1351–1358. [Google Scholar] [CrossRef]
- Marien, C.B.D.; Cottineau, T.; Robert, D.; Drogui, P. TiO2 Nanotube arrays: Influence of tube length on the photocatalytic degradation of Paraquat. Appl. Catal. B Environ. 2016, 194, 1–6. [Google Scholar] [CrossRef]
- Tian, F.; Wu, Z.; Chen, Q.; Yan, Y.; Cravotto, G.; Wu, Z. Microwave-induced crystallization of AC/TiO2 for improving the performance of rhodamine B dye degradation. Appl. Surf. Sci. 2015, 351, 104–112. [Google Scholar] [CrossRef]
- Khodadadi, M.; Ehrampoush, M.H.; Ghaneian, M.T.; Allahresani, A.; Mahvi, A.H. Synthesis and characterizations of FeNi3@SiO2@TiO2 nanocomposite and its application in photo-catalytic degradation of tetracycline in simulated wastewater. J. Mol. Liq. 2018, 255, 224–232. [Google Scholar] [CrossRef]
- Jiang, Q.; Huang, J.; Ma, B.G.; Yang, Z.C.; Zhang, T.; Wang, X. Recyclable, hierarchical hollow photocatalyst TiO2@SiO2 composite microsphere realized by raspberry-like SiO2. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125112. [Google Scholar] [CrossRef]
- Ouzzine, M.; Romero-Anaya, A.J.; Lillo-Ródenas, M.A.; Linares-Solano, A. Spherical activated carbon as an enhanced support for TiO2/AC photocatalysts. Carbon 2014, 67, 104–118. [Google Scholar] [CrossRef]
- Chekem, C.T.; Goetz, V.; Richardson, Y.; Plantard, G.; Blin, J. Modelling of adsorption/photodegradation phenomena on AC-TiO2 composite catalysts for water treatment detoxification. Catal. Today 2019, 328, 183–188. [Google Scholar] [CrossRef]
- Liu, G.Y.; Xia, Y.N.; Niu, Y.H.; Zhao, X.; Zhang, G.T.; Song, L.F.; Chen, H.X. Photocatalytic performance of doped TiO2/AC coating and its UV stability research. Prog. Org. Coat. 2020, 148, 105882. [Google Scholar] [CrossRef]
- Wang, N.; Li, X.; Yang, Y.L.; Zhou, Z.W.; Shang, Y.; Zhuang, X.X.; Zhang, T.T. Two-stage calcination composite of Bi2O3-TiO2 supported on powdered activated carbon for enhanced degradation of sulfamethazine under solar irradiation. J. Water Process Eng. 2020, 35, 101220. [Google Scholar] [CrossRef]
- Lei, B.; Cui, W.; Sheng, J.; Wang, H.; Chen, P.; Li, J.; Sun, Y.; Dong, F. Synergistic effects of crystal structure and oxygen vacancy on Bi2O3 polymorphs: Intermediates activation, photocatalytic reaction efficiency, and conversion pathway. Sci. Bull. 2020, 65, 467–476. [Google Scholar] [CrossRef]
- Ho, Y.S.; Mckay, G. The sorption of lead (II) ions on peat. Water Res. 1999, 33, 578–584. [Google Scholar]
- Liu, Y. New insights into pseudo-second-order kinetic equation for adsorption. Colloids Surf. A Physicochem. Eng. Asp. 2008, 320, 275–278. [Google Scholar] [CrossRef]
- Jiang, S.; Yu, T.; Xia, R.; Wang, X.; Gao, M.Z. Realization of super high adsorption capability of 2D δ-MnO2/GO through intra-particle diffusion. Mater. Chem. Phys. 2019, 232, 374–381. [Google Scholar] [CrossRef]
- Boyd, G.E.; Adamson, A.W.; Myers, L.S. The exchange adsorption of ions from aqueous solutions by organic zeolites. II. Kinetics1. J. Am. Chem. Soc. 1947, 69, 2836–2848. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 1, 1–2. [Google Scholar]
- Langmuir, I. Adsorption of gases on glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Wang, J.L. Comparison of linearization methods for modeling the Langmuir adsorption isotherm. J. Mol. Liq. 2019, 296, 111850. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M.; Gayatri, S.L. Batch adsorption of 4-nitrophenol by acid activated jute stick char: Equilibrium, kinetic and thermodynamic studies. Chem. Eng. J. 2010, 158, 173–180. [Google Scholar]
- Dong, F.; Li, C.; He, G.; Chen, X.; Mao, X. Kinetics and degradation pathway of sulfamethazine chlorination in pilot-scale water distribution systems. Chem. Eng. J. 2017, 321, 521–532. [Google Scholar] [CrossRef]
- Zheng, X.Y.; Xu, S.P.; Wang, Y.; Sun, X.; Gao, Y.; Gao, B.Y. Enhanced degradation of ciprofloxacin by graphitized mesoporous carbon (GMC)-TiO2 nanocomposite: Strong synergy of adsorption-photocatalysis and antibiotics degradation mechanism. J. Colloid Interf. Sci. 2018, 527, 202–213. [Google Scholar] [CrossRef]
- Gao, B.F.; Yap, P.S.; Lim, T.M.; Lim, T.T. Adsorption-photocatalytic degradation of Acid Red 88 by supported TiO2: Effect of activated carbon support and aqueous anions. Chem. Eng. J. 2011, 171, 1098–1107. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Banfield, J.F. Kinetics of crystallization and crystal growth of nanocrystalline anatase in nanometer-sized amorphous titania. Chem. Mater. 2002, 14, 4145–4154. [Google Scholar] [CrossRef]
- Liu, Y.; Xin, F.; Wang, F.; Luo, S.; Yin, X. Synthesis, characterization, and activities of visible light-driven Bi2O3-TiO2 composite photocatalysts. J. Alloys Compd. 2010, 498, 179–184. [Google Scholar] [CrossRef]
- Zhang, H.; Qu, J.H.; Liu, H.J.; Zhao, X. Characterization of isolated fractions of dissolved organic matter from sewage treatment plant and the related disinfection by-products formation potential. J. Hazard. Mater. 2009, 164, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, M.; Cruz, A.A.; Shea, K.O.; Falaras, P.; Dionysiou, D.D. Effects of water parameters on the degradation of microcystin-LR under visible light-activated TiO2 photocatalyst. Water Res. 2011, 45, 3787–3796. [Google Scholar] [PubMed]
- Feitz, A.J.; Waite, T.D.; Jones, G.J.; Boyden, B.H.; Orr, P.T. Photocatalytic degradation of the blue green algal toxin microcystin-LR in a natural organic-aqueous matrix. Environ. Sci. Technol. 1999, 33, 243–249. [Google Scholar] [CrossRef]
- Sukul, P.; Spiteller, M. Sulfonamides in the environment as veterinary drugs. Rev. Environ. Contam. Toxicol. 2006, 187, 67–101. [Google Scholar]
- Qiang, Z.M.; Adams, C. Potentiometric determination of acid dissociation constants (pKa) for human and veterinary antibiotics. Water Res. 2004, 38, 2874–2890. [Google Scholar]
- Yang, J.C.; Wang, Z.H.; Kaloush, K.E.; Dylla, H. Effect of pavement thermal properties on mitigating urban heat islands: A multi-scale modeling case study in Phoenix. Build. Environ. 2016, 108, 110–121. [Google Scholar] [CrossRef] [Green Version]
- Rincón, A.G.; Pulgarin, C. Effect of pH, inorganic ions, organic matter and H2O2 on E. coli K12 photocatalytic inactivation by TiO2: Implications in solar water disinfection. Appl. Catal. B Environ. 2004, 51, 283–302. [Google Scholar] [CrossRef]
- Yap, P.S.; Lim, T.T. Effect of aqueous matrix species on synergistic removal of bisphenol-A under solar irradiation using nitrogen-doped TiO2/AC composite. Appl. Catal. B Environ. 2011, 101, 709–717. [Google Scholar] [CrossRef]
- Harlapur, S.F.; Rashmi, B.N.; Nagaswarupa, H.P.; Prashantha, S.C.; Shashishekar, T.R.; Kumar, M.R.A. Photocatalytic studies of TiO2 nanomaterials prepared via facile wet chemical route. Mater. Today Proc. 2017, 4, 11713–11719. [Google Scholar] [CrossRef]
- Panda, R.K.; Muduli, R.; Behera, D. Electric and magnetic properties of Bi substituted cobalt ferrite nanoparticles: Evolution of grain effect. J. Alloys Compd. 2015, 634, 239–245. [Google Scholar] [CrossRef]
- Spurr, R.A.; Myers, H. Quantitative analysis of anatase-rutile mixtures with an X-ray diffractometer. Anal. Chem. 1957, 29, 760–762. [Google Scholar] [CrossRef]
- Garcia, J.C.; Nolan, M.; Deskins, N.A. The nature of interfaces and charge trapping sites in photocatalytic mixed-phase TiO2 from first principles modeling. J. Chem. Phys. 2015, 142, 024708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bacsa, R.R.; Grätzel, M. Rutile Formation in Hydrothermally Crystallized Nanosized Titania. J. Am. Ceram. Soc. 1996, 79, 2185–2188. [Google Scholar] [CrossRef]
- Scherrer, P.; Gottingen, N.G.W. Bestimmung der Größe und der inneren Struktur von Kolloidteilchen mittels Röntgenstrahlen. Math. Physik. Kl. 1918, 2, 96–100. [Google Scholar]
- Davis, E.A.; Mott, N.F. Conduction in non-crystalline systems V. Conductivity, optical absorption and photoconductivity in amorphous semiconductors. Philos. Mag. 1970, 22, 903–922. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, K.K.; Liu, T.; Xu, J.; Xu, B.G. Synthesis of one-dimensional Bi2O3-Bi2O2.33 heterojunctions with high interface quality for enhanced visible light photocatalysis in degradation of high-concentration phenol and MO dyes. Appl. Catal. B Environ. 2017, 203, 946–954. [Google Scholar] [CrossRef]
- Sood, S.; Mehta, S.K.; Umar, A.; Kansal, S.K. The visible light-driven photocatalytic degradation of alizarin red S using Bi-doped TiO2 nanoparticles. New J. Chem. 2014, 38, 3127–3136. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Fu, Q.Y.; Jiang, R.; Jiang, H.J.; Xiao, L.; Zeng, G.M.; Zhao, S.L.; Wang, Y. Adsorption removal of congo red onto magnetic cellulose/Fe3O4/activated carbon composite: Equilibrium, kinetic and thermodynamic studies. Chem. Eng. J. 2011, 173, 494–502. [Google Scholar] [CrossRef]
- Wang, W.; Ding, X.; He, M.; Wang, J.; Lou, X. Kinetic adsorption profile and conformation evolution at the DNA-gold nanoparticle interface probed by dynamic light scattering. Anal. Chem. 2014, 86, 10186–10192. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.S.; Jian, X.; Wang, S.F.; Zhang, Y.; He, Y.; Li, X.C. Photodegradation of sulfamethazine in aqueous solution by bismuth molybdate photocatalyst. Catal. Sci. Technol. 2013, 6, 1603–1611. [Google Scholar]
- Guo, C.; Xu, J.; Zhang, Y.; He, Y. Hierarchical mesoporous TiO2 microspheres for the enhanced photocatalytic oxidation of sulfonamides and their mechanism. RSC Adv. 2012, 2, 4720–4727. [Google Scholar] [CrossRef]
- Li, M.; Wang, C.; Yau, M.; Bolton, J.R.; Qiang, Z. Sulfamethazine degradation in water by the VUV/UV process: Kinetics, mechanism and antibacterial activity determination based on a mini-fluidic VUV/UV photoreaction system. Water Res. 2017, 108, 348–355. [Google Scholar]
- Payan, A.; Isari, A.A.; Gholizade, N. Catalytic decomposition of sulfamethazine antibiotic and pharmaceutical wastewater using Cu-TiO2@functionalized SWCNT ternary porous nanocomposite: Influential factors, mechanism, and pathway studies. Chem. Eng. J. 2019, 361, 1121–1141. [Google Scholar] [CrossRef]









| Composites | Pseudo-First-Order | Pseudo-Second-Order | Intra-Particle Diffusion | ||||||
|---|---|---|---|---|---|---|---|---|---|
| k1 (h−1) | qe (mg·g−1) | R2 | k2 (mg·g−1·h−1) | qe (mg·g−1) | R2 | Kd (mg·g−1·h−1) | C | R2 | |
| Bi2O3–TiO2/PAC (8%–700 °C) | 0.025 | 1.388 | 0.667 | 0.084 | 5.450 | 1.000 | 0.432 | 2.147 | 0.631 |
| Bi2O3–TiO2/PAC (10%–700 °C) | 0.052 | 1.760 | 0.820 | 0.038 | 4.817 | 0.997 | 0.474 | 1.087 | 0.818 |
| Bi2O3–TiO2/PAC (12%–700 °C) | 0.052 | 1.557 | 0.982 | 0.028 | 3.723 | 0.993 | 0.342 | 0.524 | 0.889 |
| Bi2O3–TiO2 (10%–700 °C) | 0.143 | 2.151 | 0.951 | 0.032 | 3.397 | 0.990 | 0.318 | 0.563 | 0.813 |
| TiO2(700 °C) | 0.081 | 1.379 | 0.901 | 0.068 | 5.291 | 0.994 | 0.465 | 1.704 | 0.636 |
| Samples | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) | Average Crystal Size (nm) |
|---|---|---|---|---|
| Bi2O3–TiO2/PAC(8%–700 °C) | 123.044 | 0.280 | 14.407 | 46.9 |
| Bi2O3–TiO2/PAC(10%–700 °C) | 130.736 | 0.277 | 13.740 | 48.2 |
| Bi2O3–TiO2/PAC(12%–700 °C) | 103.445 | 0.248 | 15.276 | 47.8 |
| Bi2O3–TiO2(10%–700 °C) | 84.785 | 0.351 | 16.540 | 23.3 |
| TiO2(700 °C) | 61.921 | 0.122 | 7.859 | 25.3 |
| PAC | 928.767 | 0.654 | 4.899 | - |
| Types | Models | k | qe | R2 |
|---|---|---|---|---|
| External diffusion | Boyd’s external diffusion equation | 0.111 | 4.520 (q∞) | 0.995 |
| Internal diffusion | Weber and Morris model | 0.612 | 3.455 | 0.787 |
| Adsorption onto active sites | Langmuir kinetics model | 0.006 (ka), 0.0004 (kd) | 4.502 | 0.993 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, X.; Li, X.; Yang, Y.; Wang, N.; Shang, Y.; Zhou, Z.; Li, J.; Wang, H. Enhanced Sulfamerazine Removal via Adsorption–Photocatalysis Using Bi2O3–TiO2/PAC Ternary Nanoparticles. Water 2020, 12, 2273. https://doi.org/10.3390/w12082273
Zhuang X, Li X, Yang Y, Wang N, Shang Y, Zhou Z, Li J, Wang H. Enhanced Sulfamerazine Removal via Adsorption–Photocatalysis Using Bi2O3–TiO2/PAC Ternary Nanoparticles. Water. 2020; 12(8):2273. https://doi.org/10.3390/w12082273
Chicago/Turabian StyleZhuang, Xiaoxuan, Xing Li, Yanling Yang, Nan Wang, Yi Shang, Zhiwei Zhou, Jiaqi Li, and Huiping Wang. 2020. "Enhanced Sulfamerazine Removal via Adsorption–Photocatalysis Using Bi2O3–TiO2/PAC Ternary Nanoparticles" Water 12, no. 8: 2273. https://doi.org/10.3390/w12082273





