High Loaded Bioflocculation Membrane Reactor of Novel Structure for Organic Matter Recovery from Sewage: Effect of Temperature on Bioflocculation and Membrane Fouling
Abstract
:1. Introduction
2. Materials and Methods
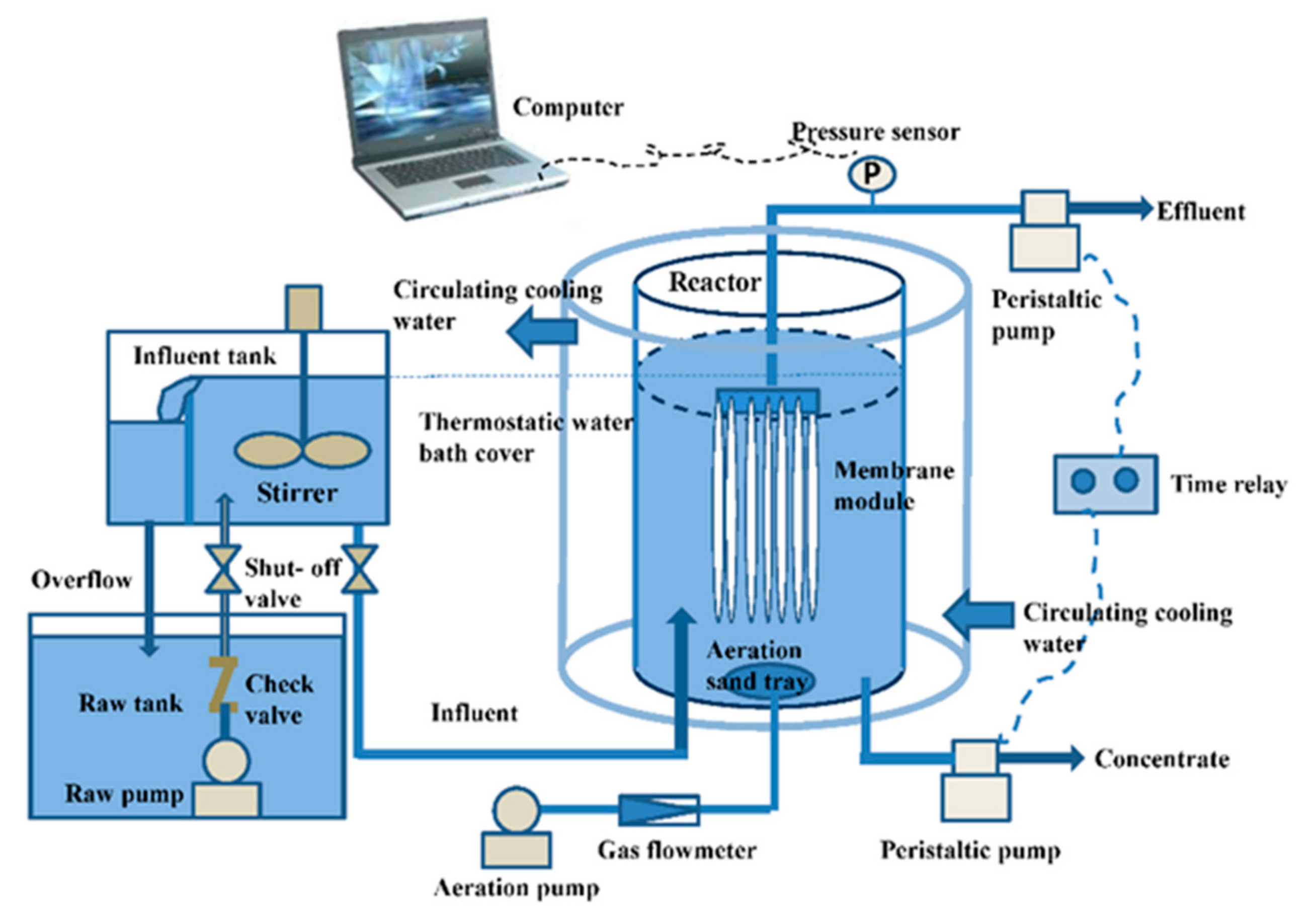
2.1. Experimental Apparatus and Operation
2.2. Samples and Analysis
3. Results
3.1. Recovery Efficiency of Organic Matter
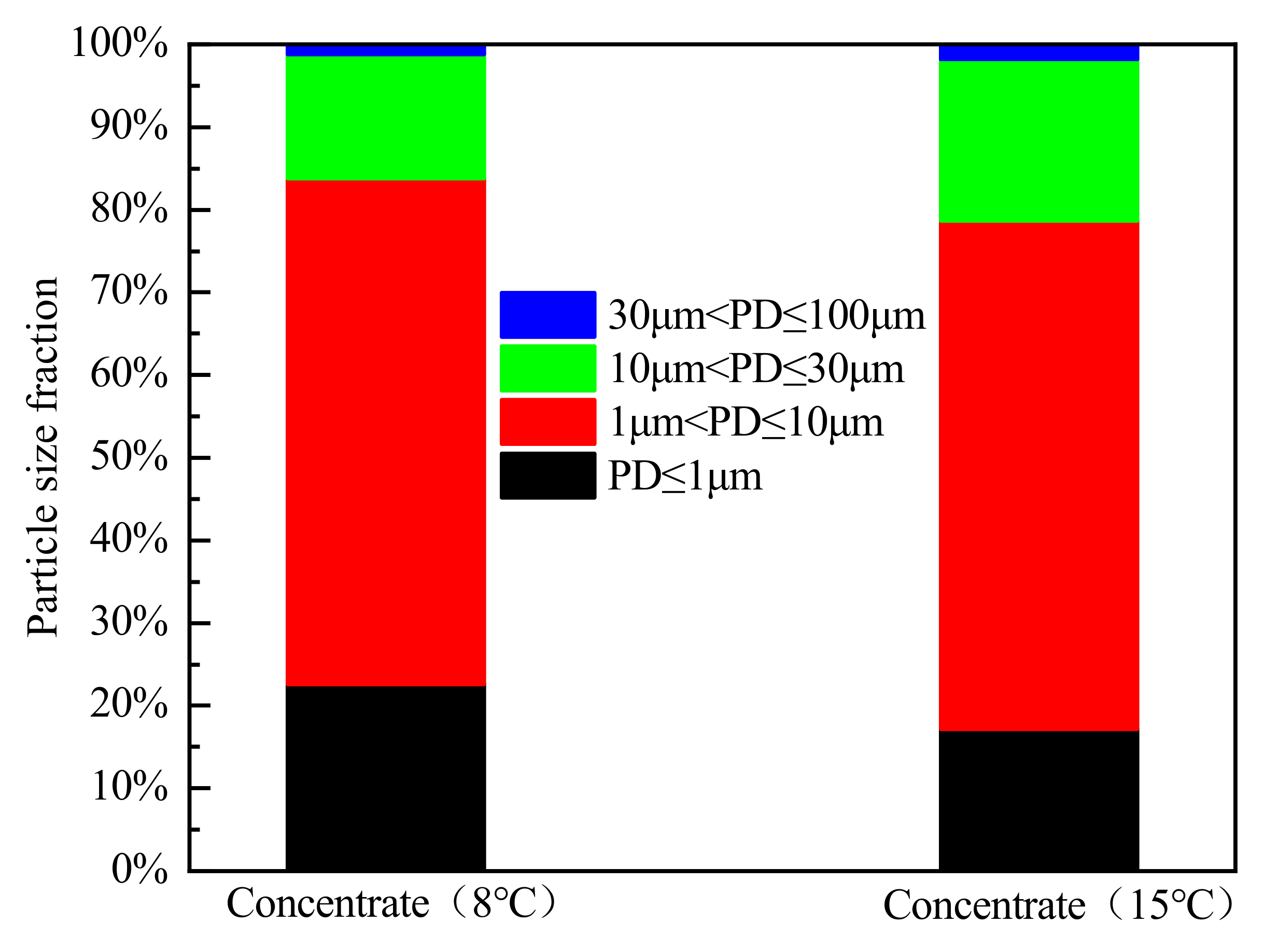
3.2. Bioflocculation Effect
3.3. EPS and Metal Cation Concentration
3.4. Trans-Membrane Pressure (TMP)
4. Discussion
4.1. The Effect of EPS and Metal Cations on Bioflocculation
4.2. The Influence of Temperature on Bioflocculation
4.3. Influence of Temperature on Membrane Fouling
4.4. Practical Significance
5. Conclusions
- The HLB-MR can achieve a highly efficient recovery of organic matter in municipal wastewater at a low temperature of 8 °C. If the loss of organic matter due to the membrane cleaning process is ignored, the recovery rate could reach more than 80% at 8 °C and 15 °C.
- The bioflocculation efficiency of the HLB-MR under a low temperature of 8 °C is 65%, which is significantly lower than the flocculation efficiency of 85% at 15 °C. A large number of fine particles and high turbidity in the supernatant of the concentrated solution in the 8 °C reactor further confirms the inhibitory effect of low temperature on bioflocculation. Under a low temperature of 8 °C, the EPS concentration and the cations (sodium, calcium, and aluminum) are lower than that at 15 °C. With the increase of the EPS concentration secreted by microorganisms in the HLB-MR, the bioflocculation is enhanced, so that the uptake of the cations (sodium, calcium, and aluminum) from the sludge matrix increases.
- Compared with 15 °C, the poor bioflocculation in HLB-MR at 8 °C leads to more serious membrane fouling. It is recommended to appropriately increase SRT, increase aeration intensity, add powdered activated carbon, or enhance the backwashing intensity during the cold season to overcome the problem of serious membrane fouling during the operation of the HLB-MR.
Author Contributions
Funding
Conflicts of Interest
References
- McCarty, P.L.; Bae, J.; Kim, J. Domestic Wastewater Treatment as a Net Energy Producer—Can This be Achieved? Environ. Sci. Technol. 2011, 45, 7100–7106. [Google Scholar] [CrossRef]
- Togna, A.P.; Schraa, O.J.; Melcer, H.; Sutton, P.M. Treating municipal wastewater with the goal of resource recovery. Water Sci. Technol. 2011, 63, 25–31. [Google Scholar]
- Freguia, S.; Teh, E.H.; Boon, N.; Leung, K.M.; Keller, J.; Rabaey, K. Microbial fuel cells operating on mixed fatty acids. Bioresour. Technol. 2010, 101, 1233–1238. [Google Scholar] [CrossRef]
- Lee, W.S.; Chua, A.S.M.; Yeoh, H.K.; Ngoh, G.C. A review of the production and applications of waste-derived volatile fatty acids. Chem. Eng. J. 2014, 235, 83–99. [Google Scholar] [CrossRef]
- Mezohegyi, G.; Bilad, M.R.; Vankelecom, I.F.J. Direct sewage up-concentration by submerged aerated and vibrated membranes. Bioresour. Technol. 2012, 118, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, H.; Okimoto, K.; Kimura, K.; Watanabe, Y. Hydrophilic fraction of natural organic matter causing irreversible fouling of microfiltration and ultrafiltration membranes. Water Res. 2014, 54, 123–136. [Google Scholar] [CrossRef]
- Huang, H.; Lee, N.; Young, T.; Gary, A.; Lozier, J.; Jacangelo, J. Natural organic matter fouling of low-pressure, hollow-fiber membranes: Effects of NOM source and hydrodynamic conditions. Water Res. 2007, 41, 3823–3832. [Google Scholar] [CrossRef] [PubMed]
- Carroll, T.; King, S.; Gray, S.; Bolto, B.A.; Booker, N. The fouling of microfiltration membranes by NOM after coagulation treatment. Water Res. 2000, 34, 2861–2868. [Google Scholar] [CrossRef]
- Howe, K.J.; Marwah, A.; Chiu, K.-P.; Adham, S.S. Effect of coagulation on the size of MF and UF membrane foulants. Environ. Sci. Technol. 2006, 40, 7908–7913. [Google Scholar] [CrossRef]
- Huang, H.; Schwab, K.; Jacangelo, J.G. Pretreatment for low pressure membranes in water treatment: A review. Environ. Sci. Technol. 2009, 43, 3011–3019. [Google Scholar] [CrossRef]
- Wan, C.; Alam, M.A.; Zhao, X.-Q.; Zhang, X.-Y.; Guo, S.-L.; Ho, S.-H.; Chang, J.-S.; Bai, F.-W. Current progress and future prospect of microalgal biomass harvest using various flocculation technologies. Bioresour. Technol. 2015, 184, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Sarioglu, M.; Sayi-Ucar, N.; Cokgor, E.; Orhon, D.; van Loosdrecht, M.C.M.; Insel, G. Dynamic modeling of nutrient removal by a MBR operated at elevated temperatures. Water Res. 2017, 123, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Yu, S.; Shi, W.; Heijman, S.G.J.; Rietveld, L.C. Effect of different temperatures on performance and membrane fouling in high concentration PAC-MBR system treating micro-polluted surface water. Bioresour. Technol. 2013, 141, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Faust, L.; Temmink, H.; Zwijnenburg, A.; Kemperman, A.J.B.; Rijnaarts, H.H.M. Effect of dissolved oxygen concentration on the bioflocculation process in high loaded MBRs. Water Res. 2014, 66, 199–207. [Google Scholar] [CrossRef]
- Faust, L.; Temmink, H.; Zwijnenburg, A.; Kemperman, A.J.B.; Rijnaarts, H.H.M. High loaded MBRs for organic matter recovery from sewage: Effect of solids retention time on bioflocculation and on the role of extracellular polymers. Water Res. 2014, 56, 258–266. [Google Scholar] [CrossRef]
- Water Environment Federation, American Public Health Association. Standard Methods for the Examination of Water and Wastewater; American Public Health Association (APHA): Washington, DC, USA, 2005. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, L.A.; Randall, R.J. Protein measurement whit the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Salehizadeh, H. Extracellular biopolymeric flocculants: Recent trends and biotechnological importance. Biotechnol. Adv. 2001, 19, 371–385. [Google Scholar] [CrossRef]
- Dignac, M.-F.; Urbain, V.; Rybacki, D.; Bruchet, A.; Snidaro, D.; Scribe, P. Chemical description of extracellular polymers: Implication on activated sludge floc structure. Water Sci. Technol. 1998, 38, 45–53. [Google Scholar] [CrossRef]
- Bura, R.; Cheung, M.; Liao, B.; Finlayson, J.; Lee, B.; Droppo, I.; Leppard, G.; Liss, S. Composition of extracellular polymeric substances in the activated sludge floc matrix. Water Sci. Technol. 1998, 37, 325–333. [Google Scholar] [CrossRef]
- Urbain, V.; Block, J.; Manem, J. Bioflocculation in activated sludge: An analytic approach. Water Res. 1993, 27, 829–838. [Google Scholar] [CrossRef]
- Wilén, B.-M.; Jin, B.; Lant, P. The influence of key chemical constituents in activated sludge on surface and flocculating properties. Water Res. 2003, 37, 2127–2139. [Google Scholar] [CrossRef]
- Bala Subramanian, S.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Extracellular polymeric substances (EPS) producing bacterial strains of municipal wastewater sludge: Isolation, molecular identification, EPS characterization and performance for sludge settling and dewatering. Water Res. 2010, 44, 2253–2266. [Google Scholar] [CrossRef] [PubMed]
- Rijnaarts, H.H.; Norde, W.; Lyklema, J.; Zehnder, A.J. DLVO and steric contributions to bacterial deposition in media of different ionic strengths. Colloids Surf. B Biointerfaces 1999, 14, 179–195. [Google Scholar] [CrossRef]
- Liu, X.-M.; Sheng, G.-P.; Yu, H.-Q. DLVO approach to the flocculability of a photosynthetic H2-producing bacterium, Rhodopseudomonas acidophila. Environ. Sci. Technol. 2007, 41, 4620–4625. [Google Scholar] [CrossRef]
- Sobeck, D.C.; Higgins, M.J. Examination of three theories for mechanisms of cation-induced bioflocculation. Water Res. 2002, 36, 527–538. [Google Scholar] [CrossRef]
- De Temmerman, L.; Maere, T.; Temmink, H.; Zwijnenburg, A.; Nopens, I. Salt stress in a membrane bioreactor: Dynamics of sludge properties, membrane fouling and remediation through powdered activated carbon dosing. Water Res. 2014, 63, 112–124. [Google Scholar] [CrossRef]
- Kara, F.; Gurakan, G.C.; Sanin, F.D. Monovalent cations and their influence on activated sludge floc chemistry, structure, and physical characteristics. Biotechnol. Bioeng. 2008, 100, 231–239. [Google Scholar] [CrossRef]
- Salta, M.; Wharton, J.A.; Blache, Y.; Stokes, K.R.; Briand, J.F. Marine biofilms on artificial surfaces: Structure and dynamics. Environ. Microbiol. 2013, 15, 2879–2893. [Google Scholar] [CrossRef]
- Li, H.; Wen, Y.; Cao, A.; Huang, J.; Zhou, Q.; Somasundaran, P. The influence of additives (Ca2+, Al3+, and Fe3+) on the interaction energy and loosely bound extracellular polymeric substances (EPS) of activated sludge and their flocculation mechanisms. Bioresour. Technol. 2012, 114, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Zheng, W.; Yang, Y.; Cao, A.; Zhou, Q. Influence of Al3+ addition on the flocculation and sedimentation of activated sludge: Comparison of single and multiple dosing patterns. Water Res. 2015, 75, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Bruus, J.H.; Nielsen, P.H.; Keiding, K. On the stability of activated sludge flocs with implications to dewatering. Water Res. 1992, 26, 1597–1604. [Google Scholar] [CrossRef]
- van den Brink, P.; Satpradit, O.-A.; van Bentem, A.; Zwijnenburg, A.; Temmink, H.; van Loosdrecht, M. Effect of temperature shocks on membrane fouling in membrane bioreactors. Water Res. 2011, 45, 4491–4500. [Google Scholar] [CrossRef] [PubMed]
- Wilén, B.-M.; Keiding, K.; Nielsen, P.H. Anaerobic deflocculation and aerobic reflocculation of activated sludge. Water Res. 2000, 34, 3933–3942. [Google Scholar] [CrossRef]
- Xiao, F.; Ma, J.; Yi, P.; Huang, J.C. Effects of low temperature on coagulation of kaolinite suspensions. Water Res. 2008, 42, 2983–2992. [Google Scholar] [CrossRef]
- Diamantis, V.; Eftaxias, A.; Bundervoet, B.; Verstraete, W. Performance of the biosorptive activated sludge (BAS) as pre-treatment to UF for decentralized wastewater reuse. Bioresour. Technol. 2014, 156, 314–321. [Google Scholar] [CrossRef]
- Hong, E.; Yeneneh, A.M.; Kayaalp, A.; Sen, T.K.; Ang, H.M.; Kayaalp, M. Rheological characteristics of municipal thickened excess activated sludge (TEAS): Impacts of pH, temperature, solid concentration and polymer dose. Res. Chem. Intermed. 2016, 42, 6567–6585. [Google Scholar] [CrossRef] [Green Version]
- Belfort, G.; Davis, R.H.; Zydney, A.L. The behavior of suspensions and macromolecular solutions in crossflow microfiltration. J. Membr. Sci. 1994, 96, 1–58. [Google Scholar] [CrossRef]
- Schippers, J.C.; Vanrolleghem, P.A.; Guinzbourg, B.F.; Kennedy, M.D.; Jiang, T. Optimising the operation of a MBR pilot plant by quantitative analysis of the membrane fouling mechanism. Water Sci. Technol. 2005, 51, 19–25. [Google Scholar]
- Remy, M.; Potier, V.; Temmink, H.; Rulkens, W. Why low powdered activated carbon addition reduces membrane fouling in MBRs. Water Res. 2010, 44, 861–867. [Google Scholar] [CrossRef]
- Remy, M.; van der Marel, P.; Zwijnenburg, A.; Rulkens, W.; Temmink, H. Low dose powdered activated carbon addition at high sludge retention times to reduce fouling in membrane bioreactors. Water Res. 2009, 43, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Teodosiu, C.C.; Kennedy, M.D.; Van Straten, H.A.; Schippers, J.C. Evaluation of secondary refinery effluent treatment using ultrafiltration membranes. Water Res. 1999, 33, 2172–2180. [Google Scholar] [CrossRef]
- Le-Clech, P.; Chen, V.; Fane, T.A.G. Fouling in membrane bioreactors used in wastewater treatment. J. Membr. Sci. 2006, 284, 17–53. [Google Scholar] [CrossRef]




| Influent | CODss | CODCO | CODSO | CODTO | |
|---|---|---|---|---|---|
| 180 | 31 | 36 | 247 ± 21 | ||
| LT(8 °C) | concentrate | 2223 ± 227 | 155 ± 12 | 96 ± 8 | 2474 ± 316 |
| effluent | 35 ± 5 | ||||
| HT(15 °C) | concentrate | 2413 ± 235 | 67 ± 6 | 72 ± 8 | 2552 ± 306 |
| effluent | 26 ± 3 | ||||
| Supernatant Concentration (mg/L) | Sediments Concentration (mg/gTSS) | |||||
|---|---|---|---|---|---|---|
| Na | Ca | Al | Na | Ca | Al | |
| LT(8 °C) | 43.85 | 9.34 | 29.24 | 3.41 | 15.63 | 13.25 |
| HT(15 °C) | 40.04 | 8.23 | 36.44 | 3.71 | 18.20 | 16.06 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, L.; Xiong, L.; Zhang, L.; Lu, W. High Loaded Bioflocculation Membrane Reactor of Novel Structure for Organic Matter Recovery from Sewage: Effect of Temperature on Bioflocculation and Membrane Fouling. Water 2020, 12, 2497. https://doi.org/10.3390/w12092497
Wan L, Xiong L, Zhang L, Lu W. High Loaded Bioflocculation Membrane Reactor of Novel Structure for Organic Matter Recovery from Sewage: Effect of Temperature on Bioflocculation and Membrane Fouling. Water. 2020; 12(9):2497. https://doi.org/10.3390/w12092497
Chicago/Turabian StyleWan, Liguo, Ling Xiong, Lijun Zhang, and Wenxi Lu. 2020. "High Loaded Bioflocculation Membrane Reactor of Novel Structure for Organic Matter Recovery from Sewage: Effect of Temperature on Bioflocculation and Membrane Fouling" Water 12, no. 9: 2497. https://doi.org/10.3390/w12092497




