Water of Increased Content of Molecular Oxygen
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Water
2.1.2. Oxygen
2.2. Methods
2.2.1. Treating Water with LPGP
2.2.1.1. Treating Plain Water
2.2.1.2. Treating Water Saturated with Oxygen with LPGP
2.2.2. Physicochemical Properties of LPGP Treated Water
2.2.2.1. pH
2.2.2.2. Conductivity
2.2.2.3. Density
2.2.2.4. Dissolved Oxygen Content
2.2.2.5. Active Oxygen
2.2.2.6. Differential Scanning Calorimetry (DSC)
2.2.2.7. Fourier Transformation Infrared—Attenuated Total Reflectance (FTIR-ATR) spectra
2.2.2.8. Determination of Ozone
2.2.2.9. Electron Spin Resonance (ESR) Spectra
2.2.2.10. Ultraviolet/Visible (UV-VIS) Spectra
2.2.2.11. Raman Spectra
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Białopiotrowicz, T.; Ciesielski, W.; Domański, J.; Doskocz, M.; Fiedorowicz, M.; Grąż, K.; Khachatryan, K.; Kołoczek, H.; Kozak, A.; Oszczęda, Z.; et al. Structure and physicochemical properties of water treated with low-temperature low-frequency plasma. Curr. Phys. Chem. 2016, 6, 312–320. [Google Scholar] [CrossRef]
- Chwastowski, J.; Ciesielska, K.; Ciesielski, W.; Khachatryan, K.; Kołoczek, H.; Kulawik, D.; Oszczeda, Z.; Tomasik, P.; Witczak, M. Structure and physicochemical properties of water treated under nitrogen with low-temperature glow plasma of low frequency. Water 2020, 12, 1314. [Google Scholar] [CrossRef]
- Ciesielska, A.; Ciesielski, W.; Khachatryan, K.; Kołoczek, H.; Kulawik, D.; Oszczęda, Z.; Soroka, J.A.; Tomasik, P. Structure and physicochemical properties of water treated under methane with low-temperature glow plasma of low frequency. Water 2020, 12, 1638. [Google Scholar] [CrossRef]
- Ciesielski, W.; Ciesielska, A.; Khachatryan, K.; Kołoczek, H.; Kulawik, D.; Oszczęda, Z.; Soroka, J.A.; Tomasik, P. Structure and physicochemical properties of water treated under carbon dioxide with low-temperature low-pressure glow plasma of low frequency. Water 2020, 12, 1920. [Google Scholar] [CrossRef]
- Zelenak, Z.M.; Berzsenyi, L.; Abramoff, F. Oxygen Enriched Liquids, Method and Apparatus for Making, and Applications Thereof. U.S. Patent 581422A, 29 September 1998. [Google Scholar]
- Messer Americas, FARMOX ™ Drop-in Oxygenation Apparatus. New, Easy-to-Deploy, Highly Efficient Solution for Oxygenation of Water. Available online: https://cdn2.hubspot.net/hubfs/189660/Messer%20US%20Website_2019/Resources/MESS-3024_FARMOX_Dropln_datasheet.pdf (accessed on 25 June 2020).
- Donsbach, K.W.; Cazares, R. Process for Making Highly Oxygenated Drinking Water and Drinking Water Made by the Process. U.S. Patent 5587191A, 24 December 1996. [Google Scholar]
- DeWald, J.J. Method and Apparatus for Adding Oxygen to Drinking Water. U.S. Patent 69361179B2, 30 August 2005. [Google Scholar]
- Compagnie Gervais Danone. Method for Enriching Water with Oxygen by an Electrolytic Process, Oxygen enrIched Water or Beverage and Uses Thereof. U.S. Patent 8,709,231, 19 December 2008.
- Pakdaman, A. Oxygen Enriched Water and Oral Oxygen Therapy (OOT). Available online: https://static.webshopapp.com/shops/035143/files/056059208/pakdaman-studies-oxygen-enriched-water.pdf (accessed on 22 June 2020).
- Pușcaș, C.; Moldovan, M.; Silaghi-Dumitrescu, L.; Ungureanu, L.; Silaghi-Dumitrescu, R. On the apparent redox reactivity of “oxygen-enriched water”. Biol. Trace Elem. Res. 2020, 1–9. [Google Scholar]
- Charton, A.; Péronnet, F.; Doutreleau, S.; Lonsdorfer, E.; Klein, A.; Jimenez, L.; Geny, B.; Diemunsch, P.; Richard, R. Effect of administration of water enriched in O2 by injection or electrolysis on transcutaneous oxygen pressure in anesthetized pigs. Drug Des. Devel. Ther. 2014, 8, 1161–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoenberg, M.H.; Hierl, T.C.; Zhao, J.; Wohlgemuth, N.; Nilsson, U.A. The generation of oxygen radicals after drinking of oxygenated water. Eur. J. Med. Res. 2002, 7, 109–116. [Google Scholar]
- Gruber, R.; Axmann, S.; Schoenberg, M.H. The influence of oxygenated water on the immune status, liver enzymes, and the generation of oxygen radicals; a prospective, randomised, blinded clinical study. Clin. Nutr. 2005, 24, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Preiato, D. What is Oxygenated Water? Benefits, Uses and Precautions, Healthline. 5 March 2020. Available online: https://www.healthline.com/nutrition/oxygenated-water (accessed on 26 June 2020).
- Tromans, D. Temperature and pressure dependent solubility of oxygen in water: A thermodynamic analysis. Hydrometallurgy 1998, 48, 327–342. [Google Scholar] [CrossRef]
- Emsley, J. Oxygen. In Nature's Building Blocks: An A-Z Guide to the Elements; Oxford University Press: Oxford, UK, 2001; pp. 297–304. [Google Scholar]
- Air solubility in water. The Engineering Toolbox. Retrieved June 20. 2020. Available online: https://www.engineeringtoolbox.com (accessed on 26 June 2020).
- Evans, D.H.; Claiborne, J.B. The Physiology of Fishes, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2005; p. 88. [Google Scholar]
- Li, Y.; Buckin, V. State of oxygen molecules in aqueous supersaturated solutions. J. Phys. Chem. B 2019, 123, 4025–4043. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, S.V.; Bruskov, V.I.; Astashev, M.E.; Chernikov, A.V.; Yaguzhinsky, L.S.; Zakharov, S.D. Oxygen—Dependent auto-oscillations of water luminescence triggered by the 1264 nm radiation. J. Phys. Chem. B 2011, 115, 7693–7698. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, E.V.; Abrosimov, V.K. Specific features of hydration of gaseous nitrogen and oxygen, revealed from data on their solubility in water H/D isotopomers. Russian J. Gen. Chem. 2005, 75, 1851–1856. [Google Scholar] [CrossRef]
- Vaz da Cruz, V.; Gelmukhanov, F.; Eckert, S.; Iannuzzi, M.; Ertan, E.; Pietzsch, A.; Couto, R.C.; Niskanen, J.; Fondell, M.; Dantz, M.; et al. Probing hydrogen bond strength in liquid water by resonant inelastic X-ray scattering. Nat. Commun. 2019, 10, 1013. [Google Scholar]
- Nieva, J.; Wentworth, P., Jr. The antibody-catalyzed water oxidation pathway—A new chemical arm to immune defense. Trends Biochem. Sci. 2004, 29, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Oszczęda, Z.; Elkin, I.; Stręk, W. Equipment for treatment of water with plasma. Polish Patent Application P-389626, 20 November 2009. [Google Scholar]
- McCaa, D.J.; Shaw, J.H. The infrared spectrum of ozone. J. Mol. Struct. 1968, 25, 374–397. [Google Scholar] [CrossRef]
- Keutsch, F.N.; Cruzan, J.D.; Saykally, R.J. The water trimer. Chem. Revs. 2003, 103, 2533–2578. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, S.V.; Astashev, M.E.; Bruskov, V.I.; Kozlov, V.A.; Zakharov, S.D.; Bunkin, N.F. Self-oscillating water chemiluminescence modes and reactive oxygen species generation induced by laser irradiation; effect of the exclusion zone created by Nafion. Entropy 2014, 16, 6166–6185. [Google Scholar] [CrossRef] [Green Version]
- Chaplin, M. Water Structure and Science. 2016. Available online: www1.lsbu.ac.uk/water/water_vibrational_spectrum.html (accessed on 20 June 2020).
- Krupienie, P.H. The spectrum of molecular oxygen. J. Phys. Chem. Ref. Data 1972, 1, 423–534. [Google Scholar] [CrossRef] [Green Version]
- Hu, Q.; Zhao, H.; Ouyang, S. Understanding water structure from Raman spectra of isotopic substitution H2O/D2O up to 573 K. Phys. Chem. Chem. Phys. 2017, 19, 21540–21547. [Google Scholar] [CrossRef] [PubMed]
- Oszczeda, Z.; Tomasik, P.; Ciesielski, W.; Kulawik, D. A Way of removal of permanent and temporary water hardness applying low-temperature, low-pressure glow plasma of low-frequency. Polish Patent Application P-426720, 20 August 2018. [Google Scholar]
- Ciesielska, K.; Ciesielski, W.; Khachatryan, K.; Kulawik, D.; Oszczęda, Z.; Tomasik, P. Structure and physicochemical properties of water treated under ammonia with low-temperature, low-pressure glow plasma of low frequency. Open Chem. 2020. accepted. [Google Scholar]
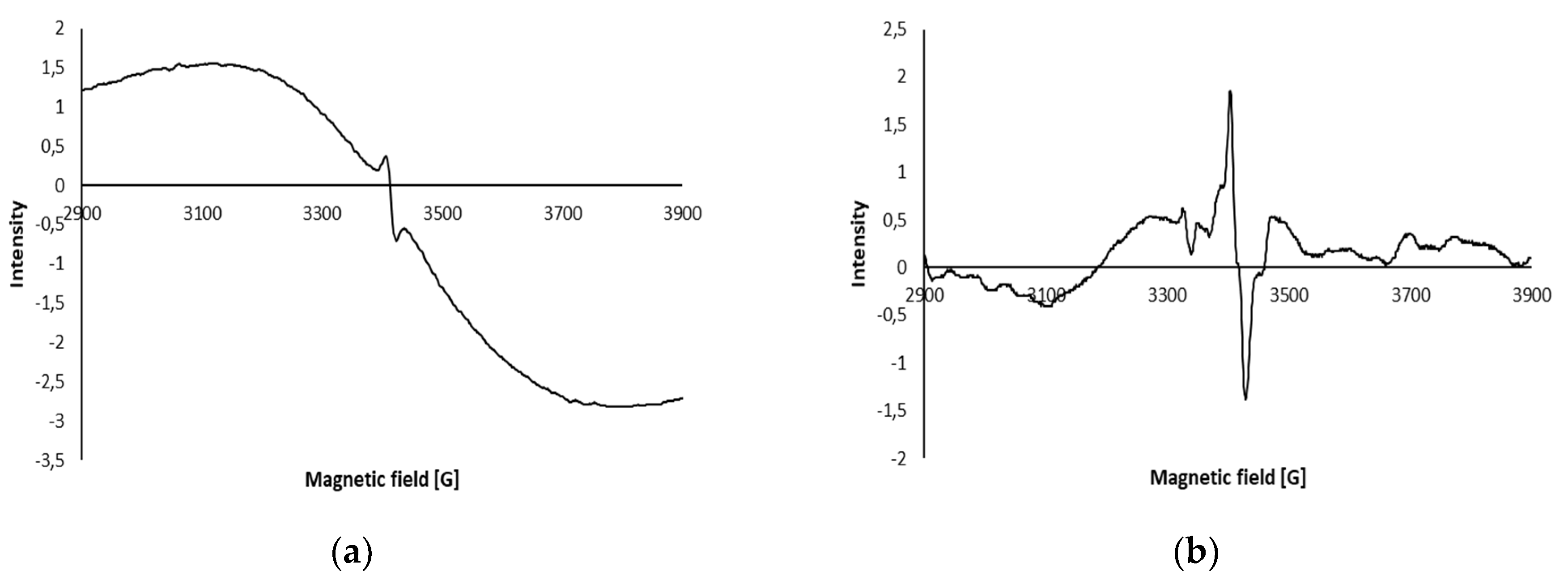
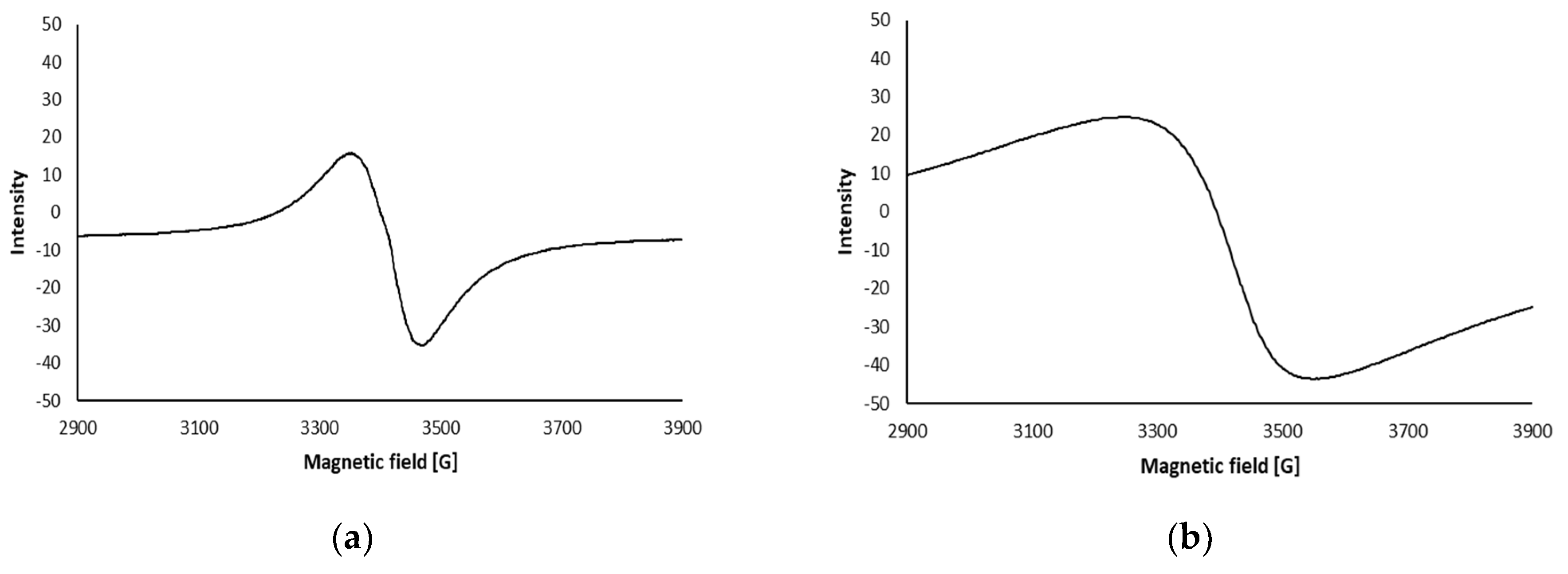
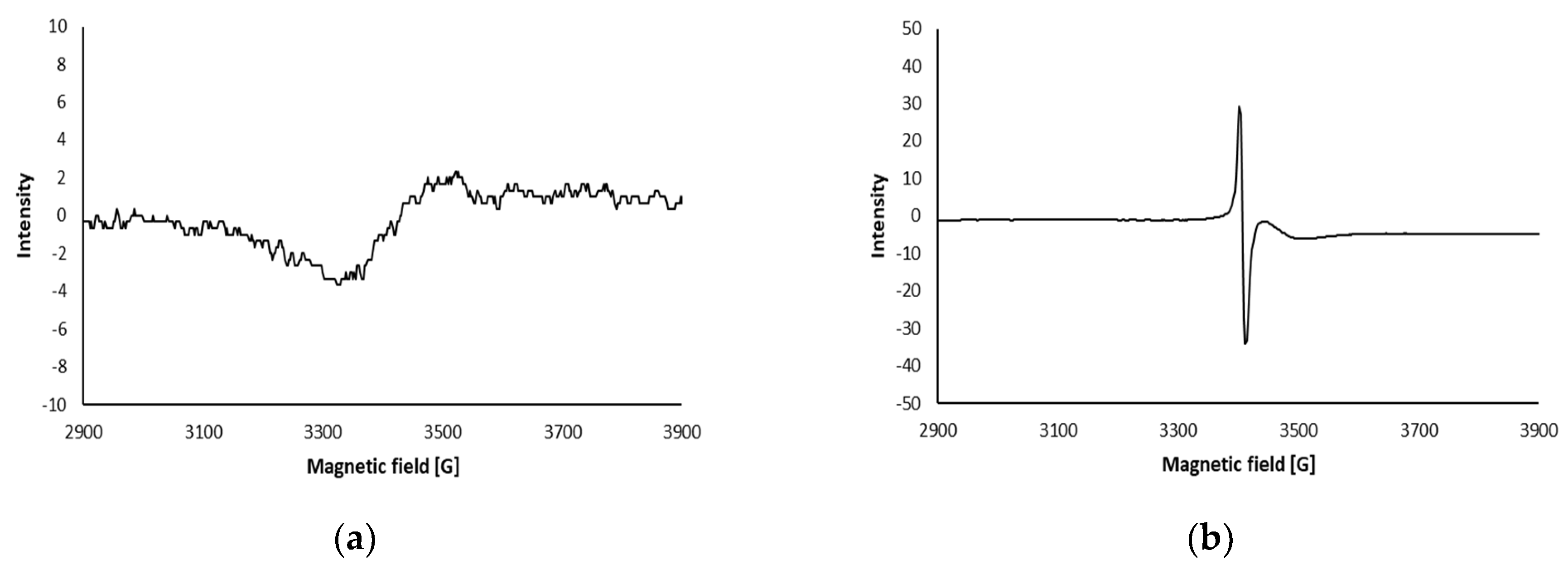

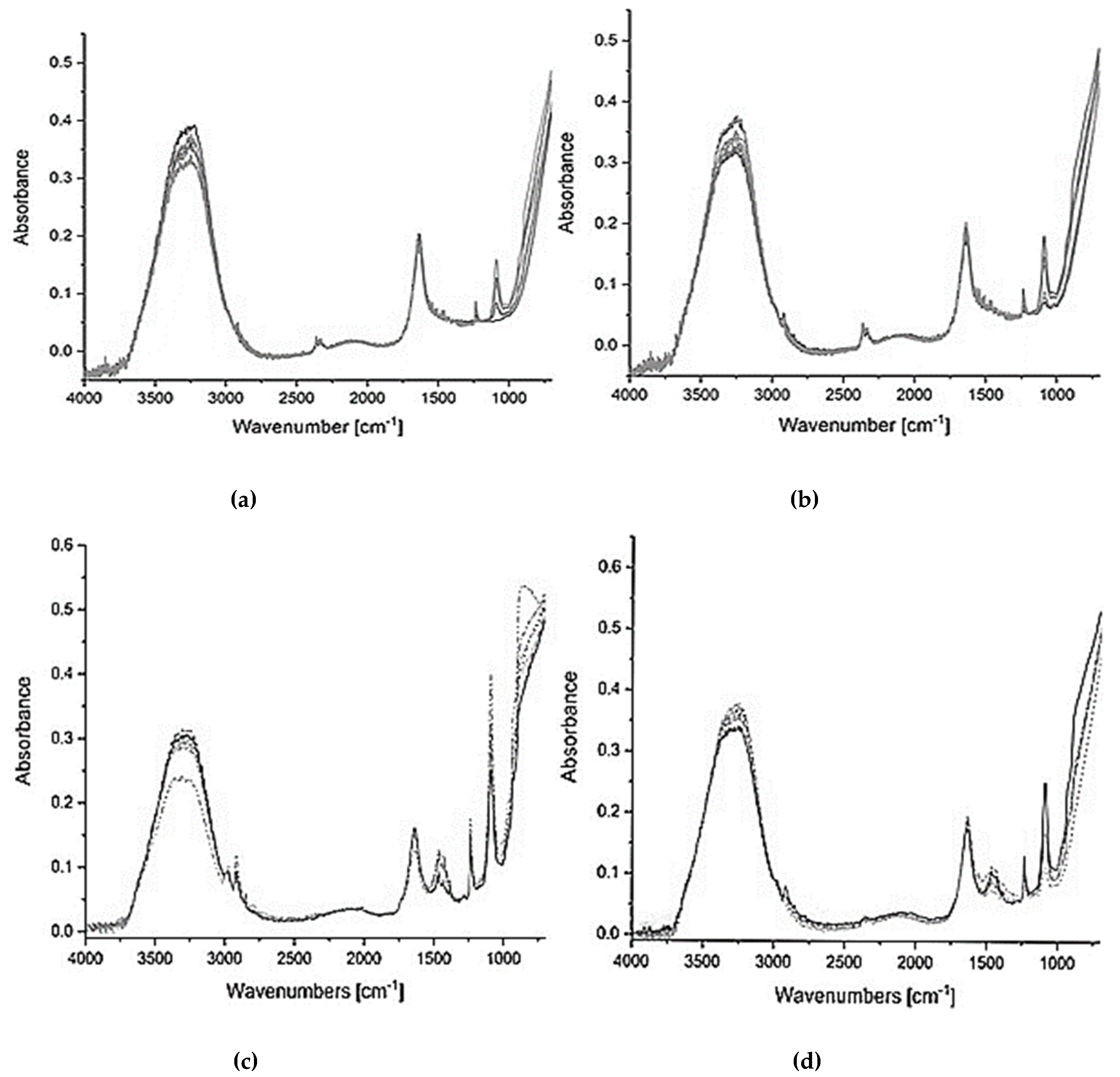
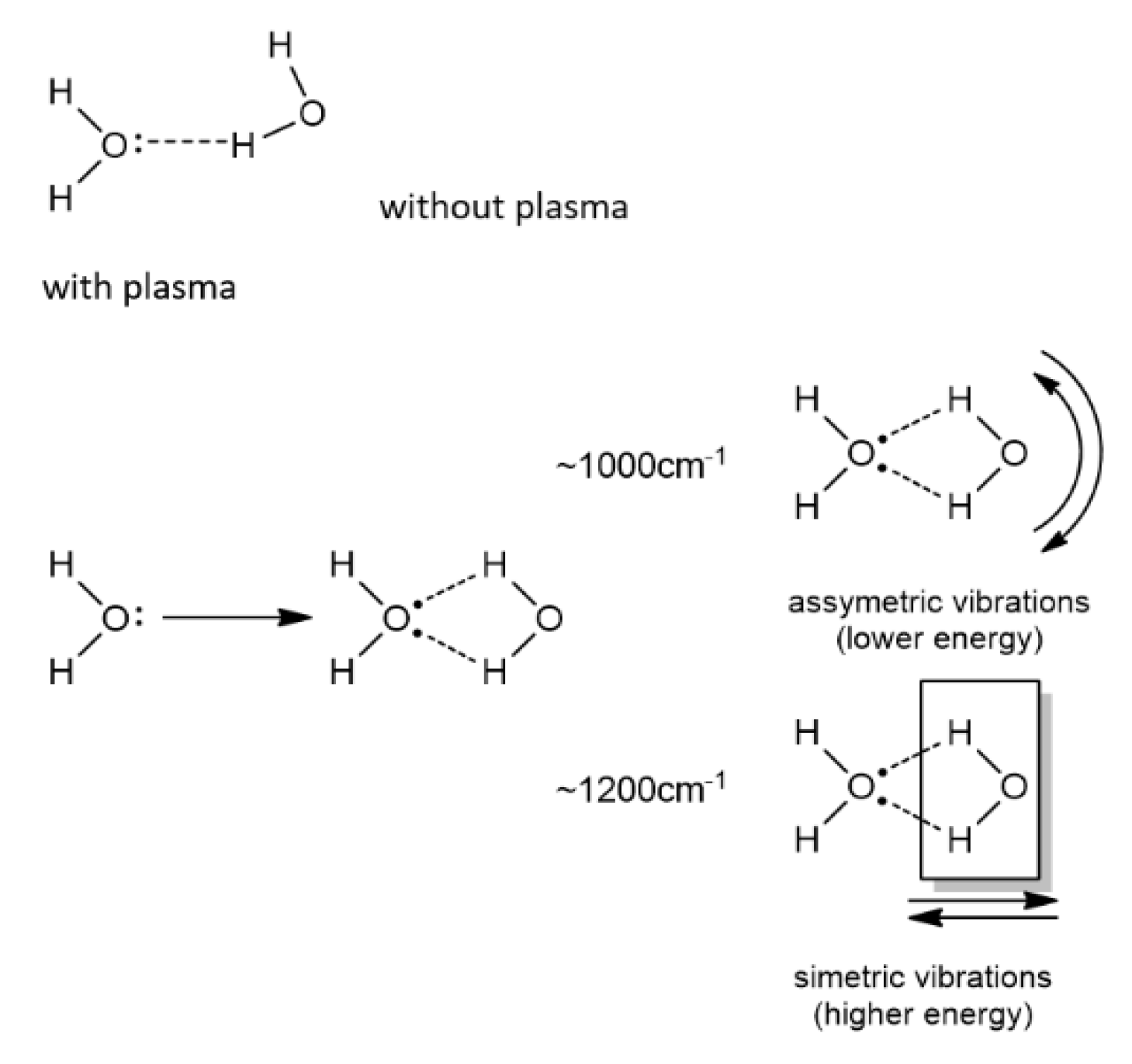




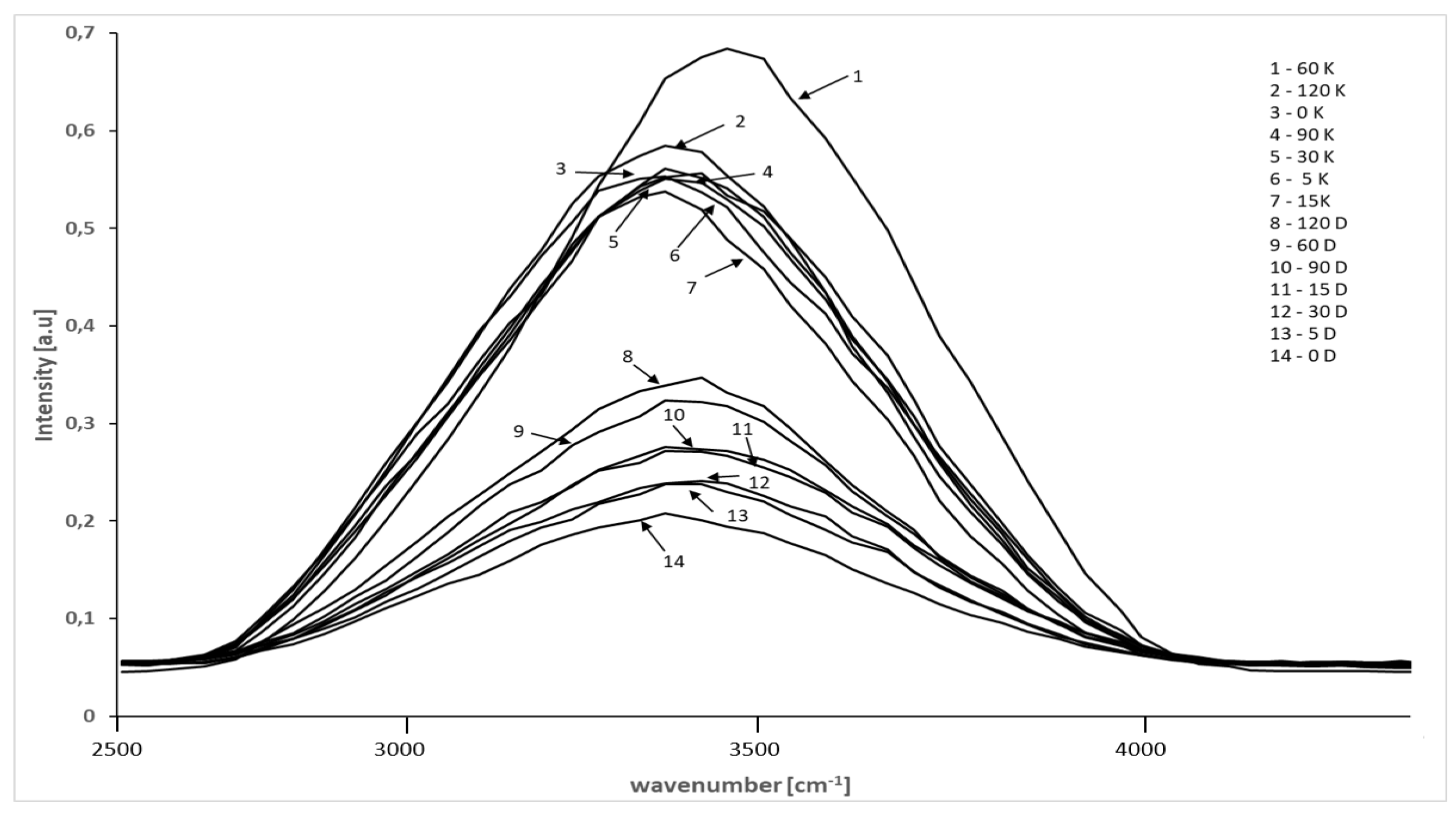
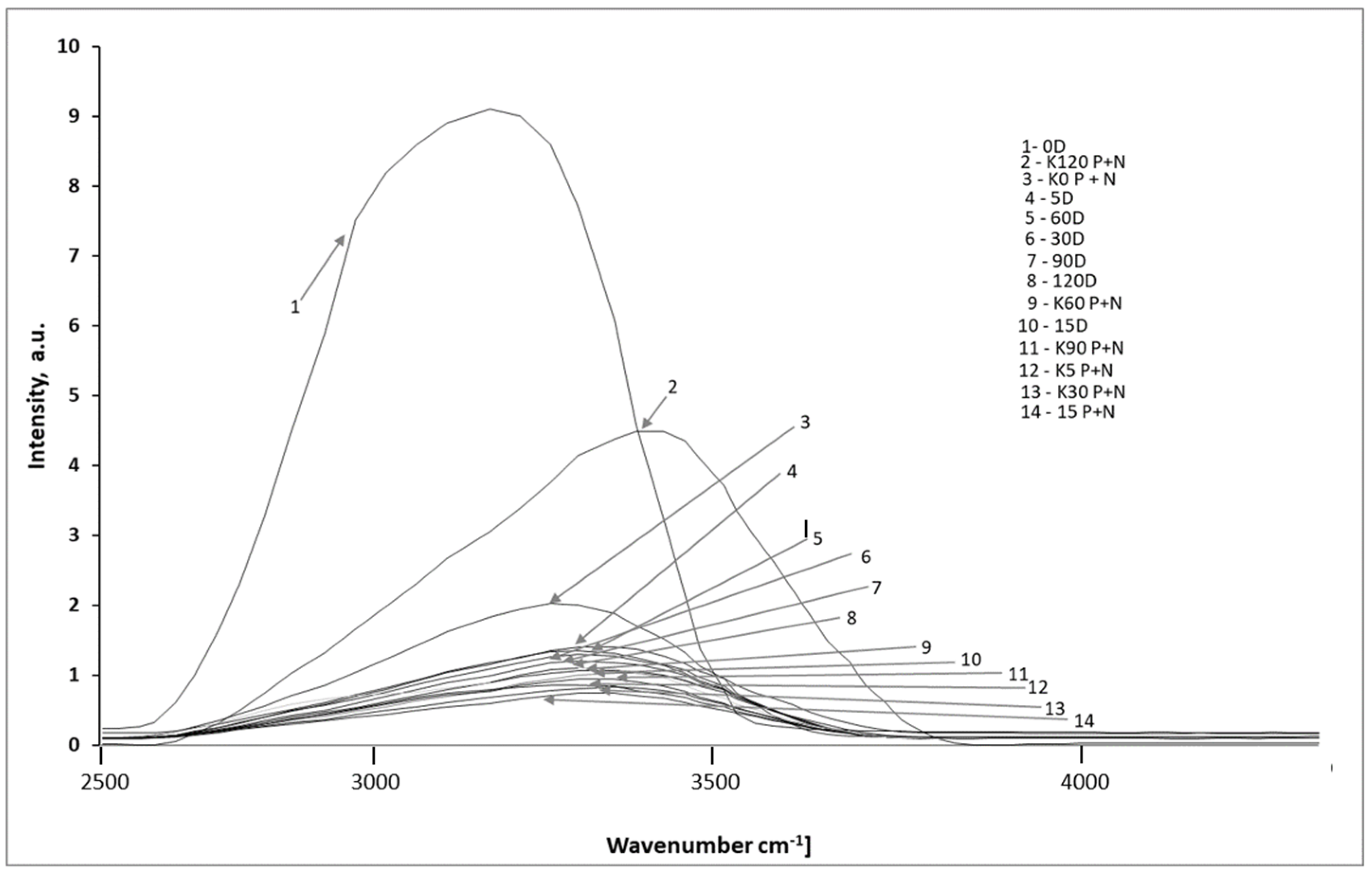
| Sample Type | LPGP Treatment Time (min) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 5 | 15 | 30 | 60 | 90 | 120 | |
| Deionized treated with LPGP b | |||||||
| Water saturated then treated (WST) Water treated then saturated (WTS) | 6.335 6.335 | 7.670 6.361 | 8.539 6.381 | 9.329 6.386 | 8.638 6.382 | 8.410 6.381 | 8.203 6.378 |
| Tap treated with LPGP c | |||||||
| Water saturated then treated (WST) Water treated then saturated (WTS) | 7.343 7.343 | 7.921 7.180 | 8.119 7.240 | 8.370 7.350 | 8.549 7.280 | 8.755 7.280 | 8.840 7.260 |
| Sample Type | LPGP Treatment Time (min) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 5 | 15 | 30 | 60 | 90 | 120 | |
| Deionized treated with LPGP b | |||||||
| Water saturated then treated (WST) Water treated then saturated (WTS) | 0.0485 0.0485 | 0.0353 0.0531 | 0.0560 0.0612 | 0.0633 0.0636 | 0.1000 0.0572 | 0.0490 0.0568 | 0.0216 0.0552 |
| Tap treated with LPGP c | |||||||
| Water saturated then treated (WST) Water treated then saturated (WTS) | 3.659 3.659 | 4.023 3.671 | 4.127 3.669 | 3.295 3.672 | 4.152 3.672 | 4.140 3.662 | 4.009 3.663 |
| Sample Type | LPGP Treatment Time (min) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 5 | 15 | 30 | 60 | 90 | 120 | |
| Deionized treated with LPGP b | |||||||
| Water saturated then treated (WST) Water treated then saturated (WTS) | 996.487 996.487 | 996.752 996.223 | 996.853 996.224 | 997.089 996.280 | 996.524 996.224 | 996.526 996.228 | 996.497 996.221 |
| Tap treated with LPGP c | |||||||
| Water saturated then treated (WST) Water treated then saturated (WTS) | 998.227 998.227 | 998.195 998.224 | 998.258 998.225 | 998.085 998.229 | 998.847 998.224 | 998.521 998.224 | 998.355 998.226 |
| Sample Type | LPGP Treatment Time (min) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 5 | 15 | 30 | 60 | 90 | 120 | |
| Deionized treated with LPGP b | |||||||
| Water saturated then treated (WST) Water treated then saturated (WTS) | 10 10 | 12 12 | 16 13 | 18 14 | 16 13 | 16 12 | 15 12 |
| Tap treated with LPGP c | |||||||
| Water saturated then treated (WST) Water treated then saturated (WTS) | 15 15 | 20 16 | 21 16 | 23 17 | 20 18 | 21 16 | 21 16 |
| Time of Treatment (min) | Oxygen (meqO2/kg) | |
|---|---|---|
| Water | ||
| Deionized | Tap | |
| Non-saturated (control) | 0.000 ± 0.001 | 0.000 ± 0.001 |
| 0 | 0.000 ± 0.001 0.00 ± 0.001 | 0.000 ± 0.001 0.00 ± 0.001 |
| 5 | 0.050 ± 0.001 0.00 ± 0.001 | 0.020 ± 0.001 0.00 ± 0.001 |
| 15 | 0.070 ± 0.001 0.00 ± 0.001 | 0.030 ± 0.001 0.00 ± 0.001 |
| 30 | 0.085 ± 0.001 0.01 ± 0.001 | 0.065 ± 0.001 0.02 ± 0.001 |
| 60 | 0.070 ± 0.001 0.00 ± 0.001 | 0.065 ± 0.001 0.00 ± 0.001 |
| 90 | 0.070 ± 0.001 0.00 ± 0.001 | 0.060 ± 0.001 0.00 ± 0.001 |
| 120 | 0.075 ± 0.001 0.00 ± 0.001 | 0.060 ± 0.001 0.00 ± 0.001 |
| Treating Time (min) | Band Intensity [a.u.] | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ~3250 cm−1 | ~1600 cm−1 | ~1200 cm−1 | ~1000 cm−1 | |||||||||||||
| CD | D | CT | T | CD | D | CT | T | CD | D | CT | T | CD | D | CT | T | |
| 0 | 0.35 | 0.39 0.305 | 0.30 | 0.375 0.33 | 0.19 | 0.21 0.16 | 0.16 | 0.195 0.17 | 0.10 | 0.00 0.11 | 0.15 | 0.055 0.125 | 0.17 | 0.065 0.25 | 0.32 | 0.07 0.25 |
| 5 | 0.35 0.31 | 0.325 0.355 | 0.18 0.15 | 0.175 0.19 | 0.075 0.15 | 0.085 0.125 | 0.12 0.25 | 0.14 0.175 | ||||||||
| 15 | 0.355 0.295 | 0.32 0.355 | 0.18 0.15 | 0.18 0.19 | 0.06 0.145 | 0.09 0.125 | 0.08 0.32 | 0.175 0.175 | ||||||||
| 30 | 0.35 0.29 | 0.315 0.355 | 0.175 0.15 | 0.175 0.19 | 0.075 0.145 | 0.08 0.125 | 0.125 0.32 | 0.13 0.175 | ||||||||
| 60 | 0.325 0.24 | 0.375 0.36 | 0.18 0.125 | 0.20 0.20 | 0.085 0.175 | 0.06 0.125 | 0.165 0.40 | 0.075 0.18 | ||||||||
| 90 | 0.36 0.30 | 0.330 0.36 | 0.175 0.15 | 0.18 0.19 | 0.055 0.145 | 0.07 0.125 | 0.07 0.30 | 0.145 0.175 | ||||||||
| 120 | 0.325 0.295 | 0.355 0.35 | 0.18 0.15 | 0.19 0.19 | 0.075 0.145 | 0.075 0.125 | 0.075 0.32 | 0.085 0.10 | ||||||||
| Treatment Time (min) | Bands Position (cm−1) and Their Ratio Intensities [a.u.] a | ||||||
|---|---|---|---|---|---|---|---|
| A(3480) | B(3260) | C(1635) | A/(A+B) | A/B | A/C | B/C | |
| Deionized water (D) | |||||||
| D0 | 0.0923 | 0.3831 | 0.2040 | 0.1942 | 0.2409 | 0.4525 | 1.8779 |
| 0.1491 | 0.2371 | 0.1604 | 0.3861 | 0.6288 | 0.9296 | 1.4782 | |
| D5 | 0.0637 | 0.3522 | 0.1884 | 0.1532 | 0.1809 | 0.3381 | 1.8694 |
| 0.1446 | 0.2562 | 0.1627 | 0.3608 | 0.5644 | 0.8888 | 1.5747 | |
| D15 | 0.0690 | 0.3610 | 0.1920 | 0.1605 | 0.1911 | 0.3594 | 1.8802 |
| 0.1010 | 0.2683 | 0.1578 | 0.2735 | 0.3764 | 0.6401 | 1.7003 | |
| D30 | 0.0586 | 0.3497 | 0.1878 | 0.1435 | 0.1676 | 0.3120 | 1.8621 |
| 0.0961 | 0.2521 | 0.1511 | 0.2760 | 0.3812 | 0.6360 | 1.6684 | |
| D60 | 0.0562 | 0.3306 | 0.1785 | 0.1453 | 0.1700 | 0.3148 | 1.8521 |
| 0.0572 | 0.2273 | 0.1290 | 0.2011 | 0.2516 | 0.4434 | 1.7620 | |
| D90 | 0.0669 | 0.3661 | 0.1979 | 0.1545 | 0.1827 | 0.3396 | 1.8584 |
| 0.0680 | 0.2817 | 0.1588 | 0.1945 | 0.2414 | 9.4782 | 1.7739 | |
| D120 | 0.0557 | 0.3268 | 0.1825 | 0.1456 | 0.1704 | 0.3052 | 1.7907 |
| 0.0687 | 0.2772 | 0.1576 | 0.1986 | 0.2478 | 0.4359 | 1.7589 | |
| Tap water (T) | |||||||
| T0 | 0.0658 | 0.3666 | 0.1990 | 0.1522 | 0.1795 | 0.3307 | 1.8422 |
| 0.1114 | 0.3044 | 0.1756 | 0.2679 | 0.3660 | 0.6344 | 1.7335 | |
| T5 | 0.0517 | 0.3221 | 0.1822 | 0.1383 | 0.1605 | 0.2838 | 1.7678 |
| 0.1246 | 0.3066 | 0.1849 | 0.2890 | 0.4964 | 0.6739 | 1.6582 | |
| T15 | 0.0528 | 0.3145 | 0.1733 | 0.1438 | 0.1679 | 0.3047 | 1.8148 |
| 0.1369 | 0.3032 | 0.1898 | 0.3111 | 0.4515 | 0.7213 | 1.5975 | |
| T30 | 0.0530 | 0.3242 | 0.1801 | 0.1405 | 0.1635 | 0.2943 | 1.8001 |
| 0.1256 | 0.3167 | 0.1886 | 0.2840 | 0.3966 | 0.6660 | 1.6792 | |
| T60 | 0.0723 | 0.3690 | 0.2015 | 0.1638 | 0.1959 | 0.3588 | 1.8313 |
| 0.1509 | 0.3364 | 0.1952 | 0.3097 | 0.4486 | 0.7731 | 1.7234 | |
| T90 | 0.0547 | 0.3380 | 0.1828 | 0.1393 | 0.1618 | 0.2992 | 1.8490 |
| 0.1293 | 0.3228 | 0.1949 | 0.2860 | 0.4006 | 0.6634 | 1.6562 | |
| T120 | 0.0550 | 0.3467 | 0.1917 | 0.1369 | 0.1586 | 0.2869 | 1.8086 |
| 0.0897 | 0.3258 | 0.1832 | 0.2159 | 0.2753 | 0.4896 | 1.7784 | |
| Sample and Treatment time (min) | Onset Temperature (°C) | Peak Maximum (°C) | Peak Abruption b (PA) (1/°C) | Maximum of Heat Flow c (MHF) (W/g) |
|---|---|---|---|---|
| Deionized water (D) | ||||
| Dd | 100.7 | 128.3 | 0.0362 | −20.2 |
| D0 | 100.5 | 128.4 | 0.0358 | −19.2 |
| 101.4 | 128.9 | 0.0364 | −20.1 | |
| D5 | 100.4 | 127.4 | 0.0370 | −20.3 |
| 101.1 | 128.6 | 0.0364 | −21.0 | |
| D15 | 100.1 | 127.1 | 0.0370 | −20.4 |
| 101.0 | 127.8 | 0.0373 | −20.6 | |
| D30 | 100.5 | 127.3 | 0.0373 | −20.4 |
| 100.9 | 128.0 | 0.0369 | −21.5 | |
| D60 | 100.5 | 127.5 | 0.0370 | −20.2 |
| 101.1 | 128.8 | 0.0361 | −20.5 | |
| D90 | 100.7 | 127.5 | 0.0373 | −20.9 |
| 100.7 | 128.3 | 0.0362 | −21.1 | |
| D120 | 100.1 | 127.8 | 0.0361 | −20.6 |
| 100.8 | 128.0 | 0.0368 | −21.4 | |
| Tap water (T) | ||||
| Td | 101.6 | 130.5 | 0.0346 | −19.4 |
| T0 | 100.3 | 126.4 | 0.0383 | −22.2 |
| 100.8 | 127.8 | 0.0370 | −21.4 | |
| T5 | 100.3 | 130.0 | 0.0337 | −19.4 |
| 100.7 | 127.8 | 0.0369 | −21.2 | |
| T15 | 100.8 | 126.9 | 0.0383 | −21.9 |
| 100.8 | 127.6 | 0.0373 | −21.9 | |
| T30 | 100.5 | 127.8 | 0.0366 | −20.5 |
| 100.8 | 128.2 | 0.0365 | −21.1 | |
| T60 | 100.6 | 128.5 | 0.0358 | −20.1 |
| 101.0 | 128.5 | 0.0364 | −21.3 | |
| T90 | 100.5 | 128.3 | 0.0360 | −19.4 |
| 101.1 | 128.0 | 0.0372 | −21.6 | |
| T120 | 100.9 | 129.3 | 0.0352 | −18.6 |
| 101.2 | 128.0 | 0.0373 | −22.1 | |
| Sample and Time of Treatment (min) | Cp b | ΔH c | ΔS d | |||
|---|---|---|---|---|---|---|
| J/gK | J/molK | J/g | J/mol | J/g K | J/mol K | |
| Deionized water (D) | ||||||
| D e | 0.363 | 0.020 | −1645.3 | −91.4056 | 4.09839 | 0.227689 |
| D0 | 11.521 | 0.640 | −1365.2 | −75.8444 | 3.39983 | 0.188879 |
| 9.568 | 0.532 | −1657.2 | −92.0667 | 4.12188 | 0.228993 | |
| D5 | 12.568 | 0.698 | −1362.5 | −75.6944 | 3.40157 | 0.188976 |
| 9.243 | 0.514 | −1656.3 | −92.0167 | 4.12271 | 0.22904 | |
| D15 | 21.543 | 1.197 | −1351.2 | −75.0667 | 3.37589 | 0.187549 |
| 10.024 | 0.557 | −1651.6 | −91.7556 | 4.11922 | 0.228845 | |
| D30 | 22.359 | 1.242 | −1349.8 | −74.9889 | 3.37071 | 0.187262 |
| 13.254 | 0.736 | −1648.5 | −91.5833 | 4.10944 | 0.228302 | |
| D60 | 20.158 | 1.120 | −1359.8 | −75.5444 | 3.39398 | 0.188555 |
| 11.478 | 0.638 | −1654.8 | −91.9333 | 4.11693 | 0.228718 | |
| D90 | 19.523 | 1.085 | −1364.5 | −75.8056 | 3.40572 | 0.189206 |
| 10.658 | 0.592 | −1655.4 | −91.9667 | 4.12355 | 0.229086 | |
| D120 | 18.924 | 1.051 | −1365.8 | −75.8778 | 3.40641 | 0.189245 |
| 10.025 | 0.557 | −1655.9 | −91.9944 | 4.12788 | 0.229327 | |
| Tap water (T) | ||||||
| T | 1.523 | 0.085 | −2014.5 | −111.917 | 4.99071 | 0.277262 |
| T0 | 10.236 | 0.569 | −2058.5 | −114.361 | 5.15205 | 0.286225 |
| 9.254 | 0.514 | −2016.8 | −112.044 | 5.03005 | 0.279447 | |
| T5 | 11.035 | 0.613 | −2057.5 | −114.306 | 5.10356 | 0.283531 |
| 10.235 | 0.569 | −2018.6 | −112.144 | 5.03454 | 0.279697 | |
| T15 | 19.536 | 1.085 | −2059.4 | −114.411 | 5.14786 | 0.285992 |
| 13.054 | 0.725 | −2013.2 | −111.844 | 5.02358 | 0.279088 | |
| T30 | 19.962 | 1.109 | −2053.5 | −114.083 | 5.12159 | 0.284533 |
| 11.235 | 0.624 | −2016.4 | −112.022 | 5.02404 | 0.279114 | |
| T60 | 17.562 | 0.976 | −2049.7 | −113.872 | 5.10320 | 0.283511 |
| 10.296 | 0.572 | −2018.4 | −112.133 | 5.02527 | 0.279182 | |
| T90 | 16.235 | 0.902 | −2056.2 | −114.233 | 5.12193 | 0.284552 |
| 9.236 | 0.513 | −2019.3 | −112.183 | 5.03378 | 0.279654 | |
| T120 | 16.014 | 0.890 | −2057.2 | −114.289 | 5.11169 | 0.283983 |
| 9.685 | 0.538 | −2019.8 | −112.211 | 5.03502 | 0.279724 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chwastowski, J.; Ciesielski, W.; Khachatryan, K.; Kołoczek, H.; Kulawik, D.; Oszczęda, Z.; Soroka, J.A.; Tomasik, P.; Witczak, M. Water of Increased Content of Molecular Oxygen. Water 2020, 12, 2488. https://doi.org/10.3390/w12092488
Chwastowski J, Ciesielski W, Khachatryan K, Kołoczek H, Kulawik D, Oszczęda Z, Soroka JA, Tomasik P, Witczak M. Water of Increased Content of Molecular Oxygen. Water. 2020; 12(9):2488. https://doi.org/10.3390/w12092488
Chicago/Turabian StyleChwastowski, Jarosław, Wojciech Ciesielski, Karen Khachatryan, Henryk Kołoczek, Damian Kulawik, Zdzisław Oszczęda, Jacek A. Soroka, Piotr Tomasik, and Mariusz Witczak. 2020. "Water of Increased Content of Molecular Oxygen" Water 12, no. 9: 2488. https://doi.org/10.3390/w12092488
APA StyleChwastowski, J., Ciesielski, W., Khachatryan, K., Kołoczek, H., Kulawik, D., Oszczęda, Z., Soroka, J. A., Tomasik, P., & Witczak, M. (2020). Water of Increased Content of Molecular Oxygen. Water, 12(9), 2488. https://doi.org/10.3390/w12092488








