Use of Electrocoagulation for Treatment of Pharmaceutical Compounds in Water/Wastewater: A Review Exploring Opportunities and Challenges
Abstract
:1. Introduction
2. Sources and Characteristics of Pharmaceuticals in Wastewater
2.1. Pharmaceutical Production Companies
2.2. Wastewater Treatment Plants
2.3. Hospitals
2.4. Landfills
2.5. Characteristic of Wastewater Associated with Pharmaceutical Industry
3. Different Removal Technologies for Pharmaceutical Contaminants
3.1. Physiochemical Treatment Technologies
3.1.1. Activated Carbon
3.1.2. Carbon Nanotubes
3.1.3. Electrocoagulation
3.1.4. Ozone Treatment
3.1.5. Advanced Oxidation Technologies
3.2. Bioremediation
3.3. Membrane Technology
4. Applicability of Electrocoagulation in Removal of Pharmaceuticals
4.1. Theory and Mechanism of EC
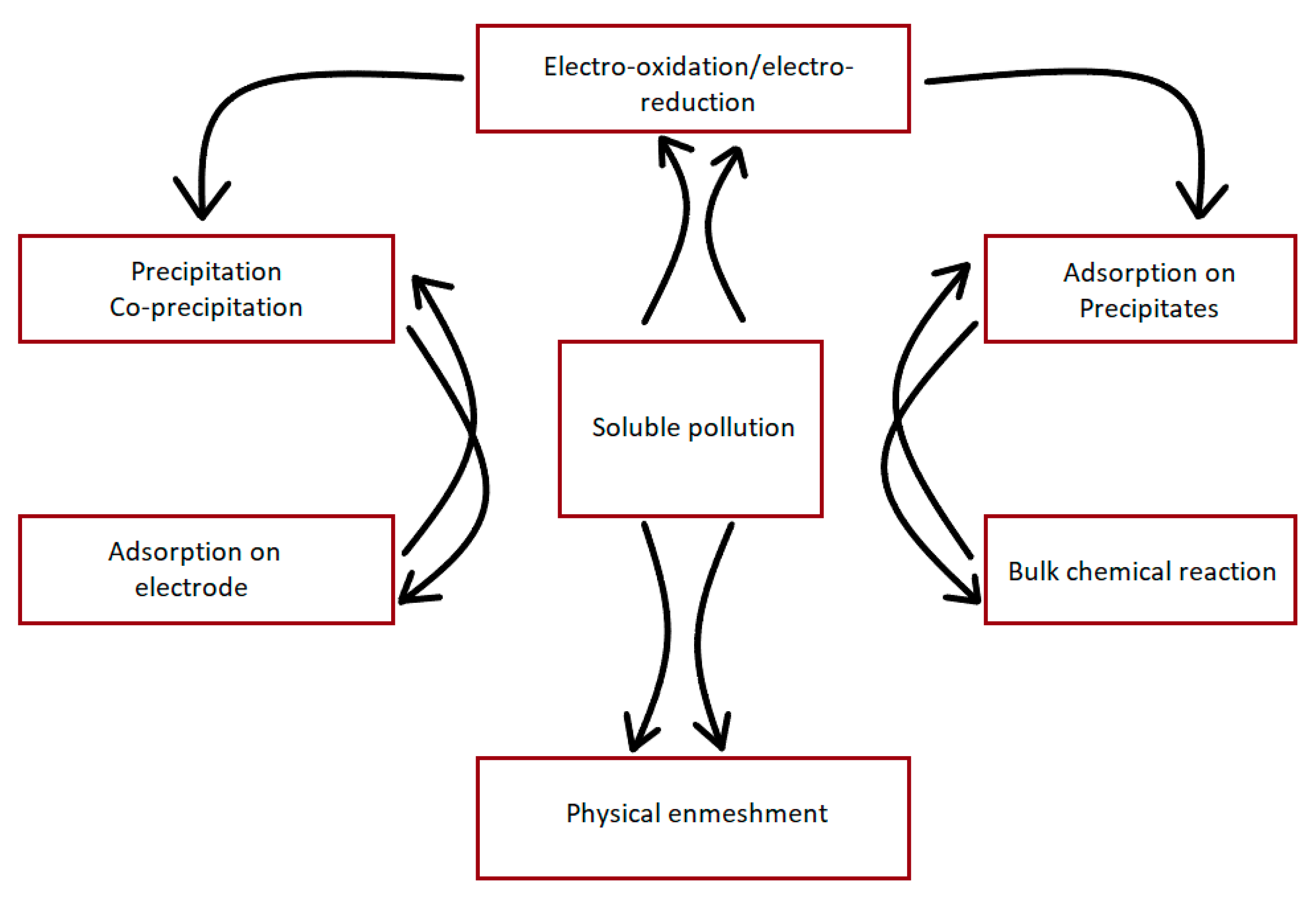
4.2. EC Mechanisms
4.3. EC Using Al Electrodes
4.4. EC Using Fe Electrodes
5. Parameters Affecting EC
5.1. Initial pH
5.2. Duration of Electrocoagulation Treatment
5.3. Current Density (CD)
5.4. Mode of Electricity Application
5.5. Electrode Material
5.6. Electrode Spacing
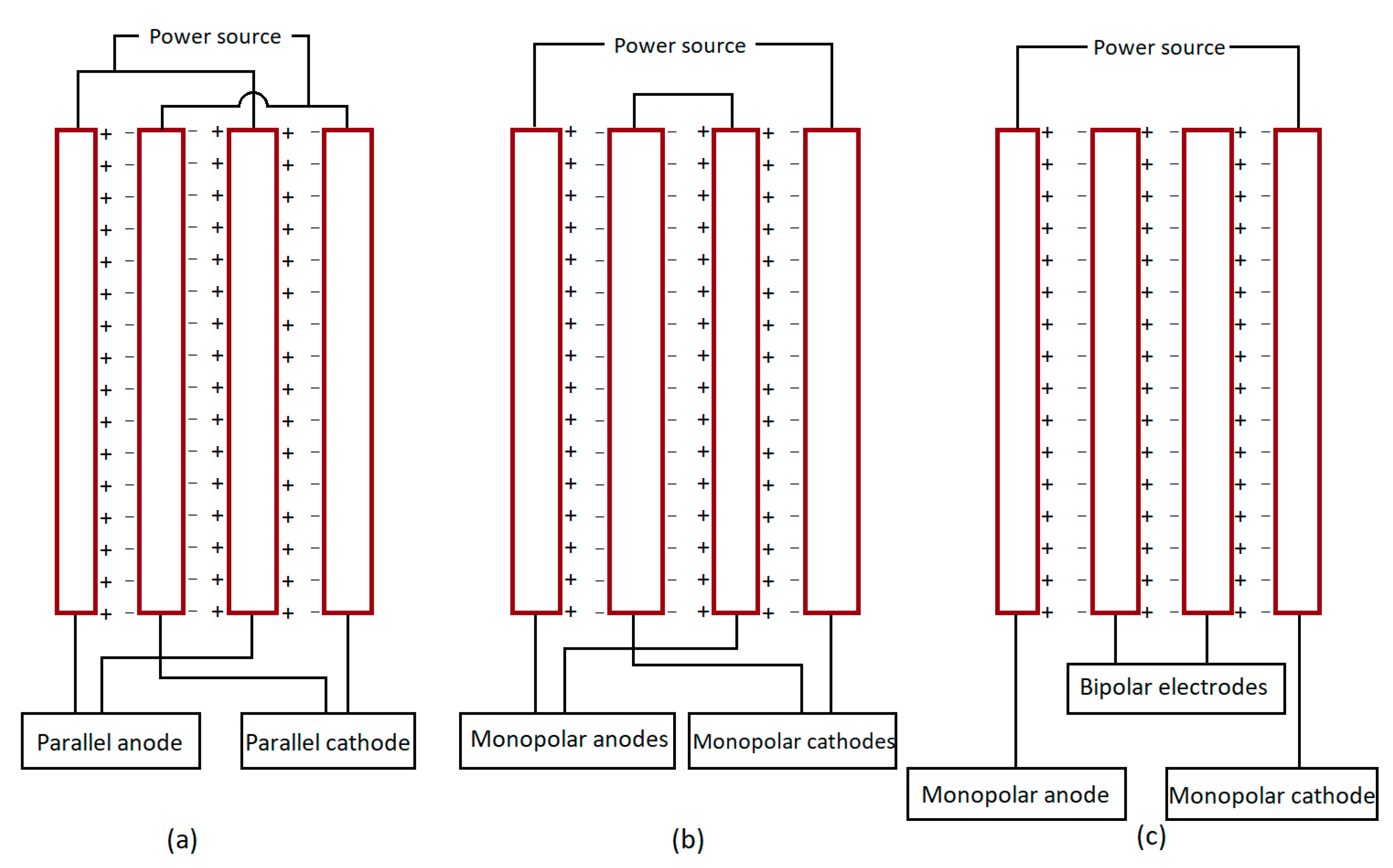
5.7. Electrode Arrangement
- Monopolar-series electrodes (MP-S): In the MP-S setup, each internal pair of sacrificial electrodes are joined to one another making an equal amount of current supply in each. However, the voltage is additive here (Figure 4b).
- Bipolar series electrodes (BP-S): In the BP-S arrangement, two of the outer monopolar electrodes are joined to the external circuit, and internal bipolar sacrificial electrodes are without a connection. Here, on the positive side, oxidation of meal takes place, and the cathodic reaction takes place on the negative side [144] (Figure 4c).
5.8. Electrode Shape
5.9. Mode of Power Supply
6. Combined Electrocoagulation Processes
6.1. Combined EC/adsorption (CEA) Process
6.2. Combined Chemical Coagulation (ECCC)/EC Process
6.3. Combined Membrane/EC Process
6.4. Combined Sono/Electrocoagulation Process
7. Cost Analysis
8. Challenges and Suggested Mitigations
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Fatta-Kassinos, D.; Meric, S.; Nikolaou, A. Pharmaceutical residues in environmental waters and wastewater: Current state of knowledge and future research. Anal. Bioanal. Chem. 2011, 399, 251–275. [Google Scholar] [CrossRef]
- Khan, U.; Nicell, J.A. Assessing the risk of exogenously consumed pharmaceuticals in land-applied human urine. Water Sci. Technol. 2010, 62, 1335–1345. [Google Scholar] [CrossRef] [PubMed]
- Lapworth, D.J.; Baran, N.; Stuart, M.E.; Ward, R.S. Emerging organic contaminants in groundwater: A review of sources, fate and occurrence. Environ. Pollut. 2012, 163, 287–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Ok, Y.S.; Kim, K.H.; Kwon, E.E.; Tsang, Y.F. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review. Sci. Total Environ. 2017, 596–597, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Chander, V.; Sharma, B.; Negi, V.; Aswal, R.S.; Singh, P.; Singh, R.; Dobhal, R. Pharmaceutical Compounds in Drinking Water. J. Xenobiotics 2016, 6, 5774. [Google Scholar] [CrossRef] [PubMed]
- Snyder, S.; Lue-Hing, C.; Cotruvo, J.; Drewes, J.E.; Eaton, A.; Pleus, R.C.; Schlenk, D. Pharmaceuticals in the Water Environment. National Association of Clean Water Environment (NACWA) and Association of Metropolitan Water Agencies (AMWA). 2009, p. 38. Available online: https://www.acs.org/content/dam/acsorg/policy/acsonthehill/briefings/pharmaceuticalsinwater/nacwa-paper.pdf (accessed on 27 May 2021).
- Abdel-Shafy, I. Issue of Pharmaceutical Compounds in Water and Wastewater: Sources, Impact and Elimination. Egypt. J. Chem. 2013, 56, 449–471. [Google Scholar]
- Khan, S.U.; Rameez, H.; Basheer, F.; Farooqi, I.H. Eco-toxicity and health issues associated with the pharmaceuticals in aqueous environments: A global scenario. In Pharmaceutical Wastewater Treatment Technologies: Concepts and Implementation Strategies; IWA Publishing: London, UK, 2021. [Google Scholar] [CrossRef]
- Jukosky, J.A.; Watzin, M.C.; Leiter, J.C. The effects of environmentally relevant mixtures of estrogens on Japanese medaka (Oryzias latipes) reproduction. Aquat. Toxicol. 2008, 86, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Gross-Sorokin, M.Y.; Roast, S.D.; Brighty, G.C. Assessment of feminization of male fish in English rivers by the Environment Agency of England and Wales. Environ. Health Perspect. 2006, 114, 147–151. [Google Scholar] [CrossRef] [Green Version]
- Crane, M.; Watts, C.; Boucard, T. Chronic aquatic environmental risks from exposure to human pharmaceuticals. Sci. Total Environ. 2006, 367, 23–41. [Google Scholar] [CrossRef]
- Quinn, B.; Gagné, F.; Blaise, C. An investigation into the acute and chronic toxicity of eleven pharmaceuticals (and their solvents) found in wastewater effluent on the cnidarian, Hydra attenuata. Sci. Total Environ. 2008, 389, 306–314. [Google Scholar] [CrossRef]
- Gaworecki, K.M.; Klaine, S.J. Behavioral and biochemical responses of hybrid striped bass during and after fluoxetine exposure. Aquat. Toxicol. 2008, 88, 207–213. [Google Scholar] [CrossRef]
- Nentwig, G. Effects of pharmaceuticals on aquatic invertebrates. Part II: The antidepressant drug fluoxetine. Arch. Environ. Contam. Toxicol. 2007, 52, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Pomati, F.; Castiglioni, S.; Zuccato, E.; Fanelli, R.; Vigetti, D.; Rossetti, C.; Calamari, D. Effects of a complex mixture of therapeutic drugs at environmental levels on human embryonic cells. Environ. Sci. Technol. 2006, 40, 2442–2447. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, E.; Pierre, M.G.; Perrodin, Y. Groundwater contamination by microbiological and chemical substances released from hospital wastewater: Health risk assessment for drinking water consumers. Environ. Int. 2009, 35, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Kumaraswamy, R.; Amha, Y.M.; Anwar, M.Z.; Henschel, A.; Rodríguez, J.; Ahmad, F. Molecular analysis for screening human bacterial pathogens in municipal wastewater treatment and reuse. Environ. Sci. Technol. 2014, 48, 11610–11619. [Google Scholar] [CrossRef] [PubMed]
- Miège, C.; Choubert, J.M.; Ribeiro, L.; Eusèbe, M.; Coquery, M. Fate of pharmaceuticals and personal care products in wastewater treatment plants--conception of a database and first results. Environ. Pollut. 2009, 157, 1721–1726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stackelberg, P.E.; Furlong, E.T.; Meyer, M.T.; Zaugg, S.D.; Henderson, A.K.; Reissman, D.B. Persistence of pharmaceutical compounds and other organic wastewater contaminants in a conventional drinking-water-treatment plant. Sci. Total Environ. 2004, 329, 99–113. [Google Scholar] [CrossRef] [Green Version]
- Kümmerer, K. Drugs in the environment: Emission of drugs, diagnostic aids and disinfectants into wastewater by hospitals in relation to other sources—A review. Chemosphere 2001, 45, 957–969. [Google Scholar] [CrossRef]
- Farhadi, S.; Aminzadeh, B.; Torabian, A.; Khatibikamal, V.; Alizadeh Fard, M. Comparison of COD removal from pharmaceutical wastewater by electrocoagulation, photoelectrocoagulation, peroxi-electrocoagulation and peroxi-photoelectrocoagulation processes. J. Hazard. Mater. 2012, 219–220, 35–42. [Google Scholar] [CrossRef]
- Gebhardt, W.; Schröder, H.F. Liquid chromatography-(tandem) mass spectrometry for the follow-up of the elimination of persistent pharmaceuticals during wastewater treatment applying biological wastewater treatment and advanced oxidation. J. Chromatogr. 2007, 1160, 34–43. [Google Scholar] [CrossRef]
- Klavarioti, M.; Mantzavinos, D.; Kassinos, D. Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ. Int. 2009, 35, 402–417. [Google Scholar] [CrossRef]
- Ensano, B.M.B.; Borea, L.; Naddeo, V.; Belgiorno, V.; De Luna, M.D.G.; Ballesteros, F.C. Removal of Pharmaceuticals from Wastewater by Intermittent Electrocoagulation. Water 2017, 9, 85. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Galvis, E.M.; Cardenas-Gutierrez, I.Y.; Contreras-Ropero, J.E.; García-Martínez, J.B.; Barajas-Solano, A.F.; Zuorro, A. An innovative low-cost equipment for electro-concentration of microalgal biomass. App. Sci. 2020, 10, 4841. [Google Scholar] [CrossRef]
- Khan, S.U.; Mahtab, M.S.; Farooqi, I.H. Enhanced lead (II) removal with low energy consumption in an electrocoagulation column employing concentric electrodes: Process optimisation by RSM using CCD. Int. J. Environ. Anal. Chem. 2021, 1–18. [Google Scholar] [CrossRef]
- Emamjomeh, M.M.; Mousazadeh, M.; Mokhtari, N.; Jamali, H.A.; Makkiabadi, M.; Naghdali, Z.; Hashim, K.S.; Ghanbari, R. Simultaneous removal of phenol and linear alkylbenzene sulfonate from automotive service station wastewater: Optimization of coupled electrochemical and physical processes. Sep. Sci. Technol. 2020, 55, 3184–3194. [Google Scholar] [CrossRef]
- Khetan, S.K.; Collins, T.J. Human Pharmaceuticals in the Aquatic Environment: A Challenge to Green Chemistry. Chem. Rev. 2007, 107, 2319–2364. [Google Scholar] [CrossRef]
- Rana, R.S.; Singh, P.; Kandari, V.; Singh, R.; Dobhal, R.; Gupta, S. A review on characterization and bioremediation of pharmaceutical industries’ wastewater: An Indian perspective. Appl. Water Sci. 2017, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Rezania, S.; Park, J.; Md Din, M.F.; Mat Taib, S.; Talaiekhozani, A.; Kumar Yadav, K.; Kamyab, H. Microplastics pollution in different aquatic environments and biota: A review of recent studies. Mar. Pollut. Bull. 2018, 133, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Lübbert, C.; Baars, C.; Dayakar, A.; Lippmann, N.; Rodloff, A.C.; Kinzig, M.; Sörgel, F. Environmental pollution with antimicrobial agents from bulk drug manufacturing industries in Hyderabad, South India, is associated with dissemination of extended-spectrum beta-lactamase and carbapenemase-producing pathogens. Infection 2017, 45, 479–491. [Google Scholar] [CrossRef]
- Fick, J.; Söderström, H.; Lindberg, R.H.; Phan, C.; Tysklind, M.; Larsson, D.G.J. Contamination of surface, ground, and drinking water from pharmaceutical production. Environ. Toxicol. Chem. 2009, 28, 2522–2527. [Google Scholar] [CrossRef]
- Bengtsson-Palme, J.; Boulund, F.; Fick, J.; Kristiansson, E.; Larsson, D.G.J. Shotgun metagenomics reveals a wide array of antibiotic resistance genes and mobile elements in a polluted lake in India. Front. Microbiol. 2014, 5, 648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marathe, N.; Regina, V.R.; Walujkar, S.A.; Charan, S.; Moore, E.R.B.; Joakim Larsson, D.G.; Shouche, Y.S. A treatment plant receiving waste water from multiple bulk drug manufacturers is a reservoir for highly multi-drug resistant integron-bearing bacteria. PLoS ONE 2013, 8, 77310. [Google Scholar] [CrossRef] [Green Version]
- Bill. 2020. Available online: http://moef.gov.in/wp-content/uploads/2019/02/silent-valley.pdf (accessed on 27 May 2021).
- Heberer, T. Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: A review of recent research data. Toxicol. Lett. 2002, 131, 5–17. [Google Scholar] [CrossRef]
- Martín, J.; Camacho-Muñoz, D.; Santos, J.L.; Aparicio, I.; Alonso, E. Occurrence of pharmaceutical compounds in wastewater and sludge from wastewater treatment plants: Removal and ecotoxicological impact of wastewater discharges and sludge disposal. J. Hazard. Mater. 2012, 239–240, 40–47. [Google Scholar] [CrossRef]
- Liu, J.L.; Wong, M.H. Pharmaceuticals and personal care products (PPCPs): A review on environmental contamination in China. Environ. Int. 2013, 59, 208–224. [Google Scholar] [CrossRef]
- Li, B.; Zhang, T. Biodegradation and Adsorption of Antibiotics in the Activated Sludge Process. Environ. Sci. Technol. 2010, 44, 3468–3473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, B.; Yuan, H.; Ma, R.; Huang, J.; Deng, S.; Wang, Y.; Yu, G. Discharge inventory of pharmaceuticals and personal care products in Beijing, China. Emerg. Contam. 2016, 2, 148–156. [Google Scholar] [CrossRef] [Green Version]
- Hodges, J.E.N.; Holmes, C.M.; Vamshi, R.; Mao, D.; Price, O.R. Estimating chemical emissions from home and personal care products in China. Environ. Pollut. 2012, 165, 199–207. [Google Scholar] [CrossRef]
- Robinson, I.; Junqua, G.; Van Coillie, R.; Thomas, O. Trends in the detection of pharmaceutical products, and their impact and mitigation in water and wastewater in North America. Anal. Bioanal. Chem. 2007, 387, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, M.; Alda, D.; Maria, J.L.; Diaz-Cruz, S.; Postigo, C.; Radjenovic, J.; Gros, M.; Barcelo, D. Fate and removal of pharmaceuticals and illicit drugs in conventional and membrane bioreactor wastewater treatment plants and by riverbank filtration. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2009, 367, 3979–4003. [Google Scholar] [CrossRef]
- Deegan, A.M.; Shaik, B.; Nolan, K.; Urell, K.; Tobin, J.; Morrissey, A. Treatment options for wastewater effluents from pharmaceutical companies. Int. J. Environ. Sci. Technol. 2011, 8, 649–666. [Google Scholar] [CrossRef] [Green Version]
- Gome, A.; Upadhyay, K. Biodegradability Assessment of Pharmaceutical Wastewater Treated by Ozone. Int. Res. J. Environ. Sci. 2013, 2, 21–25. [Google Scholar]
- Arsand, D.R.; Kummerer, K.; Martins, A.F. Removal of dexamethasone from aqueous solution and hospital wastewater by electrocoagulation. Sci. Total Environ. 2013, 443, 351–357. [Google Scholar] [CrossRef]
- Dindas, G.B.; Caliskan, Y.; Celebi, E.E.; Tekbas, M.; Bektas, N.; Yatmaz, H.C. Treatment of pharmaceutical wastewater by combination of electrocoagulation, electro-fenton and photocatalytic oxidation processes. J. Environ. Chem. Eng. 2020, 8, 103777. [Google Scholar] [CrossRef]
- Deshpande, A.M.; Satyanarayan, S.; Ramakant, S. Treatment of high-strength pharmaceutical wastewater by electrocoagulation combined with anaerobic process. Water Sci. Technol. 2010, 61, 463–472. [Google Scholar] [CrossRef]
- Boroski, M.; Rodrigues, A.C.; Garcia, J.C.; Sampaio, L.C.; Nozaki, J.; Hioka, N. Combined electrocoagulation and TiO2 photoassisted treatment applied to wastewater effluents from pharmaceutical and cosmetic industries. J. Hazard. Mater. 2009, 162, 448–454. [Google Scholar] [CrossRef]
- Idris, M.; Kolo, M.; Garba, S.; Ismail, M. Physico-chemical analysis of pharmaceutical effluent and surface water of River Gorax in Minna, Niger State, Nigeria. Bull. Environ. Pharmacol. Life Sci. 2013, 2, 45–49. [Google Scholar]
- Imran, H. Wastewater Monitoring of Pharmaceutical Industry. Electron. J. Environ. Agric. Food Chem. 2005, 60, 994–1004. [Google Scholar]
- Cetecioglu, Z.; Ince, B.; Gros, M.; Rodriguez-Mozaz, S.; Barceló, D.; Ince, O.; Orhon, D. Biodegradation and reversible inhibitory impact of sulfamethoxazole on the utilization of volatile fatty acids during anaerobic treatment of pharmaceutical industry wastewater. Sci. Total Environ. 2015, 536, 667–674. [Google Scholar] [CrossRef]
- Hu, H.; Jiang, C.; Ma, H.; Ding, L.; Geng, J.; Xu, K.; Huang, H.; Ren, H. Removal characteristics of DON in pharmaceutical wastewater and its influence on the N-nitrosodimethylamine formation potential and acute toxicity of DOM. Water Res. 2017, 109, 114–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, X.; Ng, K.K.; Li, X.R.; Ng, H.Y. Investigation of intertidal wetland sediment as a novel inoculation source for anaerobic saline wastewater treatment. Environ. Sci. Technol. 2015, 49, 6231–6239. [Google Scholar] [CrossRef]
- Rodriguez, E.; Campinas, M.; Acero, J.L.; Rosa, M.J. Investigating PPCP Removal from Wastewater by Powdered Activated Carbon/Ultrafiltration. Water Air Soil Pollut. 2016, 227, 177. [Google Scholar] [CrossRef]
- Mailler, R.; Gasperi, J.; Coquet, Y.; Deshayes, S.; Zedek, S.; Cren-Olivé, C.; Cartiser, N.; Eudes, V.; Bressy, A.; Caupos, E.; et al. Study of a large scale powdered activated carbon pilot: Removals of a wide range of emerging and priority micropollutants from wastewater treatment plant effluents. Water Res. 2015, 72, 315–330. [Google Scholar] [CrossRef] [Green Version]
- Saucier, C.; Adebayo, M.A.; Lima, E.C.; Cataluña, R.; Thue, P.S.; Prola, L.D.T.; Puchana-Rosero, M.J.; Machado, F.M.; Pavan, F.A.; Dotto, G.L. Microwave-assisted activated carbon from cocoa shell as adsorbent for removal of sodium diclofenac and nimesulide from aqueous effluents. J. Hazard. Mater. 2015, 289, 18–27. [Google Scholar] [CrossRef]
- Flores-Cano, J.V.; Sánchez-Polo, M.; Messoud, J.; Velo-Gala, I.; Ocampo-Pérez, R.; Rivera-Utrilla, J. Overall adsorption rate of metronidazole, dimetridazole and diatrizoate on activated carbons prepared from coffee residues and almond shells. J. Environ. Manag. 2016, 169, 116–125. [Google Scholar] [CrossRef]
- El-Shafey, E.S.I.; Al-Lawati, H.A.J.; Al-Hussaini, A.Y. Adsorption of fexofenadine and diphenhydramine on dehydrated and activated carbons from date palm leaflets. Chem. Ecol. 2014, 30, 765–783. [Google Scholar] [CrossRef]
- Torrellas, S.A.; García Lovera, R.; Escalona, N.; Sepúlveda, C.; Sotelo, J.L.; García, J. Chemical-activated carbons from peach stones for the adsorption of emerging contaminants in aqueous solutions. Chem. Eng. J. 2015, 279, 788–798. [Google Scholar] [CrossRef]
- Sheng, C.; Nnanna, A.G.A.; Liu, Y.; Vargo, J.D. Removal of Trace Pharmaceuticals from Water using coagulation and powdered activated carbon as pretreatment to ultrafiltration membrane system. Sci. Total Environ. 2016, 550, 1075–1083. [Google Scholar] [CrossRef]
- Herrero Latorre, C.; Álvarez Méndez, J.; Barciela García, J.; García Martín, S.; Peña Crecente, R.M. Carbon nanotubes as solid-phase extraction sorbents prior to atomic spectrometric determination of metal species: A review. Anal. Chim. Acta 2012, 749, 16–35. [Google Scholar] [CrossRef]
- Tuzen, M.; Saygi, K.O.; Soylak, M. Solid phase extraction of heavy metal ions in environmental samples on multiwalled carbon nanotubes. J. Hazard. Mater. 2008, 152, 632–639. [Google Scholar] [CrossRef]
- Mubarak, N.M.; Sahu, J.N.; Abdullah, E.C.; Jayakumar, N.S. Removal of Heavy Metals from Wastewater Using Carbon Nanotubes. Sep. Purif. Rev. 2014, 43, 311–338. [Google Scholar] [CrossRef]
- Gong, J.L.; Wang, B.; Zeng, G.; Yang, C.; Niu, C.; Niu, Q.; Zhou, W.; Liang, Y. Removal of cationic dyes from aqueous solution using magnetic multi-wall carbon nanotube nanocomposite as adsorbent. J. Hazard. Mater. 2009, 164, 1517–1522. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wu, W.; Jing, Q.; Zhu, L. Aqueous Adsorption of Aniline, Phenol, and their Substitutes by Multi-Walled Carbon Nanotubes. Environ. Sci. Technol. 2008, 42, 7931–7936. [Google Scholar] [CrossRef] [PubMed]
- Shan, D.; Deng, S.; Zhao, T.; Yu, G.; Winglee, J.; Wiesner, M.R. Preparation of regenerable granular carbon nanotubes by a simple heating-filtration method for efficient removal of typical pharmaceuticals. Chem. Eng. J. 2016, 294, 353–361. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Lo, S.; Liou, Y.; Hu, C. Removal of nonsteroidal anti-inflammatory drugs (NSAIDs) by electrocoagulation-flotation with a cationic surfactant. Sep. Purif. Technol. 2015, 152, 148–154. [Google Scholar] [CrossRef]
- Akmehmet Balcioğlu, I.; Otker, M. Treatment of pharmaceutical wastewater containing antibiotics by O3 and O3/H2O2 processes. Chemosphere 2003, 50, 85–95. [Google Scholar] [CrossRef]
- Davididou, K.; Monteagudo, J.M.; Chatzisymeon, E.; Duran, A.; Expósitob, A.J. Degradation and mineralization of antipyrine by UV-A LED photo-Fenton reaction intensified by ferrioxalate with addition of persulfate. Sep. Purif. Technol. 2017, 172, 227–235. [Google Scholar] [CrossRef] [Green Version]
- Luo, S.; Wei, Z.; Spinney, R.; Zhang, Z.; Dionysiou, D.; Gao, L.; Chai, L.; Wang, D.; Xiao, R. UV direct photolysis of sulfamethoxazole and ibuprofen: An experimental and modelling study. J. Hazard. Mater. 2018, 343, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Ziylan-Yavas, A.; Ince, N.H. Single, simultaneous and sequential applications of ultrasonic frequencies for the elimination of ibuprofen in water. Ultrason. Sonochem. 2018, 40, 17–23. [Google Scholar] [CrossRef]
- Loos, G.; Scheers, T.; Van Eyck, K.; Van Schepdael, A.; Adams, E.; der Bruggen, B.; Cabooter, D.; Dewil, R. Electrochemical oxidation of key pharmaceuticals using a boron doped diamond electrode. Sep. Purif. Technol. 2018, 195, 184–191. [Google Scholar] [CrossRef]
- Liu, N.; Lei, Z.D.; Wang, T.; Wang, J.-J.; XD, Z.; Xu, G.; Tang, L. Radiolysis of carbamazepine aqueous solution using electron beam irradiation combining with hydrogen peroxide: Efficiency and mechanism. Chem. Eng. J. 2016, 295, 484–493. [Google Scholar] [CrossRef]
- Adityosulindro, S.; Barthe, L.; González-Labrada, K.; Jáuregui Haza, U.J.; Delmas, H.; Julcour, C. Sonolysis and sono-Fenton oxidation for removal of ibuprofen in (waste)water. Ultrason. Sonochem. 2017, 39, 889–896. [Google Scholar] [CrossRef] [Green Version]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. J. Environ. Manag. 2018, 219, 189–207. [Google Scholar] [CrossRef]
- Misal, S.A.; Lingojwar, D.P.; Shinde, R.M.; Gawai, K.R. Purification and characterization of azoreductase from alkaliphilic strain Bacillus badius. Process. Biochem. 2011, 46, 1264–1269. [Google Scholar] [CrossRef]
- Fernandes, J.P.; Duarte, P.; Almeida, C.M.R.; Carvalho, M.F.; Mucha, A.P. Potential of bacterial consortia obtained from different environments for bioremediation of paroxetine and bezafibrate. J. Environ. Chem. Eng. 2020, 8, 103881. [Google Scholar] [CrossRef]
- Watkinson, A.J.; Murby, E.J.; Costanzo, S.D. Removal of antibiotics in conventional and advanced wastewater treatment: Implications for environmental discharge and wastewater recycling. Water Res. 2007, 41, 4164–4176. [Google Scholar] [CrossRef]
- Reif, R.; Omil, F.; Lema, J.M. Chapter 9–Removal of Pharmaceuticals by Membrane Bioreactor (MBR) Technology. In Analysis, Removal, Effects and Risk of Pharmaceuticals in the Water Cycle; Petrovic, M., Barcelo, D., Pérez, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 62, pp. 287–317. [Google Scholar]
- Kasonga, T.K.; Coetzee, M.A.A.; Kamika, I.; Momba, M.N.B. Assessing a co-culture fungal granule ability to remove pharmaceuticals in a sequencing batch reactor. Environ. Technol. 2020, 1–16. [Google Scholar] [CrossRef]
- Bardi, A.; Yuan, Q.; Tigini, V.; Spina, F.; Varese, G.; Spennati, F.; Becarelli, S.; Gregorio, S.; Petroni, G.; Munz, G. Recalcitrant Compounds Removal in Raw Leachate and Synthetic Effluents Using the White-Rot Fungus. Water 2017, 9, 824. [Google Scholar] [CrossRef] [Green Version]
- Das, M.P.; Bashwant, M.; Kumar, K.; Das, J. Control of pharmaceutical effluent parameters through bioremediation. J. Chem. Pharm. Res. 2012, 4, 1061–1065. [Google Scholar]
- Radjenovic, J.; Petrovic, M.; Barceló, D. Analysis of pharmaceuticals in wastewater and removal using a membrane bioreactor. Anal. Bioanal. Chem. 2007, 387, 1365–1377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giwa, A.; Hasan, S.W.; Yousuf, A.; Chakraborty, S.; Johnson, D.J.; Hilal, N. Biomimetic membranes: A critical review of recent progress. Desalination 2017, 420, 403–424. [Google Scholar] [CrossRef] [Green Version]
- Shekarian, E.; Saljoughi, E.; Naderi, A. Polyacrylonitrile (PAN)/IGEPAL blend asymmetric membranes: Preparation, morphology, and performance. J. Polym. Res. 2013, 20, 162. [Google Scholar] [CrossRef]
- Zazouli, M.A.; Susanto, H.; Nasseri, S.; Ulbricht, M. Influences of solution chemistry and polymeric natural organic matter on the removal of aquatic pharmaceutical residuals by nanofiltration. Water Res. 2009, 43, 3270–3280. [Google Scholar] [CrossRef]
- Yoon, Y.; Westerhoff, P.; Snyder, S.A.; Wert, E.C. Removal of endocrine disrupting compounds and pharmaceuticals by nanofiltration and ultrafiltration membranes. Desalination 2007, 202, 16–23. [Google Scholar] [CrossRef]
- Javier Benitez, F.; Acero, J.L.; Real, F.J.; Roldán, G.; Rodriguez, E. Ultrafiltration and nanofiltration membranes applied to the removal of the pharmaceuticals amoxicillin, naproxen, metoprolol and phenacetin from water. J. Chem. Technol. Biotechnol. 2011, 86, 858–866. [Google Scholar] [CrossRef]
- Sun, S.P.; Hatton, T.A.; Chan, S.Y.; Chung, T.S. Novel thin-film composite nanofiltration hollow fiber membranes with double repulsion for effective removal of emerging organic matters from water. J. Membr. Sci. 2012, 401–402, 152–162. [Google Scholar] [CrossRef]
- Omidvar, M.; mahmoud Mousavi, S.; Soltanieh, M.; Safekordi, A.A. Preparation and characterization of poly (ethersulfone) nanofiltration membranes for amoxicillin removal from contaminated water. J. Environ. Heal. Sci. Eng. 2014, 12, 18. [Google Scholar] [CrossRef] [Green Version]
- Snyder, S.A.; Adham, S.; Redding, A.M.; Cannon, F.S.; DeCarolis, J.; Oppenheimer, J.; Wert, E.C.; Yoon, Y. Role of membranes and activated carbon in the removal of endocrine disruptors and pharmaceuticals. Desalination 2007, 202, 156–181. [Google Scholar] [CrossRef]
- Ghernaout, D. Advanced oxidation phenomena in electrocoagulation process: A myth or a reality? Desalin. Water Treat. 2013, 51, 40–42. [Google Scholar] [CrossRef]
- Ghernaout, D.; Irki, S.; Boucherit, A. Removal of Cu2+ and Cd2+, and humic acid and phenol by electrocoagulation using iron electrodes. Desalin. Water Treat. 2014, 52, 3256–3270. [Google Scholar] [CrossRef]
- Yasri, N.; Hu, J.; Kibria, M.G.; Roberts, E.P.L. Electrocoagulation Separation Processes. Multidiscip. Adv. Effic. Sep. Process. 2020, 1348, 167–203. [Google Scholar]
- Ghernaout, D.; Benblidia, C.; Khemici, F. Microalgae removal from Ghrib Dam (Ain Defla, Algeria) water by electroflotation using stainless steel electrodes. Desalin. Water Treat. 2015, 54, 3328–3337. [Google Scholar] [CrossRef]
- Ghernaout, D. Electrocoagulation as a Pioneering Separation Technology–Electric Field Role. Green Sustain. Chem. 2020, 7, 1–19. [Google Scholar] [CrossRef]
- Kobya, M.; Can, O.T.; Bayramoglu, M. Treatment of textile wastewaters by electrocoagulation using iron and aluminum electrodes. J. Hazard. Mater. 2003, 100, 163–178. [Google Scholar] [CrossRef]
- Hakizimana, J.N.; Gourich, B.; Chafi, M.; Stiriba, Y.; Vial, C.; Drogui, P.; Naja, J. Electrocoagulation process in water treatment: A review of electrocoagulation modeling approaches. Desalination 2017, 404, 1–21. [Google Scholar] [CrossRef]
- Ghernaout, D.; Elboughdiri, N. Dealing with Cyanobacteria and Cyanotoxins: Engineering Viewpoints. Open Access Libr. J. 2020, 7, 1. [Google Scholar] [CrossRef]
- Ghernaout, D.; Elboughdiri, N. Eliminating Cyanobacteria and Controlling Algal Organic Matter–Short Notes. Open Access Libr. J. 2020, 7, 1–17. [Google Scholar] [CrossRef]
- Mousazadeh, M.; Alizadeh, S.M.; Frontistis, Z.; Kabdaşlı, I.; Karamati Niaragh, E.; Al Qodah, Z.; Naghdali, Z.; Mahmoud, A.E.; Sandoval, M.A.; Butler, E.; et al. Electrocoagulation as a Promising Defluoridation Technology from Water: A Review of State of the Art of Removal Mechanisms and Performance Trends. Water 2021, 13, 656. [Google Scholar] [CrossRef]
- Faheem, K.; Khan, S.U.; Washeem, M.; Khan, S.U. Energy efficient removal of COD from landfill leachate wastewater using electrocoagulation: Parametric optimization using RSM. Int. J. Environ. Sci. Technol. 2021, 1–12. [Google Scholar] [CrossRef]
- Den, W.; Chia-Jung, W. Removal of silica from brackish water by electrocoagulation pretreatment to prevent fouling of reverse osmosis membranes. Sep. Purif. Technol. 2008, 59, 318–325. [Google Scholar] [CrossRef]
- Hu, C.Y.; Lo, S.L.; Kuan, W.H. Simulation the kinetics of fluoride removal by electrocoagulation (EC) process using aluminum electrodes. J. Hazard. Mater. 2007, 145, 180–185. [Google Scholar] [CrossRef]
- Bagga, A.; Chellam, S.; Clifford, D.C. Evaluation of iron chemical coagulation and electrocoagulation pretreatment for surface water microfiltration. J. Memb. Sci. 2008, 309, 82–93. [Google Scholar] [CrossRef]
- Gu, Z.; Liao, Z.; Schulz, M.; Davis, J.R.; Baygents, J.C.; Farrell, J. Estimating Dosing Rates and Energy Consumption for Electrocoagulation Using Iron and Aluminum Electrodes. Ind. Eng. Chem. Res. 2009, 48, 3112–3117. [Google Scholar] [CrossRef]
- Mansouri, K.; Ibrik, K.; Bensalah, N.; Abdel-Wahab, A. Anodic Dissolution of Pure Aluminum during Electrocoagulation Process: Influence of Supporting Electrolyte, Initial pH, and Current Density. Ind. Eng. Chem. Res. 2011, 50, 13362–13372. [Google Scholar] [CrossRef]
- Rebhun, M.; Lurie, M. Control of Organic Matter by Coagulation and Floc Separation. Water Sci. Technol. 1993, 27, 1–20. [Google Scholar] [CrossRef]
- Djuricic, T.; Malinovic, B.; Bjelić, D. The phosphate removal efficiency electrocoagulation wastewater using iron and aluminum electrodes. Bull. Chem. Technol. Bosnia Herzeg. 2017, 47, 33–38. [Google Scholar]
- Vepsäläinen, M.; Selin, J.; Rantala, P.; Pulliainen, M.; Särkkä, H.; Kuhmonen, K.; Bhatnagar, A.; Sillanpää, M. Precipitation of dissolved sulphide in pulp and paper mill wastewater by electrocoagulation. Environ. Technol. 2011, 32, 1393–1400. [Google Scholar] [CrossRef]
- Ferreira, A.D.; Marchesiello, M.; Thivel, P.X. Removal of copper, zinc and nickel present in natural water containing Ca2+ and HCO3− ions by electrocoagulation. Sep. Purif. Technol. 2013, 107, 109–117. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, H.; Ni, J. Fluoride distribution in electrocoagulation defluoridation process. Sep. Purif. Technol. 2007, 56, 184–191. [Google Scholar] [CrossRef]
- Khan, S.U.; Farooqi, I.H.; Usman, M.; Basheer, F. Energy Efficient Rapid Removal of Arsenic in an Electrocoagulation Reactor with Hybrid Fe/Al Electrodes: Process Optimization Using CCD and Kinetic Modeling. Water 2020, 12, 10. [Google Scholar] [CrossRef]
- Hu, C.Y.; Lo, S.L.; Kuan, W.H. Effects of co-existing anions on fluoride removal in electrocoagulation (EC) process using aluminum electrodes. Water Res. 2003, 37, 4513–4523. [Google Scholar] [CrossRef]
- Ben Sasson, M.; Calmano, W.; Adin, A. Iron-oxidation processes in an electroflocculation (electrocoagulation) cell. J. Hazard. Mater. 2009, 171, 704–709. [Google Scholar] [CrossRef]
- Jimenez, C.; Saez, C.; Martinez, F.; Canizares, P.; Rodrigo, M.A. Electrochemical dosing of iron and aluminum in continuous processes: A key step to explain electro-coagulation processes. Sep. Purif. Technol. 2012, 98, 102–108. [Google Scholar] [CrossRef]
- Malakootian, M.; Mansoorian, H.J.; Moosazadeh, M. Performance evaluation of electrocoagulation process using iron-rod electrodes for removing hardness from drinking water. Desalination 2010, 255, 67–71. [Google Scholar] [CrossRef]
- Lakshmanan, D.; Clifford, D.A.; Samanta, G. Ferrous and Ferric Ion Generation During Iron Electrocoagulation. Environ. Sci. Technol. 2009, 43, 3853–3859. [Google Scholar] [CrossRef] [PubMed]
- Akyol, A. Treatment of paint manufacturing wastewater by electrocoagulation. Desalination 2012, 285, 91–99. [Google Scholar] [CrossRef]
- Kobya, M.; Senturk, E.; Bayramoglu, M. Treatment of poultry slaughterhouse wastewaters by electrocoagulation. J. Hazard. Mater. 2006, 133, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Parga, J.R.; Cocke, D.L.; Valenzuela, J.L.; Gomes, J.A.; Kesmez, M.; Irwin, G.; Moreno, H.; Weir, M. Arsenic removal via electrocoagulation from heavy metal contaminated groundwater in La Comarca Lagunera México. J. Hazard. Mater. 2005, 124, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Al-Shannag, M.; Al-Qodah, Z.; Bani-Melhem, K.; Qtai shat, M.R.; Alkasrawi, M. Heavy metal ions removal from metal plating wastewater using electrocoagulation: Kinetic study and process performance. Chem. Eng. J. 2015, 260, 749–756. [Google Scholar] [CrossRef]
- Bensadok, K.; Benammar, S.; Lapicque, F.; Nezzal, G. Electrocoagulation of cutting oil emulsions using aluminium plate electrodes. J. Hazard. Mater. 2008, 152, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Mouedhen, G.; Feki, M.; Wery, M.D.P.; Ayedi, H.F. Behavior of aluminum electrodes in electrocoagulation process. J. Hazard. Mater. 2008, 150, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Ouaissa, Y.A.; Chabani, M.; Amrane, A.; Bensmaili, A. Removal of tetracycline by electrocoagulation: Kinetic and isotherm modeling through adsorption. J. Environ. Chem. Eng. 2014, 2, 177–184. [Google Scholar] [CrossRef] [Green Version]
- Asaithambi, P.; Aziz, A.R.A.; Daud, W.M.A.B.W. Integrated ozone—electrocoagulation process for the removal of pollutant from industrial effluent: Optimization through response surface methodology. Chem. Eng. Process. Process. Intensif. 2016, 105, 92–102. [Google Scholar] [CrossRef]
- Al-Qodah, Z.; Al-Shannag, M. Heavy metal ions removal from wastewater using electrocoagulation processes: A comprehensive review. Sep. Sci. Technol. 2017, 52, 2649–2676. [Google Scholar] [CrossRef]
- Kobya, M.; Hiz, H.; Senturk, E.; Aydiner, C.; Demirbas, E. Treatment of potato chips manufacturing wastewater by electrocoagulation. Desalination 2006, 190, 201–211. [Google Scholar] [CrossRef]
- Kobya, M.; Delipinar, S. Treatment of the baker’s yeast wastewater by electrocoagulation. J. Hazard. Mater. 2008, 154, 1133–1140. [Google Scholar] [CrossRef]
- Amrose, S.E.; Bandaru, S.R.S.; Delaire, C.; van Genuchten, C.M.; Dutta, A.; DebSarkar, A.; Orr, C.; Roy, J.; Das, A.; Gadgil, A.J. Electro-chemical arsenic remediation: Field trials in West Bengal. Sci. Total Environ. 2014, 488–489, 539–546. [Google Scholar] [CrossRef]
- Essadki, A.H.; Bennajah, M.; Gourich, B.; Vial, C.; Azzi, C.; Delmas, H. Electrocoagulation/electroflotation in an external-loop airlift reactor—Application to the decolorization of textile dye wastewater: A case study. Chem. Eng. Process. Process. Intensif. 2008, 47, 1211–1223. [Google Scholar] [CrossRef]
- Kobya, M.; Demirbas, E.; Ulu, F. Evaluation of operating parameters with respect to charge loading on the removal efficiency of arsenic from potable water by electrocoagulation. J. Environ. Chem. Eng. 2016, 4, 1484–1494. [Google Scholar] [CrossRef]
- Nariyan, E.; Aghababaei, A.; Sillanpää, M. Removal of pharmaceutical from water with an electrocoagulation process; effect of various parameters and studies of isotherm and kinetic. Sep. Purif. Technol. 2017, 188, 266–281. [Google Scholar] [CrossRef]
- Ensano, B.M.B.; Borea, L.; Naddeo, V.; Belgiorno, V.; de Luna, M.D.G.; Balakrishnan, M.; Ballesteros, F.C., Jr. Applicability of the electrocoagulation process in treating real municipal wastewater containing pharmaceutical active compounds. J. Hazard. Mater. 2019, 361, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Sahu, O.; Mazumdar, B.; Chaudhari, P.K. Treatment of wastewater by electrocoagulation: A review. Environ. Sci. Pollut. Res. Int. 2014, 21, 2397–2413. [Google Scholar] [CrossRef]
- Burns, S.E.; Yiacoumi, S.; Tsouris, C. Microbubble generation for environmental and industrial separations. Sep. Purif. Technol. 1997, 11, 221–232. [Google Scholar] [CrossRef]
- Adhoum, N.; Monser, L. Decolourization and removal of phenolic compounds from olive mill wastewater by electrocoagulation. Chem. Eng. Process. Process. Intensif. 2004, 43, 1281–1287. [Google Scholar] [CrossRef]
- Daneshvar, N.; Sorkhabi, H.A.; Kasiri, M.B. Decolorization of dye solution containing Acid Red 14 by electrocoagulation with a comparative investigation of different electrode connections. J. Hazard. Mater. 2004, 112, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Nasrullah, M.; Singh, L.; Wahid, Z.A. Treatment of sewage by electrocoagulation and the effect of high current density Treatment of Sewage by Electrocoagulation and the Effect of High Current Density. Energy Environ. Eng. J. 2017, 1, 27–31. [Google Scholar]
- Khandegar, V.; Saroha, A.K. Electrocoagulation for the treatment of textile industry effluent—A review. J. Environ. Manag. 2013, 128, 949–963. [Google Scholar] [CrossRef]
- Mollah, M.Y.A.; Morkovsky, P.; Gomes, J.A.G.; Kesmez, M.; Parga, J.; Cocke, D.L. Fundamentals, present and future perspectives of electrocoagulation. J. Hazard. Mater. 2004, 114, 199–210. [Google Scholar] [CrossRef]
- Moussa, D.T.; El-Naas, M.H.; Nasser, M.; Al-Marri, M.J. A comprehensive review of electrocoagulation for water treatment: Potentials and challenges. J. Environ. Manag. 2017, 186, 24–41. [Google Scholar] [CrossRef]
- Demirci, Y.; Pekel, L.C.; Alpbaz, M. Investigation of Different Electrode Connections in Electrocoagulation of Textile Wastewater Treatment. Int. J. Electrochem. Sci. 2015, 10, 2685–2693. [Google Scholar]
- Fouad, Y.O.A.; Konsowa, A.H.; Farag, H.A.; Sedahmed, G.H. Performance of an electrocoagulation cell with horizontally oriented electrodes in oil separation compared to a cell with vertical electrodes. Chem. Eng. J. 2009, 145, 436–440. [Google Scholar] [CrossRef]
- Tachamango, S.R.; Darchen, A. Investigation and optimization of a new electrocoagulation reactor with horizontal bipolar electrodes: Effect of electrode structure on the reactor performances. J. Environ. Chem. Eng. 2018, 6, 4546–4554. [Google Scholar] [CrossRef]
- Kuroda, Y.; Kawada, Y.; Takahashi, T.; Ehara, Y.; Ito, T.; Zukeran, A.; Kono, Y.; Yasumoto, K. Effect of electrode shape on discharge current and performance with barrier discharge type electrostatic precipitaor. J. Electrostat. 2003, 57, 407–415. [Google Scholar] [CrossRef]
- Ahmadzadeh, S.; Asadipour, A.; Pournamdari, M.; Behnam, B.; Rahimi, H.R.; Dolatabadi, M. Removal of ciprofloxacin from hospital wastewater using electrocoagulation technique by aluminum electrode: Optimization and modelling through response surface methodology. Process. Saf. Environ. Prot. 2017, 109, 538–547. [Google Scholar] [CrossRef]
- Mohora, E.; Rončević, S.; Agbaba, J.; Tubić, A.; Mitić, M.; Klašnja, M.; Dalmacija, B. Removal of arsenic from groundwater rich in natural organic matter (NOM) by continuous electrocoagulation/flocculation (ECF). Sep. Purif. Technol. 2014, 136, 150–156. [Google Scholar] [CrossRef]
- Sharma, D.; Chaudhari, P.K.; Prajapati, A.K. Removal of chromium (VI) and lead from electroplating effluent using electrocoagulation. Sep. Sci. Technol. 2020, 55, 321–331. [Google Scholar] [CrossRef]
- Bao, J.; Yu, W.J.; Liu, Y.; Wang, X.; Liu, Z.Q.; Duan, Y.F. Removal of perfluoroalkanesulfonic acids (PFSAs) from synthetic and natural groundwater by electrocoagulation. Chemosphere 2020, 248, 125951. [Google Scholar] [CrossRef] [PubMed]
- Castellaños-Estupiñana, M.A.; Sánchez-Galvisa, E.M.; García-Martínezb, J.B.; Barajas-Ferreirab, C.; Zuorroc, A.; Barajas-Solano, A.F. Design of an electroflotation system for the concentration and harvesting of freshwater microalgae. Chem. Eng. 2018, 64. [Google Scholar] [CrossRef]
- Linares-Hernández, I.; Barrera-Díaz, C.; Roa-Morales, G.; Bilyeu, B.; Ureña-Núñez, F. A combined electrocoagulation-sorption process applied to mixed industrial wastewater. J. Hazard. Mater. 2007, 144, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Aouni, A.; Lafi, R.; Hafiane, A. Feasibility evaluation of combined electrocoagulation/adsorption process by optimizing operating parameters removal for textile wastewater treatment. Desalin. Water Treat. 2017, 60, 78–87. [Google Scholar] [CrossRef]
- Shamaei, L.; Khorshidi, B.; Perdicakis, B.; Sadrzadeh, B. Treatment of oil sands produced water using combined electrocoagulation and chemical coagulation techniques. Sci. Total Environ. 2018, 645, 560–572. [Google Scholar] [CrossRef]
- Radić, S.; Vujčić, V.; Cvetković, Z.; Cvjetko, P.; Oreščanin, V. The efficiency of combined CaO/electrochemical treatment in removal of acid mine drainage induced toxicity and genotoxicity. Sci. Total Environ. 2014, 466–467, 84–89. [Google Scholar] [CrossRef]
- Can, O.T.; Kobya, M.; Demirbas, E.; Bayramoglu, M. Treatment of the textile wastewater by combined electrocoagulation. Chemosphere 2006, 62, 181–187. [Google Scholar] [CrossRef]
- Salih Muharam, S.M.; Rahmah, C.I.; Yuningsih, L.M. Simultaneous Combination of Electrocoagulation and Chemical Coagulation Methods for Medical Wastewater Treatment. Makara J. Sci. 2017, 21, 113–118. [Google Scholar]
- Kermet-Said, H.; Moulai-Mostefa, N. Optimization of Turbidity and COD Removal from Pharmaceutical Wastewater by Electrocoagulation. Isotherm Modeling and Cost Analysis. Pol. J. Environ. Stud. 2015, 24, 1049–1061. [Google Scholar]
- Oulebsir, A.; Chaabane, T.; Tounsi, H.; Omine, K.; Sivasankar, V.; Flilissa, A.; Darchen, A. Treatment of artificial pharmaceutical wastewater containing amoxicillin by a sequential electrocoagulation with calcium salt followed by nanofiltration. J. Environ. Chem. Eng. 2020, 8, 104597. [Google Scholar] [CrossRef]
- Padmaja, K.; Cherukuri, J.; Reddy, M.A. A comparative study of the efficiency of chemical coagulation and electrocoagulation methods in the treatment of pharmaceutical effluent. J. Water Process. Eng. 2020, 34, 101153. [Google Scholar] [CrossRef]
- Balark, D.; Chandrika, K.; Attaolahi, M. Assessment of Effective Operational Parameters on Removal of Amoxicillin from Synthetic Wastewater Using Electrocoagulation Process. J. Pharm. Res. Int. 2019. [Google Scholar] [CrossRef] [Green Version]
- Ren, M.; Song, Y.; Xiao, S.; Zeng, P.; Peng, J. Treatment of berberine hydrochloride wastewater by using pulse electro-coagulation process with Fe electrode. Chem. Eng. J. 2011, 169, 84–90. [Google Scholar] [CrossRef]
- Al-qodah, Z.; Tawalbeh, M.; Al-shannag, M.; Al-anber, Z.; Bani-Melhem, K. Combined electrocoagulation processes as a novel approach for enhanced pollutants removal: A state-of-the-art review. Sci. Total Environ. 2020, 744, 140806. [Google Scholar] [CrossRef]
- Hakizimana, J.N.; Gourich, B.; Vial, C.; Drogui, P.; Oumani, A.; Naja, J.; Hilali, L. Assessment of hardness, microorganism and organic matter removal from seawater by electrocoagulation as a pretreatment of desalination by reverse osmosis. Desalination 2016, 393, 90–101. [Google Scholar] [CrossRef]
- Sardari, K.; Fyfe, P.; Wickramasinghe, S.R. Integrated electrocoagulation–Forward osmosis–Membrane distillation for sustainable water recovery from hydraulic fracturing produced water. J. Membr. Sci. 2018, 574, 325–337. [Google Scholar] [CrossRef]
- Asaithambi, P.; Aziz, A.R.A.; Sajjadi, B.; Daud, W.M.A.B.W. Sono assisted electrocoagulation process for the removal of pollutant from pulp and paper industry effluent. Environ. Sci. Pollut. Res. 2017, 24, 5168–5178. [Google Scholar] [CrossRef]
- Maha Lakshmi, P.; Sivashanmugam, P. Treatment of oil tanning effluent by electrocoagulation: Influence of ultrasound and hybrid electrode on COD removal. Sep. Purif. Technol. 2013, 116, 378–384. [Google Scholar] [CrossRef]
- Raschitor, A.; Fernandez, C.M.; Cretescu, I.; Rodrigo, M.A.; Cañizares, P. Sono-electrocoagulation of wastewater polluted with Rhodamine 6G. Sep. Purif. Technol. 2014, 135, 110–116. [Google Scholar] [CrossRef]
- Asaithambi, P. Studies on various operating parameters for the removal of COD from pulp and paper industry using electrocoagulation process. Desalin. Water Treat. 2016, 57, 11746–11755. [Google Scholar] [CrossRef]
- Mousazadeh, M.; Niaragh, E.K.; Usman, M.; Khan, S.U.; Sandoval, M.A.; Al-Qodah, Z.; Khalid, B.K.; Gilhotra, V.; Emamjomeh, M.M. A critical review of state-of-the-art electrocoagulation technique applied to COD-rich industrial wastewaters. Environ. Sci. Pollut. Res. 2021, 1–30. [Google Scholar] [CrossRef]
- Khan, S.U.; Asif, M.; Alam, F.; Khan, N.A.; Farooqi, I.H. Optimizing fluoride removal and energy consumption in a batch reactor using electrocoagulation: A smart treatment technology. In Smart Cities—Opportunities and Challenges; Springer: Singapore, 2020; pp. 767–778. [Google Scholar]
- Ilhan, F.; Kurt, U.; Apaydin, O.; Gonullu, M.T. Treatment of leachate by electrocoagulation using aluminum and iron electrodes. J. Hazard. Mater. 2008, 154, 381–389. [Google Scholar] [CrossRef]
- Kobya, M.; Bayramoglu, M.; Eyvaz, M. Techno-economical evaluation of electrocoagulation for the textile wastewater using different electrode connections. J. Hazard. Mater. 2007, 148, 311–318. [Google Scholar] [CrossRef]
- Khan, S.U.; Islam, D.T.; Farooqi, I.H.; Ayub, S.; Basheer, F. Hexavalent chromium removal in an electrocoagulation column reactor: Process optimization using CCD, adsorption kinetics and pH modulated sludge formation. Process. Saf. Environ. Prot. 2019, 122, 118–130. [Google Scholar] [CrossRef]
- Kobya, M.; Demirbas, E.; Bayramoglu, M.; Sensoy, M.T. Optimization of Electrocoagulation Process for the Treatment of Metal Cutting Wastewaters with Response Surface Methodology. Water Air Soil Pollut. 2011, 215, 399–410. [Google Scholar] [CrossRef]
- Espinoza-Quiñones, F.R.; Fornari, M.M.T.; Módenes, A.N.; Palácio, S.M.; Fernando, G.; da Silva, F.G., Jr.; Szymanski, N.; Kroumov, A.D.; Trigueros, D.E.G. Pollutant removal from tannery effluent by electrocoagulation. Chem. Eng. J. 2009, 151, 59–65. [Google Scholar] [CrossRef]
- Khaled, B.; Wided, B.; Béchir, H.; Elimame, E.; Mouna, L.; Zied, T. Investigation of electrocoagulation reactor design parameters effect on the removal of cadmium from synthetic and phosphate industrial wastewater. Arab. J. Chem. 2019, 12, 1848–1859. [Google Scholar] [CrossRef]
- Merzouk, B.; Gourich, B.; Madani, K.; Vial, C.; Sekki, A. Removal of a disperse red dye from synthetic wastewater by chemical coagulation and continuous electrocoagulation. A comparative study. Desalination 2011, 272, 246–253. [Google Scholar] [CrossRef]
- Chafi, M.; Gourich, B.; Essadki, A.H.; Vial, C.; Fabregat, A. Comparison of electrocoagulation using iron and aluminium electrodes with chemical coagulation for the removal of a highly soluble acid dye. Desalination 2011, 281, 285–292. [Google Scholar] [CrossRef]
- Oncel, M.S.; Muhcu, A.; Demirbas, E.; Kobya, M. A comparative study of chemical precipitation and electrocoagulation for treatment of coal acid drainage wastewater. J. Environ. Chem. Eng. 2013, 1, 989–995. [Google Scholar] [CrossRef]
- Bayramoglu, M.; Kobya, M.; Can, O.T.; Sozbir, M. Operating cost analysis of electrocoagulation of textile dye wastewater. Sep. Purif. Technol. 2004, 37, 117–125. [Google Scholar] [CrossRef]
- Bayramoglu, M.; Eyvaz, M.; Kobya, M. Treatment of the textile wastewater by electrocoagulation: Economical evaluation. Chem. Eng. J. 2007, 128, 155–161. [Google Scholar] [CrossRef]
- Kobya, M.; Ciftci, C.; Bayramoglu, C.; Sensoy, M.T. Study on the treatment of waste metal cutting fluids using electrocoagulation. Sep. Purif. Technol. 2008, 60, 285–291. [Google Scholar] [CrossRef]
- Drogui, P.; Meunier, N.; Mercier, G.; Blais, J.F. Removal of Pb2+ and Zn2+ ions from acidic soil leachate: A comparative study between electrocoagulation, adsorption and chem. Int. J. Environ. Waste Manag. 2011, 8, 241–257. [Google Scholar] [CrossRef]
- Walter, D.; Chihpin, H. Electrocoagulation of Silica Nanoparticles in Wafer Polishing Wastewater by a Multichannel Flow Reactor: A Kinetic Study. J. Environ. Eng. 2006, 132, 1651–1658. [Google Scholar]
- Calvo, L.S.; Leclerc, J.P.; Tanguy, G.; Cames, M.C.; Paternotte, G.; Valentin, G.; Rostan, A.; Lapicque, F. An electrocoagulation unit for the purification of soluble oil wastes of high COD. Environ. Prog. 2003, 22, 57–65. [Google Scholar] [CrossRef]
- Pulkka, S.; Martikainen, M.; Bhatnagar, A.; Sillanpaa, M. Electrochemical methods for the removal of anionic contaminants from water–A review. Sep. Purif. Technol. 2014, 132, 252–271. [Google Scholar] [CrossRef]
- Mateen, Q.S.; Khan, S.U.; Islam, D.T.; Khan, N.A.; Farooqi, I.H. Copper (II) removal in a column reactor using electrocoagulation: Parametric optimization by response surface methodology using central composite design. Water Environ. Res. 2020, 92, 1350–1362. [Google Scholar] [PubMed]
- Fernandes, A.; Chamem, O.; Pacheco, M.J.; Ciríaco, L.; Zairi, M.; Lopes, A. Performance of Electrochemical Processes in the Treatment of Reverse Osmosis Concentrates of Sanitary Landfill Leachate. Molecules 2019, 24, 2905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominguez-Ramos, A.; Aldaco, R.; Irabien, A. Photovoltaic solar electrochemical oxidation (PSEO) for treatment of lignosulfonate wastewater. J. Chem. Technol. Biotechnol. 2010, 85, 821–830. [Google Scholar] [CrossRef]
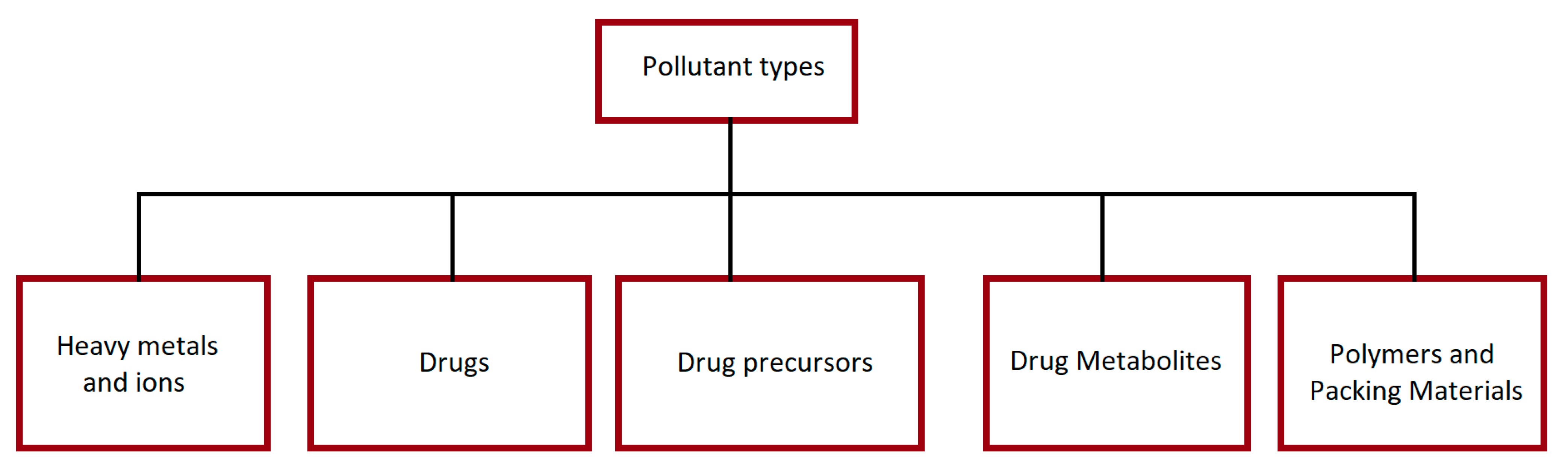

| Drugs | Chemical Class | Pharmacological Class |
|---|---|---|
| Citalopram | SSRIs | Antidepressant |
| Cocaine | Tropane alkaloid | CNS stimulant/narcotic |
| Ibuprofen | Propionic acid derivative | NSAIDs |
| Propranolol | Beta blocker | Antihypertensive |
| Clotrimazole | Imidazoles | Antifungal |
| Diclofenac | Acetic acid derivative | NSAIDs |
| Metoprolol | Beta blocker | Antihypertensive |
| Indomethacin | Indole derivative | NSAIDs |
| Atenolol | Beta blocker | Antihypertensive |
| Paracetamol | Para-aminophenol derivative | NSAIDs |
| Ranitidine | H2 receptor blocker | Antihistaminic |
| Gemfibrozil | Fibric acid derivative | Lipid and cholesterol regulating |
| Sulfadiazine | Sulfonamide | Antibiotic |
| Clofibric acid | Clofibrate metabolite | Lipid and cholesterol regulating |
| Norfloxacin | Fluoroquinolone | Antibiotic |
| Carbamazepine | Tricyclic anti-depressant | Psychiatric/Anticonvulsant |
| Amoxicillin | Penicillin | Antibiotic |
| sulfamethoxazole | Sulfonamide | Antibiotic |
| Chloramphenicol | Amphenicol-class antibacterial | Antibiotic |
| Ofloxacin | Fluoroquinolone | Antibiotic |
| Trimethoprim | Aminopyrimidine | Antibiotic |
| Ciprofloxacin | Fluoroquinolone | Antibiotic |
| Fibrates | Amphipathic carboxylic acid | Blood and lipid regulating |
| Parameter | References | ||||||
|---|---|---|---|---|---|---|---|
| [45] | [46] | [47] | [48] | [49] | [50] | [51] | |
| BOD (mg/L) | 120 | 304 | 900 | 22,000 | 200 | - | 263–330 |
| COD (mg/L) | 490 | 420 | 4000 | 34,400 | 1753 | - | 2565–28,640 |
| pH | 6.9 | 7 | 5.2 | 7.2 | 7.3 | 5.65–6.89 | 5.8–6.9 |
| TSS (mg/L) | 370 | 57 | 68 | 6250 | - | 29.67–123.03 | 761–1202 |
| TDS (mg/L) | 1550 | - | - | - | - | 136.33–193.05 | 1443–3788 |
| TS (mg/L) | 1920 | 484 | - | 29,150 | - | - | - |
| Ammonium nitrogen (mg/L) | - | 52 | - | - | 220 | - | - |
| TP (mg/L) | - | 7.5 | 1.7 | - | 17 | - | - |
| Chloride (mg/L) | - | 132 | - | - | 4.2 | - | - |
| Turbidity (NTU) | - | - | 18 | - | - | 17.22–28.78 | - |
| Conductivity (mS cm−1) | - | - | 0.5 | - | 20 | 157–119.36 | - |
| Temperature (°C) | - | 22 | 25 | 29–36 | - | 32–46 | 31–34 |
| Soluble Pollutants in WW | Mechanism of Removal | References |
|---|---|---|
| Organic Compounds | Complexation, co-precipitation | [109] |
| Phosphate Anions | Precipitation, Adsorption, Complexation | [110] |
| Sulphide Anions | Precipitation | [111] |
| Calcium Cations | Co–precipitation | [112] |
| Fluoride Anions | Complexation, Precipitation | [113] |
| Source of Wastewater | Pharmaceutical Contaminants | Experimental Conditions | %Removal Efficiency | References |
|---|---|---|---|---|
| Pharmaceutical Wastewater | Oxytetracycline Hydrochloride | Iron/aluminium anode (70 × 50 mm); Stainless steel cathode (70 × 50 mm); The gap between electrodes: 5 cm; 20 mAcm² current density; Time to react: 120 min; 0.19 kWh/L power consumption; 200–50 mg/L as a starting concentration. | 82.96–93.17 | [134] |
| Hospital Wastewater | Ciprofloxacin (CIP) | Aluminium anode and cathode; pH- 7.78; Inter-electrode distance: 1 cm; Time to react: 20 min; 12.5 mAcm² current density; 32.5 mg/L1 as a starting point. | 88.57 | [148] |
| Pharmaceutical Wastewater | COD | Aluminium anode and cathode (150 cm²); Distance between electrodes: 25 mm; pH: 3–9; 1.7–1.9 mAcm² current density; The electrical voltage is 40 volts. 60-min response time. | 34.2 | [21] |
| Water Containing Heavy Metals | Arsenic | Aluminium (both the anode and the cathode); Bipolar electrode configuration. | 93 | [149] |
| Chromium (VI)- Pb | Electrodes SS–SS; Current density (A/m2) 73.5; pH 3.5; Content: 55.3–3.5 [mg/L]; Electrodes SS–SS; Current density (A/m²) 73.5; pH 3.5; | 91.7–91.3 | [150] | |
| Perfluorobutane sulfonate (PFBS) Perfluorohexane sulfonate (PFHxS) Perfluorooctane sulfonate (PFOS) Perfluoroalkane sulfonic acids (PFSAs) | Electrode: Al-Zn Voltage supply:12v pH: 7 | 87.4 95.6 100 100 | [151] |
| Type of Wastewater | Electrode Material(Anode-Cathode) | Optimal Current Density | Mode of Electricity Application/Type | Electrode Arrangement | Electrodes Spacing | Initial Concentration | Initial pH and Temperature | Treatment Time | Pollutant Removal | Source |
|---|---|---|---|---|---|---|---|---|---|---|
| Synthetic PWW | Al-Fe | 0.5 mA/cm2 | Intermittent (5 min ON/20 min OFF) | - | 5 cm | 10 mg/L | pH = 7.5, T = 25 °C | 38 h | Diclofenac = 90%, carbamazepine (CBZ) = 70%, Amoxicillin (AMX) = 77% | [24] |
| Pharmaceutical industry wastewater | Fe-Fe | 15 mA/cm2 | Combined EC (EC/electro-Fenton) | MP-P | 2 cm | COD = 4000 mg/L, TOC = 1200 mg/L, BOD = 900 mg/L | pH = 7 T = 25 °C | 2 h | COD = 70.2%, TOC = 64%, BOD = 97% | [47] |
| Pharmaceutical effluents | Al-Al | 46.83 mA/ cm2 | Continuous electricity application | - | 1 cm | Conductivity = 784 µS/cm, turbidity = 784 NTU, COD = 525 mg/L | pH = 5.31, | 18 min | Turbidity = 96.7%, COD = 70.8% | [159] |
| Synthetic PWW (Oxytetracycline hydrochloride) | Al-Al Fe-Fe | 20 mA/ cm2 | Continuous electricity application | MP-P | 5 cm | 50 mg/L | - | 120 min | 87.75% 93.20% | [134] |
| Drug industry | Al-Al | 80 A/m2 | Combined EC (EC/anaerobic process) | - | 1 cm | COD = 34,400 mg/L, BOD = 22,000 mg/L | pH = 7.2 | 25 min | COD = 24%, BOD = 35%, Colour = 70.25% | [48] |
| Pharmaceutical factory wastewater | Fe-Fe | 763 A/m2 | Combined EC (EC/photocatalysis) | MP-P | 2 cm | - | pH = 6.0, T = 25 °C | 90 min | Turbidity = 91%, COD = 86% | [49] |
| Artificial PWW (Amoxicillin) | Al-Al | 0.7 A | Combined EC (EC/nanofiltration) | MP-P | 1 cm | 50 mg/L | pH = 2.5, T = 25 °C | 60 min | 52.7% | [160] |
| Pharmaceutical effluent | Fe-Al | 0.04 A | Continuous electricity application | - | 1 cm | COD = 7692 mg/L, TDS = 16,290 mg/L, chloride = 9017 mg/L | T = 25–27 °C | 15 min | COD = 92.3%, TDS = 91.5% | [161] |
| Synthetic PWW (Amoxicillin) | Al-Al | - | Continuous electricity | BP-S | 2.5 cm | 10 mg/L | pH = 7 | 75 min | 98.8% | [162] |
| Berberine hydrochloride (BH) wastewater | Fe-Fe | 19.44 mA/cm2 | Pulse EC | MP-P | 2 cm | BH = 1500 mg/L | pH = 7 | 3.5 h | BH = 72.8% | [163] |
| Type of Wastewater | Initial Conc. | Electrode Material | Removal Efficiency | Current Density | Operating Cost | Reference |
|---|---|---|---|---|---|---|
| Synthetic Wastewater | 100 mg/L | Al | 80–95% | 208–310 A/m2 | 0.34–0.52 USD/kg dye | [179] |
| Synthetic Wastewater | 50 mg/L | Al Fe | 87.5–93.4% 90.7–98.1% | 155–350 A/m2 155–350 A/m2 | 7.04–17.4 USD /kg dye 4.01–13.8 USD/kg dye | [180] |
| Coal Mine Drainage | _ | Fe | 28.7–99.96% | 200–500% A/M2 | 1.09–2.184 USD/m3 | [181] |
| Textile Dye Wastewater | 3422 mg/L COD | Al Fe | 15–62% 57–78% | 50–200 A/m2 50–200 A/m² | 0.32–0.58 USD/kg COD 0.7–0.175 USD/kg COD | [182] |
| Textile Dye Wastewater | 2031 mg/L COD | Al (MP-P) Fe (MP-P) | - - | 30–60 A/m2 30–60 A/m2 | 0.4–0.65 USD/m3 0.25–0.4 USD/m3 | [183] |
| Wastewater from Metal Industries | 3155 mg/L TOC, 17,312 mg/L COD | Al Fe | 93% COD 92% COD | 60 A/m2 60 A/m2 | 0.768 USD/m3 0.479 USD/m3 | [184] |
| Metal Removal from Soil Leachate | - | Fe | 99.4% Zn2+ 99.7% pb2+ | 68 A/m2 | 35.38 USD tst-1 | [185] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, R.; Sheob, M.; Saeed, B.; Khan, S.U.; Shirinkar, M.; Frontistis, Z.; Basheer, F.; Farooqi, I.H. Use of Electrocoagulation for Treatment of Pharmaceutical Compounds in Water/Wastewater: A Review Exploring Opportunities and Challenges. Water 2021, 13, 2105. https://doi.org/10.3390/w13152105
Alam R, Sheob M, Saeed B, Khan SU, Shirinkar M, Frontistis Z, Basheer F, Farooqi IH. Use of Electrocoagulation for Treatment of Pharmaceutical Compounds in Water/Wastewater: A Review Exploring Opportunities and Challenges. Water. 2021; 13(15):2105. https://doi.org/10.3390/w13152105
Chicago/Turabian StyleAlam, Rahat, Mohd Sheob, Bilal Saeed, Saif Ullah Khan, Maryam Shirinkar, Zacharias Frontistis, Farrukh Basheer, and Izharul Haq Farooqi. 2021. "Use of Electrocoagulation for Treatment of Pharmaceutical Compounds in Water/Wastewater: A Review Exploring Opportunities and Challenges" Water 13, no. 15: 2105. https://doi.org/10.3390/w13152105
APA StyleAlam, R., Sheob, M., Saeed, B., Khan, S. U., Shirinkar, M., Frontistis, Z., Basheer, F., & Farooqi, I. H. (2021). Use of Electrocoagulation for Treatment of Pharmaceutical Compounds in Water/Wastewater: A Review Exploring Opportunities and Challenges. Water, 13(15), 2105. https://doi.org/10.3390/w13152105








