Woven-Fiber Microfiltration (WFMF) and Ultraviolet Light Emitting Diodes (UV LEDs) for Treating Wastewater and Septic Tank Effluent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wastewater Sources
2.2. Wastewater Quality Characterization
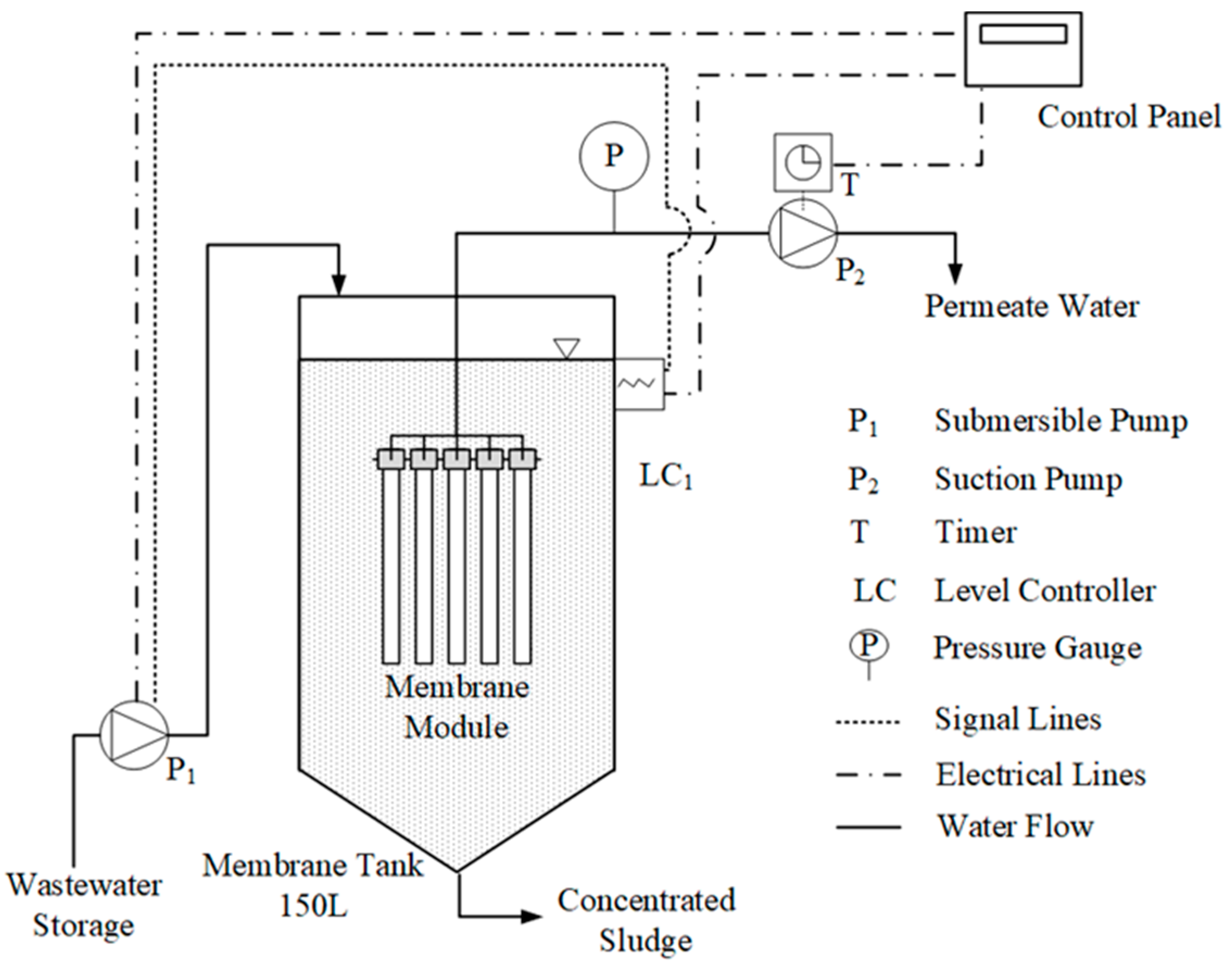
2.3. Woven-Fiber Microfiltration System
2.4. Flow-Through UV Reactors
2.5. UV Fluence Determination
2.6. Statistical Analysis
2.7. Combined Variable Approach to RED and Log Inactivation Modelling
3. Results and Discussion
3.1. WFMF Membrane System Performance
3.1.1. Domestic Wastewater
3.1.2. Septic Tank Effluent
3.2. UV Disinfection System Performance
UV Dose Response Curves
3.3. Flow-Through UV Reactor Disinfection Efficiency
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burek, P.; Satoh, Y.; Fischer, G.; Kahil, M.; Scherzer, A.; Tramberend, S.; Nava, L.; Wada, Y.; Eisner, S.; Flörke, M. Water Futures and Solution-Fast Track Initiative; IIASA: Laxenburg, Austria, 2016. [Google Scholar]
- IEA. Water Energy Nexus—Excerpt from the World Energy Outlook; IEA: Paris, France, 2016. [Google Scholar]
- West, C.; Kenway, S.; Hassall, M.; Yuan, Z. Why do residential recycled water schemes fail? A comprehensive review of risk factors and impact on objectives. Water Res. 2016, 102, 271–281. [Google Scholar] [CrossRef] [PubMed]
- WHO. Progress on Household Drinking Water, Sanitation and Hygiene 2000–2017: Special Focus on Inequalities; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Koottatep, T.; Chapagain, S.K.; Polprasert, C.; Panuvatvanich, A.; Ahn, K.-H. Sanitation situations in selected Southeast Asian countries and application of innovative technologies. Environ. Dev. Sustain. 2018, 20, 495–506. [Google Scholar] [CrossRef]
- Simachaya, W. Wastewater tariffs in Thailand. Ocean Coast. Manag. 2009, 52, 378–382. [Google Scholar] [CrossRef]
- Hutton, G.; Rodriguez, U.; Napitupulu, L.; Thang, P.; Kov, P. Economic Impacts of Sanitation in Southeast Asia: Summary. In World Bank Policy Research Working Paper; The World Bank: Washington, DC, USA, 2007; p. 44121. [Google Scholar]
- Allen, A.S.; Borchardt, M.A.; Kieke, B.A.; Dunfield, K.E.; Parker, B.L. Virus occurrence in private and public wells in a fractured dolostone aquifer in Canada. Hydrogeol. J. 2017, 25, 1117–1136. [Google Scholar] [CrossRef] [Green Version]
- Borchardt, M.A.; Bradbury, K.R.; Alexander, E.C., Jr.; Kolberg, R.J.; Alexander, S.C.; Archer, J.R.; Braatz, L.A.; Forest, B.M.; Green, J.A.; Spencer, S.K. Norovirus outbreak caused by a new septic system in a dolomite aquifer. Groundwater 2011, 49, 85–97. [Google Scholar] [CrossRef]
- Polprasert, C.; Koottatep, T. Organic Waste Recycling: Technology, Management and Sustainability; IWA Publishing: London, UK, 2017. [Google Scholar]
- Banda, L.J.; Mbewe, A.R.; Nzala, S.H.; Halwindi, H. Effect of siting boreholes and septic tanks on groundwater quality in St. Bonaventure township of Lusaka District, Zambia. Int. J. Environ. Sci. Toxicol. 2014, 2, 1–7. [Google Scholar]
- Trimper, S. The Presence and Transport of Human Enteric Viruses in Fractured Bedrock Aquifers. Master’s Thesis, Queen’s University, Kingston, ON, Canada, 2010. [Google Scholar]
- Ezugbe, E.O.; Rathilal, S. Membrane technologies in wastewater treatment: A review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef]
- Quist-Jensen, C.A.; Macedonio, F.; Drioli, E. Membrane technology for water production in agriculture: Desalination and wastewater reuse. Desalination 2015, 364, 17–32. [Google Scholar] [CrossRef]
- Chollom, M.N.; Pikwa, K.; Rathilal, S.; Pillay, V.L. Fouling mitigation on a woven fibre microfiltration membrane for the treatment of raw water. S. Afr. J. Chem. Eng. 2017, 23, 1–9. [Google Scholar] [CrossRef]
- Alfa, D.; Rathilal, S.; Pillay, V.; Pikwa, K.; Chollom, M.N. Development and evaluation of a small scale water disinfection system. J. Water Sanit Hyg. Develop. 2016, 6, 389–400. [Google Scholar] [CrossRef]
- Mecha, C.; Pillay, V.L. Development and evaluation of woven fabric microfiltration membranes impregnated with silver nanoparticles for potable water treatment. J. Membr. Sci. 2014, 458, 149–156. [Google Scholar] [CrossRef]
- Cao Ngoc Dan, T.; Nguyen, T.T.; Bui, X.T.; Vo, T.D.H.; Truong, C.H.S.; Son, N.T.; Dao, T.S.; Pham, A.D.; Nguyen, T.L.C.; Nguyen, L.H. Low-cost spiral membrane for improving effluent quality of septic tank. Desalin. Water Treat. 2016, 57, 12409–12414. [Google Scholar] [CrossRef]
- Khan, S.J.; Ali, S.; Visvanathan, C.; Pillay, V. Membrane fouling characterization in membrane-based septic tank. Desalin. Water Treat. 2013, 51, 6415–6419. [Google Scholar] [CrossRef]
- Song, K.; Mohseni, M.; Taghipour, F. Application of ultraviolet light-emitting diodes (UV-LEDs) for water disinfection: A review. Water Res. 2016, 94, 341–349. [Google Scholar] [CrossRef]
- Hijnen, W.; Beerendonk, E.; Medema, G.J. Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo) cysts in water: A review. Water Res. 2006, 40, 3–22. [Google Scholar] [CrossRef]
- Gibson, J.; Drake, J.; Karney, B. UV disinfection of wastewater and combined sewer overflows. In Ultraviolet Light in Human Health, Diseases and Environment; Springer: Berlin/Heidelberg, Germany, 2017; pp. 267–275. [Google Scholar]
- Azaizeh, H.; Linden, K.; Barstow, C.; Kalbouneh, S.; Tellawi, A.; Albalawneh, A.; Gerchman, Y. Constructed wetlands combined with UV disinfection systems for removal of enteric pathogens and wastewater contaminants. Water Sci. Technol. 2013, 67, 651–657. [Google Scholar] [CrossRef]
- Chevremont, A.-C.; Farnet, A.-M.; Coulomb, B.; Boudenne, J.-L. Effect of coupled UV-A and UV-C LEDs on both microbiological and chemical pollution of urban wastewaters. Sci. Total Environ. 2012, 426, 304–310. [Google Scholar] [CrossRef]
- Nguyen, T.M.H.; Suwan, P.; Koottatep, T.; Beck, S.E. Application of a novel, continuous-feeding ultraviolet light emitting diode (UV-LED) system to disinfect domestic wastewater for discharge or agricultural reuse. Water Res. 2019, 153, 53–62. [Google Scholar] [CrossRef]
- Vongsayalath, T. Development of Woven Fiber Microfiltration Membrane System for Water and Wastewater Treatment. Master’s Thesis, Asian Institute of Technology, Pathum Thani, Thailand, 2015. [Google Scholar]
- PCD. Effluent Standards: Standard of Discharging from Domestic Wastewater Treatment Plant in Thailand. Available online: https://www.pcd.go.th/ (accessed on 4 January 2021).
- The Royal Irrigation Department. Royal Irrigation Department Order No.73/2554; The Royal Irrigation Department: Bangkok, Thailand, 2011.
- WHO. A compendium of standards for wastewater reuse in the Eastern Mediterranean Region. In World Health Organization: Regional Office for the Eastern Mediterranean and Regional Center for Environmental Health Activities (CEHA); WHO: Geneva, Switzerland, 2006; Volume WHO-EM/CEH/142/E. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Water Works Association and Water Environment Federation: New York, NY, USA, 2012; Volume 22. [Google Scholar]
- Moodley, P.; Archer, C.; Hawksworth, D.; Leibach, L. Standard Methods for the Recovery and Enumeration of Helminth Ova in Wastewater, Sludge, Compost and Urine-Diversion Waste in South Africa: Report to the Water Research Commission; Water Research Commission: Pretoria, South Africa, 2008. [Google Scholar]
- Carré, E.; Pérot, J.; Jauzein, V.; Lopez-Ferber, M. Impact of suspended particles on UV disinfection of activated-sludge effluent with the aim of reclamation. J. Water Process. Eng. 2018, 22, 87–93. [Google Scholar] [CrossRef]
- USEPA. Wastewater Technology Fact Sheet: Ultraviolet Disinfection; U.S. Environmental Protection Agency: Washington, DC, USA, 1999.
- Tchobanoglous, G. Wastewater Engineering: Treatment and Resource Recovery; McGraw-Hill: New York, NY, USA, 2014; Volume 2. [Google Scholar]
- Beck, S.E.; Rodriguez, R.A.; Hawkins, M.A.; Hargy, T.M.; Larason, T.C.; Linden, K.G. Comparison of UV-induced inactivation and RNA damage in MS2 phage across the germicidal UV spectrum. Appl. Environ. Microbiol. 2016, 82, 1468–1474. [Google Scholar] [CrossRef] [Green Version]
- Oguma, K.; Rattanakul, S.; Bolton, J.R. Application of UV Light–Emitting Diodes to adenovirus in water. J. Environ. Eng. 2016, 142, 04015082. [Google Scholar] [CrossRef]
- USEPA. Ultraviolet Disinfection Guidance Manual for the Final Long Term 2 Enhanced Surface Water Treatment Rule: EPA 815-R-06–007; U.S Environmental Protection Agency: Washington, DC, USA, 2006.
- Sidhu, J.P.; Toze, S.G. Human pathogens and their indicators in biosolids: A literature review. Environ. Int. 2009, 35, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Amarasiri, M.; Kitajima, M.; Nguyen, T.H.; Okabe, S.; Sano, D. Bacteriophage removal efficiency as a validation and operational monitoring tool for virus reduction in wastewater reclamation. Water Res. 2017, 121, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Rattanakul, S.; Oguma, K. Inactivation kinetics and efficiencies of UV-LEDs against Pseudomonas aeruginosa, Legionella pneumophila, and surrogate microorganisms. Water Res. 2018, 130, 31–37. [Google Scholar] [CrossRef]
- NWRI. Ultraviolet Disinfection Guidelines for Drinking Water and Water Reuse, 3rd ed.; National Water Research Institute: Fountain Valley, CA, USA, 2012. [Google Scholar]
- USEPA. Method 1601: Male-Specific (F+) and Somatic Coliphage in Water by Two-Step Enrichment Procedure in EPA 821-R-01–030; U.S Environmental Protection Agency: Washington, DC, USA, 2001.
- Beck, S.E.; Ryu, H.; Boczek, L.A.; Cashdollar, J.L.; Jeanis, K.M.; Rosenblum, J.S.; Lawal, O.R.; Linden, K.G. Evaluating UV-C LED disinfection performance and investigating potential dual-wavelength synergy. Water Res. 2017, 109, 207–216. [Google Scholar] [CrossRef]
- Kheyrandish, A.; Mohseni, M.; Taghipour, F. Protocol for Determining Ultraviolet Light Emitting Diode (UV-LED) Fluence for Microbial Inactivation Studies. Environ. Sci. Technol. 2018, 52, 7390–7398. [Google Scholar] [CrossRef]
- Linden, K.L.; Wright, H.; Collins, J.; Cotton, C.; Beck, S. Guidance for Implementing Action Spectra Correction with Medium Pressure UV Disinfection; Water Research Foundation: Denver, CO, USA, 2015. [Google Scholar]
- Erdal, U.G.; Awad, J.; Vorasis, M.; Wilder, M.; Moya, M. Evaluation of UV System Performance and Challenges of UV Validation Testing at the Laguna County Sanitation District; Water Environment Federation’s Technical Exhibition and Conference: Dallas, TX, USA, 2006. [Google Scholar]
- Wright, H.; Heath, M.; Brooks, T.; Adams, J. Innovative Approaches for Validation of Ultraviolet Disinfection Reactors for Drinking Water Systems; EPA: Washington, DC, USA, 2020.
- Hull, N.M.; Herold, W.H.; Linden, K.G. UV LED water disinfection: Validation and small system demonstration study. AWWA Water Sci. 2019, 1, e1148. [Google Scholar] [CrossRef]
- Tchobanoglous, G.; Burton, F.L.; Stensel, H.D. Metcalf & Eddy. Wastewater Engineering Treatment and Reuse, 4th ed.; Tchobanoglous, F.L., Burton, H., Stensel, D., Eds.; McGraw Hill: New Delhi, India, 2003; Volume 186. [Google Scholar]
- Nam, N.H.; Visvanathan, C.; Jegatheesan, V. Performance Evaluation of Septic Tanks as Onsite Sanitation System; Southeast Water Environment 3; IWA Publishing: London, UK, 2009; pp. 141–146. [Google Scholar]
- Koottatep, T.; Connelly, S.; Pussayanavin, T.; Khamyai, S.; Sangchun, W.; Sloan, W. ‘Solar septic tank’: Evaluation of innovative decentralized treatment of blackwater in developing countries. J. Water Sanit Hyg. Dev. 2020, 10, 828–840. [Google Scholar] [CrossRef]
- Spychała, M.; Pawlak, M.; Nawrot, T. Capacity of textile filters for wastewater treatment at changeable wastewater level–A hydraulic model. Acta Sci. Pol. Form. Circumiectus 2016, 15, 421. [Google Scholar] [CrossRef]
- USEPA. Guidelines for water reuse. In Special Restricted Crop Area; USEPA: Mendoza, Argentina, 2012. [Google Scholar]
- Gullian, M.; Espinosa-Faller, F.J.; Núñez, A.; López-Barahona, N. Effect of turbidity on the ultraviolet disinfection performance in recirculating aquaculture systems with low water exchange. Aquac. Res. 2012, 43, 595–606. [Google Scholar] [CrossRef]
- Martin, H.M. Studies on the Ascaris Lumbricoides. Doctoral Dissertation, University of Nebraska, Lincoln, NE, USA, 1926. [Google Scholar]
- Tobias, A.; Bérubé, P.R. Contribution of biofilm layer to virus removal in gravity-driven membrane systems with passive fouling control. Separ. Purif. Technol. 2020, 251, 117336. [Google Scholar] [CrossRef]
- Oguma, K.; Kita, R.; Sakai, H.; Murakami, M.; Takizawa, S. Application of UV light emitting diodes to batch and flow-through water disinfection systems. Desalination 2013, 328, 24–30. [Google Scholar] [CrossRef]
- Adegoke, A.; Stenstrom, T. Septic Systems. In Global Water Pathogen Project; Rose, J.B., Jimenez-Cisneros, B., Eds.; Michigan State University: E. Lansing, MI, USA; Available online: http://www.waterpathogens.org (accessed on 4 January 2021).
- Bianco, A.; Biasin, M.; Pareschi, G.; Cavalleri, A.; Cavatorta, C.; Fenizia, F.; Galli, P.; Lessio, L.; Lualdi, M.; Redaelli, E. UV-C irradiation is highly effective in inactivating and inhibiting SARS-CoV-2 replication. Sci. Rep. 2021, 11, 1–7. [Google Scholar] [CrossRef]
- Godini, H.; Hoseinzadeh, E.; Hossini, H. Water and wastewater as potential sources of SARS-CoV-2 transmission: A systematic review. Rev. Environ. Health 2021. published ahead of print. [Google Scholar] [CrossRef]
- Rockey, N.; Young, S.; Kohn, T.; Pecson, B.; Wobus, C.E.; Raskin, L.; Wigginton, K.R. UV Disinfection of human norovirus: Evaluating infectivity using a genome-wide PCR-based approach. Environ. Sci. Technol. 2020, 54, 2851–2858. [Google Scholar] [CrossRef]
- Woo, H.; Beck, S.E.; Boczek, L.; Carlson, K.M.; Brinkman, N.E.; Linden, K.G.; Lawal, O.R.; Hayes, S.L.; Ryu, H. Ecacy of Inactivation of Human Enteroviruses by Dual-Wavelength Germicidal Ultraviolet (UV-C) Light Emitting Diodes (LEDs). Water 2019, 11, 1131. [Google Scholar] [CrossRef] [Green Version]
- Keshavarzfathy, M.; Hosoi, Y.; Oguma, K.; Taghipour, F. Experimental and computational evaluation of a flow-through UV-LED reactor for MS2 and adenovirus inactivation. Chem. Eng. J. 2021, 407, 127058. [Google Scholar] [CrossRef]
- Lui, G.Y.; Roser, D.; Corkish, R.; Ashbolt, N.; Jagals, P.; Stuetz, R. Photovoltaic powered ultraviolet and visible light emitting diodes for sustainable point-of use disinfection of drinking waters. Sci. Total Environ. 2014, 493, 185–196. [Google Scholar] [CrossRef]
- Li, B. A Sustainable, UV-LED Disinfection System for N95 masks in Haiti. Report. Available online: https://443.ece.illinois.edu/files/2020/05/COVIDReportLiWaterMarked.pdf (accessed on 18 May 2021).
- Khine, P.P. A Comparative Study of Solar Mobile Membrane Drinking Water Treatment System and Point-of-Use Filter System. Master’s Thesis, Asian Institute of Technology, Pathum Thani, Thailand, 2020. [Google Scholar]
- Linden, K.G.; Hull, N.; Speight, V. Thinking Outside the Treatment Plant: UV for Water Distribution System Disinfection. Acc. Chem. Res. 2019, 52, 1226–1233. [Google Scholar] [CrossRef]






| Parameters (Units) | Analysis Method | Raw Wastewater | Wastewater WFMF Permeate | Raw Septic Tank Effluent | Septic Tank WFMF Permeate |
|---|---|---|---|---|---|
| pH | 4500B-H+ | 7.29 ± 0.31 n = 15 | 7.46 ± 0.48 n = 15 | 7.32 ± 0.39 n = 14 | - |
| Temp (°C) | - | 29.0 ± 4.1 n = 15 | 29.3 ± 3.7 n = 15 | 29.4 ± 2.7 n = 14 | - |
| Conductivity (µS/cm2) | 2510B | 724 ± 49 n = 13 | 728 ± 54 n = 13 | 2795 ± 236 n = 14 | - |
| TSS (mg/L) | 2540D | 65.7 ± 67 n = 15 | 8.6 ± 4 n = 15 | 128.8 ± 33 n = 21 | 56.5 ± 31 n = 4 |
| Turbidity (NTU) | 2130B | 34.4 ± 22 n = 15 | 5.9 ± 6.0 n = 14 | - | - |
| BOD (mg/L) | 5210B | 51.9 ± 27.1 n = 7 | 31.3 ± 26.9 n = 7 | 302.8 ± 124 n = 9 | - |
| COD (mg/L) | 5220C | 96.2 ± 41 n = 16 | 58.3 ± 32 n = 16 | 510.6 ± 167 n = 19 | 242.8 ± 77 n = 4 |
| TKN (mg/L) | 4500-NorgC | 49.5 ± 32 n = 8 | 30.0 ± 3 n = 8 | 264.8 ± 20 n = 20 | 240.6 ± 8 n = 2 |
| NH3-N (mg/L) | 4500-NH3 C | 24.9 ± 8.7 n = 9 | 24.6 ± 7.9 n = 9 | 257.0 ± 53 n = 20 | 191.2 ± 63 n = 4 |
| NO3-N (mg/L) | 8039-HR | 3.9 ± 2.8 n = 7 | 1.7 ± 1.4 n = 7 | 5.4 ± 3.2 n = 2 | 3.4 ± 2.4 n = 2 |
| TP (mg/L) | 4500P | 24.4 ± 28 n = 9 | 18.0 ± 19 n = 9 | 368.7 ± 105 n = 2 | 320.8 ± 83 n = 2 |
| A. lumbricoides ova (eggs/L) | [31] | 0 n = 5 | 0 n = 5 | - | - |
| Total coliforms (MPN/100 mL) | 9221C | 1.0 × 106 ± 2.6 × 106 n = 9 | 3.3 × 105 ± 5.1 × 105 n = 9 | 1.3 × 107 ± 2.7 × 107 n = 17 | 1.6 × 106 ± 1.6 × 106 n = 2 |
| E. coli (MPN/100 mL) | 9221F | 3.2 × 105 ± 7.0 × 105 n = 9 | 1.5 × 105 ± 1.5 × 105 n = 9 | 2.8 × 106 ± 2.7 × 106 n = 17 | 3.5 × 105 ± 5.5 × 103 n = 2 |
| MS2 coliphage (PFU/mL) | USEPA 1602 | 16 ± 44 n = 11 | 7 ± 17 n = 11 | 19 ± 13 n = 4 | 19 ± 25 n = 4 |
| UVT254 (%) | 33.1 ± 15 n = 14 | 52.5 ± 14 n = 18 | 3.2 ± 3.1 n = 3 | 3.5 ± 1.7 n = 4 | |
| UVT280 (%) | 44.9 ± 12 n = 3 | 57.3 ± 17 n = 8 | 6.0 ± 5.1 n = 3 | 6.1 ± 2.9 n = 4 |
| This Study | Guidelines | ||||
|---|---|---|---|---|---|
| Parameters (Units) | Wastewater WFMF Effluent | Septic Tank WFMF Effluent | Thailand Irrigation Standard 1 | WHO Unrestricted Irrigation 2 | USEPA 3 Processed Food or Non-Food Crops |
| pH | 7.5 | - | 6.5–8.5 | 6.0–9.0 | |
| Conductivity (µmol/cm) | 700 | - | ≤2000 | ||
| Turbidity (NTU) | 5.9 | - | - | ≤2 | |
| TSS (mg/L) | 8.6 | 56.5 | ≤30 | ≤30 | |
| BOD (mg/L) | 31.3 | - | ≤20 | ≤30 | |
| COD (mg/L) | 58.3 | 242.8 | ≤100 | ||
| TKN (mg/L) | 30.0 | 240.6 | ≤35 | ||
| TP (mg/L) | 18.0 | 320.8 | - | ||
| FC (CFU/100 mL) | - | ≤1000 | ≤200 | ||
| Total coliforms (MPN/100 mL) | 1.0 × 106 | 1.1 × 106 | |||
| Helminth eggs (eggs/L) | 0 | - | ≤ 1 | ||
| LP UV | Estimated Inactivation Credit | ||||||
|---|---|---|---|---|---|---|---|
| Flow Rate (mL/min) | Exposure Time (s) | Log MS2 Inactivation (log10 ± 1 SD) | RED (mJ/cm2) | Norovirus 1 (log10) | Rotavirus 2 (log10) | Hepatitis A 2 (log10) | Salmonella 2 (log10) |
| 1500 | 28 | 3.2 ± 0.2 | 50.8 ± 3.8 | 6.0 | >4.1 | >5.4 | >5.6 |
| 1800 | 23 | 3.0 ± 0.3 | 48.8 ± 7.9 | 5.7 | >4.1 | >5.4 | >5.6 |
| UV LED | Estimated Inactivation Credit | |||||
|---|---|---|---|---|---|---|
| Flow Rate (mL/min) | UVT-RLE % | Estimated RED (mJ/cm2) | MS2 1 (log10) | Adenovirus 2 (log10) | Coxsackievirus 3 (log10) | Poliovirus 3 (log10) |
| 10 | 5 | 19.0 | 1.3 | 0.23 | >4 | >4 |
| 10 | 30 | 42.5 | 2.1 | 0.80 | >4 | >4 |
| 10 | 60 | 58.2 | 2.9 | 1.34 | >4 | >4 |
| 10 | 90 | 69.8 | 3.7 | 1.83 | >4 | >4 |
| 50 | 5 | 13.7 | 0.9 | 0.15 | 2.3 | 2.7 |
| 50 | 30 | 23.1 | 1.2 | 0.31 | >4 | >4 |
| 50 | 60 | 28.3 | 1.5 | 0.42 | >4 | >4 |
| 50 | 90 | 31.9 | 1.7 | 0.51 | >4 | >4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beck, S.E.; Suwan, P.; Rathnayeke, T.; Nguyen, T.M.H.; Huanambal-Sovero, V.A.; Boonyapalanant, B.; Hull, N.M.; Koottatep, T. Woven-Fiber Microfiltration (WFMF) and Ultraviolet Light Emitting Diodes (UV LEDs) for Treating Wastewater and Septic Tank Effluent. Water 2021, 13, 1564. https://doi.org/10.3390/w13111564
Beck SE, Suwan P, Rathnayeke T, Nguyen TMH, Huanambal-Sovero VA, Boonyapalanant B, Hull NM, Koottatep T. Woven-Fiber Microfiltration (WFMF) and Ultraviolet Light Emitting Diodes (UV LEDs) for Treating Wastewater and Septic Tank Effluent. Water. 2021; 13(11):1564. https://doi.org/10.3390/w13111564
Chicago/Turabian StyleBeck, Sara E., Poonyanooch Suwan, Thusitha Rathnayeke, Thi Minh Hong Nguyen, Victor A. Huanambal-Sovero, Boonmee Boonyapalanant, Natalie M. Hull, and Thammarat Koottatep. 2021. "Woven-Fiber Microfiltration (WFMF) and Ultraviolet Light Emitting Diodes (UV LEDs) for Treating Wastewater and Septic Tank Effluent" Water 13, no. 11: 1564. https://doi.org/10.3390/w13111564
APA StyleBeck, S. E., Suwan, P., Rathnayeke, T., Nguyen, T. M. H., Huanambal-Sovero, V. A., Boonyapalanant, B., Hull, N. M., & Koottatep, T. (2021). Woven-Fiber Microfiltration (WFMF) and Ultraviolet Light Emitting Diodes (UV LEDs) for Treating Wastewater and Septic Tank Effluent. Water, 13(11), 1564. https://doi.org/10.3390/w13111564







