Microbial Carbon Metabolic Functions in Sediments Influenced by Resuspension Event
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Experiments
2.2. Determination of Water Characteristics
2.3. BIOLOG ECO Microplate Test Method
2.4. Determination of Average Well-Color Development Values
2.5. Calculation of Metabolic Functional Diversity Indices
2.6. Statistical Analysis
3. Results and Discussion
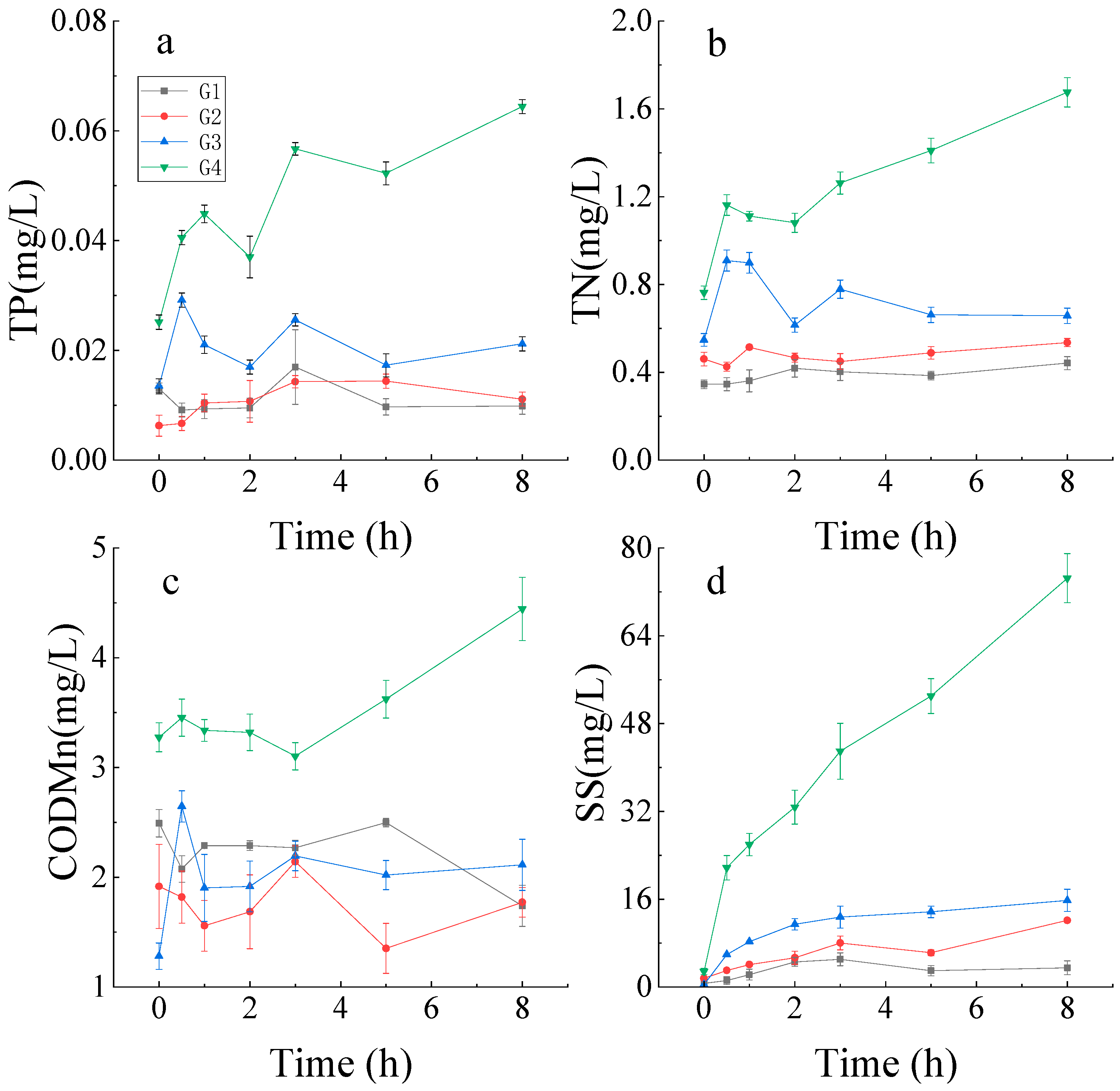
3.1. Resuspension Events Test under Four Working Conditions
3.2. The AWCD of All Carbon Sources in Sediment Microbial Communities within Incubation Time
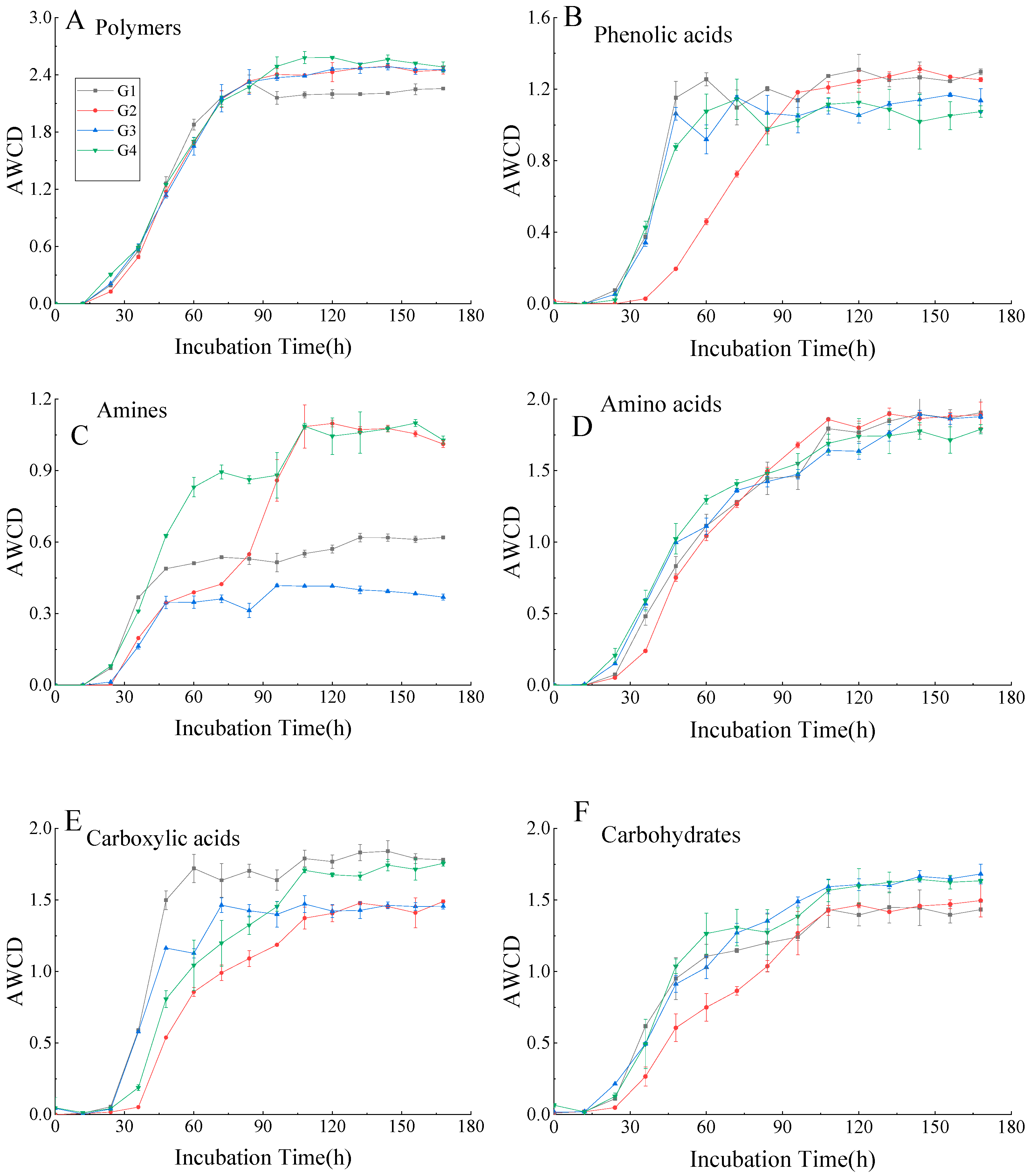
3.3. AWCD of Different Biochemical Categories of Carbon Sources
3.4. Microbial Metabolic Functional Diversity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nilsson, P.; Jansson, M. Hydrodynamic control of nitrogen and phosphorus turnover in an eutrophicated estuary in the Baltic. Water Res. 2002, 36, 4616–4626. [Google Scholar] [CrossRef]
- Green, M.O. Very small waves and associated sediment resuspension on an estuarine intertidal flat. Estuar. Coast. Shelf Sci. 2011, 93, 449–459. [Google Scholar] [CrossRef]
- You, B.-S.; Zhong, J.-C.; Fan, C.-X.; Wang, T.-C.; Zhang, L.; Ding, S.-M. Effects of hydrodynamics processes on phosphorus fluxes from sediment in large, shallow Taihu Lake. J. Environ. Sci. 2007, 19, 1055–1060. [Google Scholar] [CrossRef]
- Zhu, G.; Qin, B.; Gao, G.; Zhang, L.; Luo, L.; Zhang, Y. Effects of hydrodynamics on phosphorus concentrations in water of Lake Taihu, a large, shallow, eutrophic lake of China. Hydrobiologia 2007, 581, 53–61. [Google Scholar] [CrossRef]
- Kularatne, S.; Pattiaratchi, C. Turbulent kinetic energy and sediment resuspension due to wave groups. Cont. Shelf Res. 2008, 28, 726–736. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Leonardi, N.; Plater, A.J. Wave-driven sediment resuspension and salt marsh frontal erosion alter the export of sediments from macro-tidal estuaries. Geomorphology 2019, 325, 17–28. [Google Scholar] [CrossRef]
- Cheng, P.D.; Zhu, H.W.; Zhong, B.C.; Wang, D.Z. Sediment rarefaction resuspension and contaminant release under tidal currents. J. Hydrodyn. 2014, 26, 827–834. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, C.; Zheng, J.; Guo, L.; Yin, P.; Jia, Y. The observations of seabed sediment erosion and resuspension processes in the Jiaozhou Bay in China. Acta Oceanol. Sin. 2017, 36, 79–85. [Google Scholar] [CrossRef]
- Miles, T.; Glenn, S.M.; Schofield, O. Temporal and spatial variability in fall storm induced sediment resuspension on the Mid-Atlantic Bight. Cont. Shelf Res. 2013, 63, S36–S49. [Google Scholar] [CrossRef]
- Tiano, J.C.; Rob, W.; Bergman, M.J.N.; Pieter, V.R.; Anton, T.; Dick, V.O.; Karline, S. Acute impacts of bottom trawl gears on benthic metabolism and nutrient cycling. ICES J. Mar. Sci. 2019, 6, 1917–1930. [Google Scholar] [CrossRef]
- Van Denderen, P.D.; Bolam, S.G.; Hiddink, J.G.; Jennings, S.; Kenny, A.; Rijnsdorp, A.D.; Van Kooten, T. Similar effects of bottom trawling and natural disturbance on composition and function of benthic communities across habitats. Mar. Ecol. Prog. 2016, 541, 31–43. [Google Scholar] [CrossRef] [Green Version]
- De Juan, S.; Demestre, M. A Trawl Disturbance Indicator to quantify large scale fishing impact on benthic ecosystems. Ecol. Indic. 2012, 18, 183–190. [Google Scholar] [CrossRef]
- Geng, D.; Yang, F.; Wei, C.; Ji, H. Effects of wind-wave disturbance on the partition of arsenic between the water-suspended solids phase of Lake Taihu. Acta Entiae Circumstantiae 2015, 35, 1358–1365. [Google Scholar]
- Li, Y.; Tang, C.; Wang, J.; Acharya, K.; Du, W.; Gao, X.; Luo, L.; Li, H.; Dai, S.; Mercy, J.; et al. Effect of wave-current interactions on sediment resuspension in large shallow Lake Taihu, China. Environ. Sci. Pollut. Res. 2016, 24, 4029–4039. [Google Scholar] [CrossRef]
- Qin, B.; Zhu, G.; Zhang, L.; Luo, L.; Gao, G.; Gu, B. Estimation of internal nutrient release in large shallow Lake Taihu, China. Sci. China Ser. D 2006, 49, 38–50. [Google Scholar] [CrossRef]
- Li, D.P.; Huang, Y.; Li, Y.; Pan, Y. Impacts of sediment disturbance time on the distribution of phosphorus forms in suspended solids. Huan Jing Ke Xue = Huanjing Kexue 2012, 33, 379–384. [Google Scholar] [PubMed]
- Tang, C.; Li, Y.; He, C.; Acharya, K. Dynamic behavior of sediment resuspension and nutrients release in the shallow and wind-exposed Meiliang Bay of Lake Taihu. Sci. Total Environ. 2020, 708, 135131. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Xing, P.; Jiang, W.W.; Liu, Z.W. Effects of Sediment Resuspension on Bacterioplankton Community Composition. Huan Jing Ke Xue = Huanjing Kexue 2010, 31, 1909–1917. [Google Scholar] [PubMed]
- Kang, Y.; Song, X.; Liu, Z. Sediment resuspension dampens the effect of nutrient inputs on the phytoplankton community: A mesocosm experiment study. Hydrobiologia 2012, 710, 117–127. [Google Scholar] [CrossRef]
- Libin, Z.; Feizhou, C. Effect of sediment resuspension on predation of planktivorous fish on zooplankton. J. Lake Sci. 2015, 27, 911–916. [Google Scholar] [CrossRef] [Green Version]
- Battin, T.J.; Kaplan, L.A.; Newbold, J.D.; Hansen, C.M.E. Contributions of microbial biofilms to ecosystem processes in stream mesocosms. Nature 2003, 426, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Battin, T.J.; Besemer, K.; Bengtsson, M.M.; Romani, A.M.; Packmann, A.I. The ecology and biogeochemistry of stream biofilms. Nat. Rev. Microbiol. 2016, 14, 251–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Q.; Cheng, X.; Hui, D.; Zhang, Q.; Li, M.; Zhang, Q. Soil microbial community and its interaction with soil carbon and nitrogen dynamics following afforestation in central China. Sci. Total Env. 2016, 541, 230–237. [Google Scholar] [CrossRef]
- Comer-Warner, S.A.; Gooddy, D.C.; Ullah, S.; Glover, L.; Kettridge, N.; Wexler, S.K.; Kaiser, J.; Krause, S. Seasonal variability of sediment controls of nitrogen cycling in an agricultural stream. Biogeochemistry 2020, 148, 31–48. [Google Scholar] [CrossRef] [Green Version]
- Seeley, M.E.; Song, B.; Passie, R.; Hale, R.C. Microplastics affect sedimentary microbial communities and nitrogen cycling. Nat. Commun. 2020, 11, 2372. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Bae, H.S.; Reddy, K.R.; Ogram, A. Distributions, abundances and activities of microbes associated with the nitrogen cycle in riparian and stream sediments of a river tributary. Water Res. 2016, 106, 51–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Li, Y.; Wang, P.; Niu, L.; Zhang, W.; Wang, C. Response of bacterial community compositions to different sources of pollutants in sediments of a tributary of Taihu Lake, China. Environ. Sci. Pollut. Res. Int. 2016, 23, 13886–13894. [Google Scholar] [CrossRef]
- Rieling, T. Benthic microbial decomposition of organic matter and nutrient fluxes at the sediment-water interface in a shallow coastal inlet of the southern Baltic Sea (Nordrügensche Bodden). In Proceedings of the Marine Science, Providence, RI, USA, 11–14 September 2000; pp. 175–184. [Google Scholar]
- Kraal, P.; Burton, E.D.; Rose, A.L.; Cheetham, M.D.; Bush, R.T.; Sullivan, L.A. Decoupling between water column oxygenation and benthic phosphate dynamics in a shallow eutrophic estuary. Environ. Sci. Technol. 2013, 47, 3114–3121. [Google Scholar] [CrossRef]
- Stoliker, D.L.; Repert, D.A.; Smith, R.L.; Song, B.; LeBlanc, D.R.; McCobb, T.D.; Conaway, C.H.; Hyun, S.P.; Koh, D.C.; Moon, H.S.; et al. Hydrologic Controls on Nitrogen Cycling Processes and Functional Gene Abundance in Sediments of a Groundwater Flow-Through Lake. Environ. Sci. Technol. 2016, 50, 3649–3657. [Google Scholar] [CrossRef]
- Oberbeckmann, S.; Kreikemeyer, B.; Labrenz, M. Environmental Factors Support the Formation of Specific Bacterial Assemblages on Microplastics. Front. Microbiol. 2018, 8, 2709. [Google Scholar] [CrossRef] [Green Version]
- Zhiwen, G.; Hengjun, D.; Yulong, G.; Weifen, Q. Analysis on Metabolic Functions of Stored Rice Microbial Communities by BIOLOG ECO Microplates. Front. Microbiol. 2018, 9, 1375. [Google Scholar]
- Marisol, G.U.; Helene, M.; Celine, L.; Claire, C.; Marc, B.; Asma, S.H.; Olivier, P. Consequences of contamination on the interactions between phytoplankton and bacterioplankton. Chemosphere 2018, 195, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Kleeberg, A.; Hupfer, M.; Gust, G.; Salka, I.; Pohlmann, K.; Grossart, H.-P. Intermittent riverine resuspension: Effects on phosphorus transformations and heterotrophic bacteria. Limnol. Oceanogr. 2013, 58, 635–652. [Google Scholar] [CrossRef]
- Chengxin, F.; Lu, Z. Lake Taihu: Principles of Sediment Pollution and Remediation; Science Press: Beijing, China, 2016. [Google Scholar]
- Liu, S.; Wang, C.; Hou, J.; Wang, P.; Miao, L.; Fan, X.; You, G.; Xu, Y. Effects of Ag and Ag2S nanoparticles on denitrification in sediments. Water Res. 2018, 137, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, C.; Hou, J.; Wang, P.; Miao, L. Effects of Ag NPs on denitrification in suspended sediments via inhibiting microbial electron behaviors. Water Res. 2020, 171, 115436. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Wang, P.; Wang, C.; Hou, J.; Qian, J. Distribution of metals in water and suspended particulate matter during the resuspension processes in Taihu Lake sediment, China. Quat. Int. 2013, 286, 94–102. [Google Scholar] [CrossRef]
- Huang, J.; Ge, X.; Yang, X.; Zheng, B.; Wang, D. Remobilization of heavy metals during the resuspension of Liangshui River sediments using an annular flume. Chin. Sci. Bull. 2012, 57, 3567–3572. [Google Scholar] [CrossRef] [Green Version]
- Cloutier, D.; LeCouturier, M.N.; Amos, C.L.; Hill, P.R. The effects of suspended sediment concentration on turbulence in an annular flume. Aquat. Ecol. 2006, 40, 555–565. [Google Scholar] [CrossRef]
- Qian, J.; Zheng, S.-S.; Wang, P.-F.; Wang, C. Experimental Study on Sediment Resuspension in Taihu Lake Under Different Hydrodynamic Disturbances. J. Hydrodyn. 2011, 23, 826–833. [Google Scholar] [CrossRef]
- Han, H.J.; Hu, W.P.; Jin, Y.Q. Numerical experiments on influence of wind speed on current in lake. Oceanol. Et Limnol. Sin. 2008, 39, 567–576. [Google Scholar]
- State Environmental Protection Administration of China. Methods of Monitoding and Analyzing for Water and Wastewater, 4th ed.; China Environmental Science Press: Beijing, China, 2002.
- Miki, T.; Yokokawa, T.; Ke, P.-J.; Hsieh, I.F.; Hsieh, C.-H.; Kume, T.; Yoneya, K.; Matsui, K. Statistical recipe for quantifying microbial functional diversity from EcoPlate metabolic profiling. Ecol. Res. 2017, 33, 249–260. [Google Scholar] [CrossRef]
- Jianhuang, L. Research on soil microbial characteristics of soil-Elsholtzia harchowensis system in copper mine tailings. Acta Pedol. Sin. 2004, 41, 120–125. [Google Scholar]
- Xu, W.H.; Ge, Z.W.; Poudel, D.R. Application and Optimization of Biolog EcoPlates in Functional Diversity Studies of Soil Microbial Communities. In Proceedings of the MATEC Web of Conferences, Antwerp, Belgium, 14–18 September 2015. [Google Scholar]
- Gryta, A.; Frąc, M.; Oszust, K. The Application of the Biolog EcoPlate Approach in Ecotoxicological Evaluation of Dairy Sewage Sludge. Appl. Biochem. Biotechnol. 2014, 174, 1434–1443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, Z.; Fengxing, X.; Fengfeng, Z.; Haibo, S.; Yujie, Z.; Qiong, Z. Effects of Preprocessing Methods of Sediment on Analysis Results of Biolog EcoPlates. Tianjin Agric. Ences 2019. [Google Scholar] [CrossRef]
- Lyons, M.M.; Dobbs, F.C. Differential utilization of carbon substrates by aggregate-associated and water-associated heterotrophic bacterial communities. Hydrobiologia 2012, 686, 181–193. [Google Scholar] [CrossRef]
- Hou, J.; Li, T.; Miao, L.; You, G.; Xu, Y.; Liu, S. Dynamic responses of community structure and microbial functions of periphytic biofilms during chronic exposure to TiO2 NPs. Environ. Sci. Nano 2020, 7, 665–675. [Google Scholar] [CrossRef]
- Miao, L.; Guo, S.; Liu, Z.; Liu, S.; You, G.; Qu, H.; Hou, J. Effects of Nanoplastics on Freshwater Biofilm Microbial Metabolic Functions as Determined by BIOLOG ECO Microplates. Int. J. Environ. Res. Public Health 2019, 16, 4639. [Google Scholar] [CrossRef] [Green Version]
- Lv, T.; Carvalho, P.N.; Zhang, L.; Zhang, Y.; Button, M.; Arias, C.A.; Weber, K.P.; Brix, H. Functionality of microbial communities in constructed wetlands used for pesticide remediation: Influence of system design and sampling strategy. Water Res. 2017, 110, 241–251. [Google Scholar] [CrossRef]
- Liao, K.; Bai, Y.; Huo, Y.; Jian, Z.; Hu, W.; Zhao, C.; Qu, J. Use of convertible flow cells to simulate the impacts of anthropogenic activities on river biofilm bacterial communities. Sci. Total Environ. 2019, 653, 148–156. [Google Scholar] [CrossRef]
- Shabbir, S.; Faheem, M.; Ali, N.; Kerr, P.G.; Wu, Y. Periphyton biofilms: A novel and natural biological system for the effective removal of sulphonated azo dye methyl orange by synergistic mechanism. Chemosphere 2017, 167, 236–246. [Google Scholar] [CrossRef]
- Zheng, S.-S.; Wang, P.-F.; Wang, C.; Hou, J. Sediment resuspension under action of wind in Taihu Lake, China. Int. J. Sediment Res. 2015, 30, 48–62. [Google Scholar] [CrossRef]
- Roberts, B.A.; Fritschi, F.B.; Horwath, W.R.; Bardhan, S. Nitrogen Mineralization Potential as Influenced by Microbial Biomass, Cotton Residues and Temperature. J. Plant Nutr. 2013, 38, 311–324. [Google Scholar] [CrossRef]
- Trevathan-Tackett, S.M.; Seymour, J.R.; Nielsen, D.A.; Macreadie, P.I.; Jeffries, T.C.; Jonathan, S.; Jeff, B.; Howes, J.M.; Steven, A.D.L.; Ralph, P.J. Sediment anoxia limits microbial-driven seagrass carbon remineralization under warming conditions. Fems Microbiol. Ecol. 2017, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grierson, P.F.; Comerford, N.B.; Jokela, E.J. Phosphorus mineralization and microbial biomass in a Florida Spodosol: Effects of water potential, temperature and fertilizer application. Biol. Fertil. Soils 1999, 28, 244–252. [Google Scholar] [CrossRef]
- Wang, Y.; Ouyang, Z.; Zheng, H.; Wang, X.; Chen, F.; Zeng, J. Carbon metabolism of soil microbial communities of restored forests in Southern China. J. Soils Sediments 2011, 11, 789–799. [Google Scholar] [CrossRef]
- Ling, Q.; Bao, J.; Li, R.; Tao, Y.; Mao, Q. Analysis of Carbon Metabolism Diversity Characters of Air Microbes in Huangshan Scenic Spot Using Biolog-Eco Method. J. Basic Sci. Eng. 2012, 20, 56–63. [Google Scholar]
- Li, F.; Liu, Z.; Jia, T.; Li, S.; Shang, Z. Functional diversity of soil microbial community carbon metabolism with the degradation and restoration of alpine wetlands and meadows. Acta Ecol. Sin. 2018, 38, 6006–6015. [Google Scholar]
- Kong, X.; Wang, C.; Ji, M. Analysis of microbial metabolic characteristics in mesophilic and thermophilic biofilters using Biolog plate technique. Chem. Eng. J. 2013, 230, 415–421. [Google Scholar] [CrossRef]
- Shao, J.; Huang, Y.; Dong, L.; Zhang, Y.; Tittel, F.K. Automated rapid blood culture sensor system based on diode laser wavelength-modulation spectroscopy for microbial growth analysis. Sens. Actuators B. Chem. 2018, 273, 656–663. [Google Scholar] [CrossRef]
- Tian-Yuan, Z.; Yin-Hu, W.; Lin-Lan, Z.; Xiao-Xiong, W.; Hong-Ying, H. Screening heterotrophic microalgal strains by using the Biolog method for biofuel production from organic wastewater. Algal Res. 2014, 6, 175–179. [Google Scholar] [CrossRef]
- Yan-yan, J.; Gui-Long, Z.; Rui, Z.; Yu-Sheng, L.; Dian-Lin, Y.; Cai-Ling, W. Effects of Different Tillage Modes on Metabolic Functional Diversity of Soil Microbial Community. Chin. Agric. Sci. Bull. 2013, 29, 117–123. [Google Scholar]
- Pusceddu, A.; Fiordelmondo, C.; Polymenakou, P.; Polychronaki, T.; Tselepides, A.; Danovaro, R. Effects of bottom trawling on the quantity and biochemical composition of organic matter in coastal marine sediments (Thermaikos Gulf, northwestern Aegean Sea). Cont. Shelf Res. 2005, 25, 2491–2505. [Google Scholar] [CrossRef]
- Su, D.; Zhang, K.; Chen, F.L.; Li, R.D.; Zheng, H. Effects of nitrogen application on carbon metabolism of soil microbial communities in eucalyptus plantations with different levels of soil organic carbon. Acta Ecol. Sin. 2015, 35, 5940–5947. [Google Scholar]


| Samples | K | P |
|---|---|---|
| G1 | 1.72 | 0.039 |
| G2 | 1.717 | 0.035 |
| G3 | 1.769 | 0.035 |
| G4 | 1.718 | 0.026 |
| Chemical Guild | Plate Number | Substrates | Chemical Formula |
|---|---|---|---|
| Polymers | C1 | Tween 40 | - |
| D1 | Tween 80 | - | |
| E1 | α-Cyclodextrin | C36H60O30 | |
| F1 | Glycogen | (C6H10O5)n | |
| Carbohydrates | G1 | d-Cellobiose | C12H12O11 |
| H1 | α-d-Lactose | C12H12O11 | |
| A2 | Methyl-d-glucoside | C7H14O6 | |
| B2 | d-Xylose | C5H10O5 | |
| C2 | i-Erythritol | C4H10O4 | |
| D2 | d-Mannitol | C6H14O6 | |
| E2 | N-Acetyl-d-glucosamine | C8H15NO6 | |
| H2 | d,l-α-Glycerol phosphate | C3H9O6P | |
| B1 | Glucose-1-phosphate | C6H13O9P | |
| G2 | Pyruvic acid methyl ester | C4H6O3 | |
| Carboxylic acids | F2 | d-Glucosaminic acid | C6H13NO6 |
| A3 | d-Galactonic acid latone | C6H10O6 | |
| B3 | d-Galacturonic acid | C6H10O7 | |
| E3 | γ-Hydroxy butyric acid | C4H8O3 | |
| F3 | Itaconic acid | C5H6O4 | |
| G3 | α-Keto butyric acid | C4H6O3 | |
| H3 | d-Malic acid | C4H6O5 | |
| Amino acids | A4 | l-Arginine | C4H14N4O2 |
| B4 | l-Asparagine | C4H8N2O3 | |
| C4 | l-Phenylalanine | C9H11NO2 | |
| D4 | l-Serine | C3H7NO3 | |
| E4 | l-Threonine | C4H9NO3 | |
| F4 | Glycyl-l-glutamic acid | C7H12N2O5 | |
| Amines/amides | G4 | Phenylethylamine | C8H11N |
| H4 | Putrescine | C4H12N2 | |
| Phenolic acids | C3 | 2-Hydroxy benzoic acid | C7H6O3 |
| D3 | 4-Hydroxy benzoic acid | C7H6O3 |
| Samples | Chemical Guild | K | P |
|---|---|---|---|
| G1 | Polymers | 2.228 | 0.118 |
| Phenolic acids | 1.245 | 0.266 | |
| Amines | 0.578 | 0.156 | |
| Amino acids | 1.875 | 0.057 | |
| Carboxylic acids | 1.753 | 0.205 | |
| Carbohydrates | 1.312 | 0.101 | |
| G2 | Polymers | 2.442 | 0.094 |
| Phenolic acids | 1.273 | 0.084 | |
| Amines | 1.107 | 0.053 | |
| Amino acids | 1.868 | 0.067 | |
| Carboxylic acids | 1.425 | 0.069 | |
| Carbohydrates | 1.583 | 0.049 | |
| G3 | Polymers | 2.453 | 0.086 |
| Phenolic acids | 1.093 | 0.320 | |
| Amines | 0.383 | 0.211 | |
| Amino acids | 1.793 | 0.523 | |
| Carboxylic acids | 1.426 | 0.143 | |
| Carbohydrates | 1.711 | 0.055 | |
| G4 | Polymers | 2.535 | 0.078 |
| Phenolic acids | 1.070 | 0.186 | |
| Amines | 1.022 | 0.091 | |
| Amino acids | 1.704 | 0.075 | |
| Carboxylic acids | 1.686 | 0.065 | |
| Carbohydrates | 1.557 | 0.085 |
| Sample | Shannon Diversity (H′) | Shannon | Simpson |
|---|---|---|---|
| Evenness (E) | Diversity (D) | ||
| G1 | 3.233 ± 0.034 | 0.941 ± 0.010 | 0.958 ± 0.002 |
| G2 | 3.249 ± 0.048 | 0.946 ± 0.014 | 0.959 ± 0.002 |
| G3 | 3.245 ± 0.041 | 0.941 ± 0.010 | 0.959 ± 0.001 |
| G4 | 3.166 ± 0.041 | 0.922 ± 0.011 | 0.956 ± 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.; Zhang, M.; Ding, W.; Lan, L.; Liu, Z.; Miao, L.; Hou, J. Microbial Carbon Metabolic Functions in Sediments Influenced by Resuspension Event. Water 2021, 13, 7. https://doi.org/10.3390/w13010007
Wu M, Zhang M, Ding W, Lan L, Liu Z, Miao L, Hou J. Microbial Carbon Metabolic Functions in Sediments Influenced by Resuspension Event. Water. 2021; 13(1):7. https://doi.org/10.3390/w13010007
Chicago/Turabian StyleWu, Miao, Ming Zhang, Wei Ding, Lin Lan, Zhilin Liu, Lingzhan Miao, and Jun Hou. 2021. "Microbial Carbon Metabolic Functions in Sediments Influenced by Resuspension Event" Water 13, no. 1: 7. https://doi.org/10.3390/w13010007
APA StyleWu, M., Zhang, M., Ding, W., Lan, L., Liu, Z., Miao, L., & Hou, J. (2021). Microbial Carbon Metabolic Functions in Sediments Influenced by Resuspension Event. Water, 13(1), 7. https://doi.org/10.3390/w13010007






