Diversity, Distribution, and Habitat Occurrence of the Diaptomid Copepods (Crustacea: Copepoda: Diaptomidae) in Freshwater Ecosystems of Thailand
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
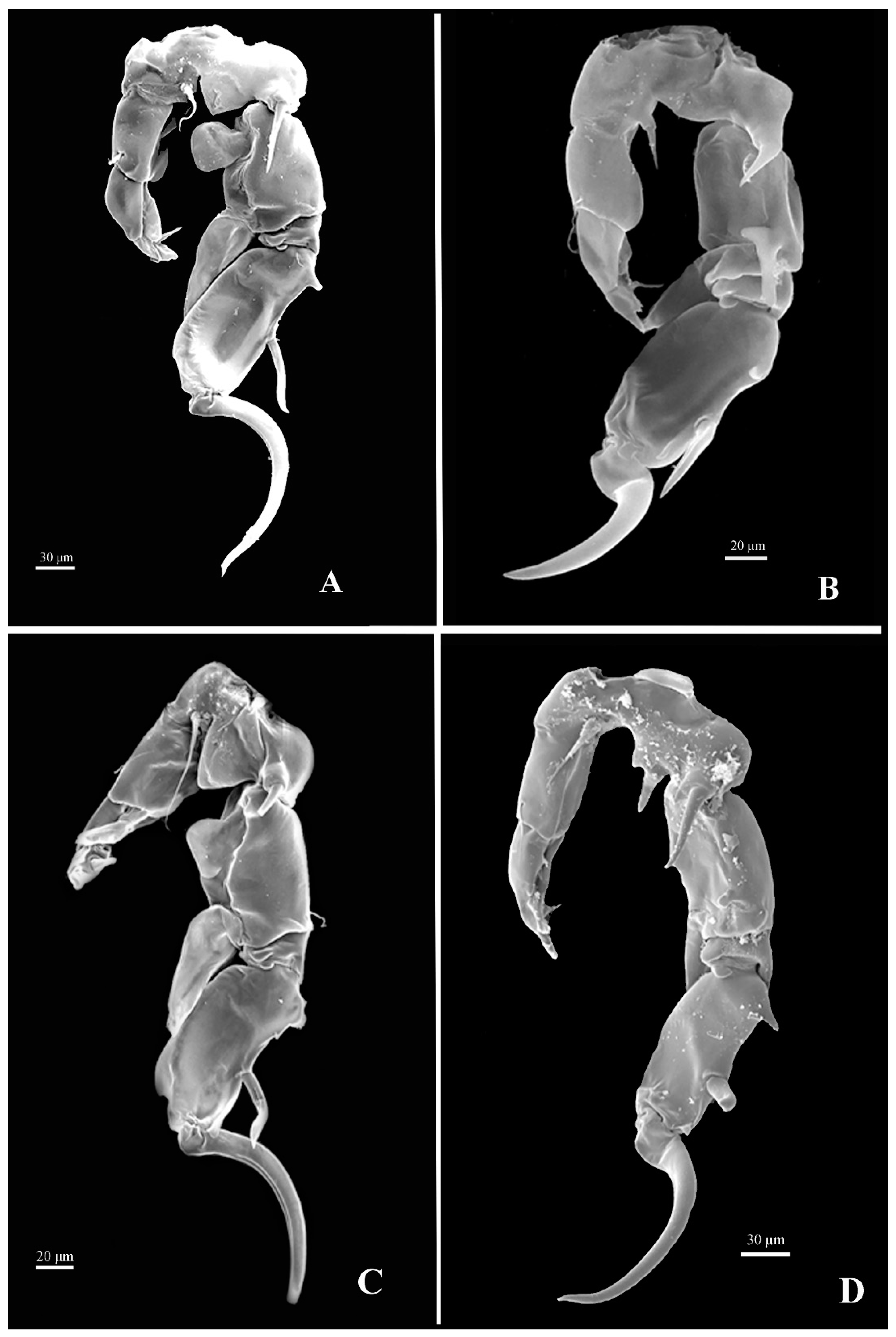
2.2. Sample Collections and Identifications
2.3. Data Analysis
3. Results
3.1. Species Diversity
3.2. Relative Occurrences and Distribution
3.3. Taxonomic Checklist
3.3.1. Genus Allodiaptomus Kiefer, 1936
3.3.2. Genus Arctodiaptomus Kiefer, 1932
3.3.3. Genus Dentodiaptomus Shen and Tai, 1964
3.3.4. Genus Eodiaptomus Kiefer, 1932
3.3.5. Genus Heliodiaptomus Kiefer, 1932
3.3.6. Genus Mongolodiaptomus Kiefer, 1937
3.3.7. Genus Neodiaptomus Kiefer, 1932
3.3.8. Genus Phyllodiaptomus Kiefer, 1936
3.3.9. Genus Tropodiaptomus Kiefer, 1932
3.3.10. Genus Vietodiaptomus Dang, 1977
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dussart, B.; Defaye, D. World Directory of Crustacea Copepoda of Inland Waters, I-Calaniformes; Backhuys Publishers: Leiden, The Netherlands, 2002; pp. 1–276. [Google Scholar]
- Boxshall, G.A.; Defaye, D. Global diversity of copepods (Crustacea: Copepoda) in freshwater. Hydrobiologia 2008, 595, 195–207. [Google Scholar] [CrossRef]
- Lai, H.C.; Fernando, C.H. Zoogeographical distribution of Southeast Asian freshwater Calanoida. Hydrobiologia 1980, 74, 53–66. [Google Scholar] [CrossRef]
- Dumont, H.J.; Ranga Reddy, Y. Phyllodiaptomus praedictus n. sp. (Copepoda, Calanoida) from Thailand. Hydrobiologia 1994, 273, 101–110. [Google Scholar] [CrossRef]
- Dumont, H.J.; Ranga Reddy, Y.; Sanoamuang, L. Description of Phyllodiaptomus christineae n. sp. from Thailand, and distinction of two subgenera within Phyllodiaptomus Kiefer, 1936 (Copepoda, Calanoida). Hydrobiologia 1996, 323, 139–148. [Google Scholar] [CrossRef]
- Ranga Reddy, Y.; Dumont, H.J. A review of the genus Eodiaptomus Kiefer, 1932, with the description of E. sanuamuangae n. sp. from Thailand, and a redescription of E. lumholtzi (Sars, 1889) from Australia (Copepoda, Calanoida). Hydrobiologia 1998, 361, 169–189. [Google Scholar] [CrossRef]
- Ranga Reddy, Y.; Sanoamuang, L.; Dumont, H.J. A note on the Diaptomidae of Thailand, including redescription of three species and description of a new species (Copepoda, Calanoida). Hydrobiologia 1998, 361, 201–223. [Google Scholar] [CrossRef]
- Ranga Reddy, Y.; Sanoamuang, L.; Dumont, H.J. Amended delimitation of Mongolodiaptomus against Neodiaptomus and Allodiaptomus and redescription of the little known Mongolodiaptomus uenoi (Kikuchi, 1936) from Thailand (Copepoda: Calanoida: Diaptomidae). Hydrobiologia 2000, 418, 99–109. [Google Scholar] [CrossRef]
- Sanoamuang, L. Species composition and distribution of freshwater Calanoida and Cyclopoida (Copepoda) of north-east Thailand. In Crustaceans and Biodiversity Crisis; Schram, F.R., Klein, J.V.C., Eds.; Brill Academic Publishers: Leiden, The Netherlands, 1999; pp. 217–230. [Google Scholar]
- Sanoamuang, L. Distributions of three Eodiaptomus species (Copepoda: Calanoida) in Thailand, with a redescription of E. draconisignivomi Brehm, 1952. Hydrobiologia 2001, 453, 565–576. [Google Scholar] [CrossRef]
- Sanoamuang, L. Eodiaptomus phuphanensis, n. sp., a new freshwater copepod (Calanoida: Diaptomidae) from the Phu Phan National Park, Thailand. Int. Rev. Hydrobiol. 2001, 86, 219–228. [Google Scholar] [CrossRef]
- Sanoamuang, L. Mongolodiaptomus dumonti n. sp., a new freshwater copepod (Calanoida, Diaptomidae) from Thailand. Hydrobiologia 2001, 448, 41–52. [Google Scholar] [CrossRef]
- Sanoamuang, L. Heliodiaptomus phuthaiorum n. sp., a new freshwater copepod (Calanoida, Diaptomidae) from temporary ponds in northeast Thailand. Int. Rev. Hydrobiol. 2004, 89, 392–406. [Google Scholar] [CrossRef]
- Sanoamuang, L.; Yindee, W. A new species of Phyllodiaptomus (Copepoda, Diaptomidae) from northeast Thailand. Crustaceana 2001, 74, 435–448. [Google Scholar]
- Sanoamuang, L.; Athibai, S. A new species of Neodiaptomus (Copepoda, Diaptomidae) from temporary waters in northeast Thailand. Hydrobiologia 2002, 489, 71–82. [Google Scholar] [CrossRef]
- Sanoamuang, L.; Sivongxay, N. Description of Eodiaptomus phuvongi, n. sp. (Copepoda, Calanoida) from Thailand and Laos. Crustaceana 2004, 77, 1223–1236. [Google Scholar] [CrossRef]
- Sanoamuang, L.; Teeramaethee, J. Phyllodiaptomus thailandicus, a new freshwater copepod (Copepoda, Calanoida, Diaptomidae) from Thailand. Crustaceana 2006, 79, 475–487. [Google Scholar]
- Proongkiat, I.; Sanoamuang, L. Description of Neodiaptomus siamensis, a new diaptomid copepod (Copepoda, Calanoida) from temporary pools in northern Thailand. Crustaceana 2008, 81, 177–189. [Google Scholar]
- Watiroyram, S.; Sanoamuang, L. A new species of Mongolodiaptomus Kiefer, 1938 from northeast Thailand and a key to the species (Crustacea: Copepoda, Calanoida, Diaptomidae). Zookeys 2017, 710, 15–32. [Google Scholar] [CrossRef]
- Sanoamuang, L.; Watiroyram, S. Mongolodiaptomus mekongensis, a new species of copepod (Copepoda, Calanoida, Diaptomidae) from temporary waters in the floodplain of the lower Mekong River Basin. Raffles Bull. Zool. 2018, 66, 782–796. [Google Scholar]
- Sanoamuang, L.; Watiroyram, S. Phyllodiaptomus (Phyllodiaptomus) roietensis, a new diaptomid copepod (Copepoda: Calanoida) from temporary waters in Thailand and Cambodia, with a key to the species. ZooKeys 2020, 911, 1–20. [Google Scholar] [CrossRef]
- Saetang, T.; Sanoamuang, L.; Maiphae, S. A new species of genus Tropodiaptomus Kiefer, 1932 (Crustacea: Copepoda: Calanoida: Diaptomidae) from Thailand. J. Nat. Hist. 2020, 54, 2297–2322. [Google Scholar] [CrossRef]
- Brehm, V. Cladocera and Copepoda Calanoida von Cambodja. Cybium 1951, 6, 95–124. [Google Scholar]
- Sanoamuang, L.; Watiroyram, S. Allodiaptomus nongensis, a new diaptomid copepod (Copepoda: Calanoida) from a tributary of the Mekong River, with notes on its consumption by local people in Central Laos. J. Limnol. 2019, 78, 185–200. [Google Scholar] [CrossRef]
- Lim, R.P.; Lai, H.C. Crustacea: Copepoda, Calanoida. In Freshwater Invertebrates of the Malaysian Region; Yule, C.M., Sen, Y.H., Eds.; Academy of Sciences Malaysia: Kuala Lumpur, Malaysia, 2014; pp. 254–266. [Google Scholar]
- Umi, W.A.D.; Yusoff, F.M.; Aris, A.Z.; Sharip, Z.; Sinev, A.Y. Planktonic microcrustacean community structure varies with trophic status and environmental variables in tropical shallow lakes in Malaysia. Diversity 2020, 12, 322. [Google Scholar] [CrossRef]
- Alekseev, V.R.; Yusoff, F.M.; Fefilova, E.B. Continental copepod biodiversity in North-Eastern Borneo, Malaysia. Arthropoda Sel. 2016, 25, 187–197. [Google Scholar] [CrossRef]
- Schmoker, C.; Mahjoub, M.S.; Calbet, A.; Hsiao, S.H.; Russo, F.; Larsen, O.; Trottet, A.; Drillet, G. A review of the zooplankton in Singapore waters. Raffles Bull. Zool. 2014, 62, 726–749. [Google Scholar]
- Mamaril, A.C. Zooplankton diversity in Philippine lakes. In Conservation and Ecological Management of Philippine Lakes in Relation to Fisheries and Aquaculture; Santiago, C.B., Cuvin-Aralar, M.L., Basiao, Z.U., Eds.; Southeast Asian Fisheries Development Center, Aquaculture Department: Iloilo, Philippines; Philippine Council for Aquatic and Marine Research and Development: Los Baños, Laguna; Bureau of Fisheries and Aquatic Resources: Quezon City, Philippines, 2001; pp. 81–93. [Google Scholar]
- Lopez, M.L.D.; Pascual, J.A.F.; Dela Paz, E.S.P.; Rizo, E.Z.C.; Tordesillas, D.T.; Guinto, S.K.; Han, B.; Dumont, H.J.; Mamaril, A.C.; Papa, R.D.S. Annotated checklist and insular distribution of freshwater microcrustaceans (Copepoda: Calanoida & Cyclopoida; Cladocera: Anomopoda & Ctenopoda in the Philippines. Raffles Bull. Zool. 2017, 65, 623–654. [Google Scholar]
- Alekseev, V.R.; Haffner, D.G.; Vaillant, J.J.; Yusoff, F.M. Cyclopoid and calanoid copepod biodiversity in Indonesia. J. Limnol. 2013, 72, 245–274. [Google Scholar] [CrossRef]
- Tran, D.L.; Dang, N.T.; Ho, T.H. An annotated checklist of the family Diaptomidae Sars, 1903 (Copepoda, Calanoida) in Vietnam. Tap Chi Sinh Hoc. 2016, 38, 384–399. [Google Scholar] [CrossRef]
- Bricker, K.S.; Wongrat, L.; Gannon, J.E. Composition and distribution of Crustacean plankton in twelve inland water bodies of Thailand. J. Fish. Environ. 1978, 10, 1–14. [Google Scholar]
- Lai, H.C.; Fernando, C.H. The freshwater Calanoida (Crustacea: Copepoda) of Thailand. Hydrobiologia 1981, 76, 161–178. [Google Scholar] [CrossRef]
- Boonsom, J. The freshwater zooplankton of Thailand (Rotifera and Crustacea). Hydrobiologia 1984, 113, 223–229. [Google Scholar] [CrossRef]
- Wikipedia. Regions of Thailand, Wikimedia Foundation. Available online: https://en.wikipedia.org/wiki/RegionsofThailand (accessed on 26 June 2021).
- Kiefer, F. Indische Ruderfusskrebse (Crustacea, Copepoda). Zool. Anz. 1936, 113, 136–142. [Google Scholar]
- Li, H.; Dumont, H.J.; Han, B.P.; Lin, Q. Updated checklist and distribution of the diaptomid copepods (Copepoda, Calanoida, Diaptomidae) of China. Crustaceana 2018, 91, 335–352. [Google Scholar] [CrossRef]
- Tordesillas, D.T.; Paredes, P.M.F.; Villaruel, K.P.E.; Queneri, C.A.A.M.; Rico, J.L.; Ban, S.; Papa, R.D.S. Effects of food concentration on the reproductive capacity of the invasive freshwater calanoid copepod Arctodiaptomus dorsalis (Marsh, 1907) in the Philippines. J. Crust. Biol. 2018, 38, 101–106. [Google Scholar] [CrossRef]
- Reid, J.W. Arctodiaptomus dorsalis (Marsh): A case history of copepod dispersal. Banisteria 2007, 1860, 3–18. [Google Scholar]
- Papa, R.D.S.; Li, H.; Tordesillas, D.T.; Han, B.; Dumont, H.J. Massive invasion of Arctodiaptomus dorsalis (Copepoda, Calanoida, Diaptomidae) in Philippine lakes: A threat to Asian zooplankton biodiversity? Biol. Invasions 2012, 14, 2471–2478. [Google Scholar] [CrossRef]
- Grochmalicki, J. Beitrag zur Kenntnis der süsswassefauna Javas: Phyllopoda, Copepoda und Ostracod. Bulletin International de l’Académie des Sciences de Cracovie, Classe des Sciences Mathématiques et Naturelles. Serie B Sci. Nat. 1915, 1915, 217–242. [Google Scholar]
- Shen, C.J.; Tai, A.Y. Descriptions of eight new species of freshwater copepods chiefly from the Pearl River delta, south China. Acta Zootaxo. Sin. 1964, 16, 225–239. [Google Scholar]
- Sanoamuang, L.; Watiroyram, S. New species of copepod (Copepoda, Calanoida) from the floodplain of the lower Mekong River Basin in Thailand and Cambodia, with amended diagnosis of the genus Dentodiaptomus Shen & Tai, 1964. Raffles Bull. Zool. 2021, in press. [Google Scholar]
- Brehm, V. Vorläufige Mitteilung über einen neuen Eodiaptomus aus Hinterindien. Anz. Öst. Akad. Wiss. Wien. 1952, 89, 215–217. [Google Scholar]
- Chaicharoen, R. Diversity and Distribution of Freshwater Calanoid and Cyclopoid Copepods in 7 Provinces of Cambodia. Ph.D. Thesis, Graduate School, Khon Kaen University, Khon Kaen, Thailand, 2011; pp. 1–216. [Google Scholar]
- Sanoamuang, L. Freshwater Zooplankton: Calanoid Copepods in Thailand; Klangnanatham Publishers: Khon Kaen, Thailand, 2002; pp. 1–159. [Google Scholar]
- Mashiko, K. Studies of the freshwater plankton of central China. II. Sci Rep. Kanazawa Univ. 1951, 1, 137–154. [Google Scholar]
- Kiefer, F. Beiträge zur Copepodenkunde (XVII). Zool. Anz. 1935, 109, 88–93. [Google Scholar]
- Ranga Reddy, Y.; Dumont, H.J. Redescription of Heliodiaptomus elegans Kiefer, 1935, a rare south-east Asian calanoid copepod. Hydrobiologia 1999, 394, 145–152. [Google Scholar] [CrossRef]
- Gurney, R. On some Freshwater Entomostraca from Ceylon. Proc. Zool. Soc. Lond. 1916, 86, 333–343. [Google Scholar] [CrossRef]
- Ranga Reddy, Y. Copepoda: Calanoida: Diaptomidae. Key to the Genera Heliodiaptomus, Allodiaptomus, Neodiaptomus, Phyllodiaptomus, Eodiaptomus, Arctodiaptomus and Sinodiaptomus; SPB Academic: The Hague, Philippines, 1994; pp. 1–221. [Google Scholar]
- Kiefer, F. Zwei neue Ruderfusskrebse (Crustacea, Copepoda) aus Malaysia. Beitr. Naturk. Forsch. Südwestdeutschl. 1974, 33, 219–222. [Google Scholar]
- Shen, C.J.; Tai, A.Y. Descriptions of six new species of freshwater copepods chiefly from the Pearl River delta, South China. Acta Zool. Sin. 1965, 2, 126–140. [Google Scholar]
- Lai, H.C.; Fernando, C.H. The freshwater Calanoida (Crustacea: Copepoda) of Singapore and peninsular Malaysia. Hydrobiologia 1978, 61, 113–127. [Google Scholar] [CrossRef]
- Lai, H.C.; Fernando, C.H. Redescription of Neodiaptomus botulifer Kiefer and one of its related species. Hydrobiologia 1978, 59, 229–235. [Google Scholar] [CrossRef]
- Kikuchi, K. A new species of Diaptomus from Formosa. Proc. Imp. Acad. Jpn. 1936, 12, 198–200. [Google Scholar] [CrossRef]
- Kiefer, F. Eine neue Diaptomidenart aus Malaysia (Crustacea, Copepoda, Calanoida). Zool. Anz. 1974, 192, 420–424. [Google Scholar]
- Kiefer, F. Zwei neue Diaptomiden (Copepoda, Calanoida) aus Indien. Zool. Anz. 1932, 100, 265–270. [Google Scholar]
- Ranga Reddy, Y. Neodiaptomus meggitti Kiefer, 1932. A rare south-east Asian species from the Andaman Islands, India (Copepoda, Calanoida, Diaptomidae). Crustaceana 2000, 73, 257–272. [Google Scholar] [CrossRef]
- Poppe, S.A.; Richard, J. Description du Diaptomus schmackeri n. sp., recueilli par M. Schmacker dans le lac Tahoo (Chine). Bull. Soc. Zool. Fr. 1892, 17, 149–151. [Google Scholar]
- Walter, T.C.; Boxshall, G. World of Copepods Database. Neodiaptomus Kiefer. 1932. Available online: http://www.marinespecies.org/copepoda/aphia.php?p=taxdetails&id=347568 (accessed on 29 June 2021).
- Ranga Reddy, Y. Neodiaptomus prateek n. sp., a new freshwater copepod from Assam, India, with critical review of the generic assignment of Neodiaptomus spp. and a note on diaptomid species richness (Calanoida: Diaptomidae). J. Crust. Biol. 2013, 33, 849–865. [Google Scholar] [CrossRef]
- Alfonso, G.; Russo, R.; Belmonte, G. First record of the Asian diaptomid Neodiaptomus schmackeri (Poppe & Richard, 1892) (Crustacea: Copepoda: Calanoida) in Europe. J. Limnol 2014, 73, 584–592. [Google Scholar]
- Krupa, E.; Aubakirova, M. Checklist and distribution of Calanoida (Crustacea: Copepoda) in Kazakhstan (Central Asia). Water 2021, 13, 2015. [Google Scholar] [CrossRef]
- Kiefer, F. Neue ruderfüßkrebse von den Sunda-Inseln. Zool. Anz. 1930, 86, 185–189. [Google Scholar]
- Lim, R.P.; Fernando, C.H. A review of Malaysian freshwater Copepoda with notes on new records and little known species. Hydrobiologia 1985, 128, 71–89. [Google Scholar] [CrossRef]
- Ranga Reddy, Y. Tropodiaptomus signatus Kiefer, 1982, a little-known species from Loktak Lake, Manipur State, India (Copepoda, Calanoida, Diaptomidae). Crustaceana 2013, 86, 1675–1688. [Google Scholar] [CrossRef]
- Kiefer, F. Vergleichende Untersuchungen über Morphologie, Taxonomie und geographische Verbreitung der Arten der Gattung Tropodiaptomus Kiefer (Copepoda: Calaoida) aus asiatischen Binnengewässern. Hydrobiologia 1982, 93, 223–253. [Google Scholar] [CrossRef]
- Teeramaethee, J. Species Diversity and Abundance of Rotifers, Cladocerans and Copepods in Two Wetlands: Bueng Boraphet, Nakhon Sawan Province and Bueng Khong Long, Nong Khai Province. Ph.D. Thesis, Graduate School, Khon Kaen University, Khon Kaen, Thailand, 2006; pp. 1–161. [Google Scholar]
- Kiefer, F. Süsswassercopoden aus Ostasien. II. Neue Diaptomiden und Cyclopiden von der Insel Formosa. Zool. Anz. 1937, 119, 58–64. [Google Scholar]
- Kiefer, F. Beitrage zur Copepodenkunde. 38. Zur Kenntnis des Diaptomus orientalis Brady-Sars. Zool. Anz. 1930, 87, 120–122. [Google Scholar]
- Dang, N.T. New diaptomid crustacean from Vietnam. Tap Chi Sinh Vat-Dia Hoc. 1977, 15, 97–99, (In Vietnamese with English summary). [Google Scholar]
- Gurney, R. On two new Entomostraca from Ceylon. Spolia Zeylan. 1906, 4, 126–134. [Google Scholar]
- Rama Devi, C.; Ranga Reddy, Y. The complete postembryonic development of Paradiaptomus greeni (Gurney, 1906) (Copepoda, Calanoida) reared in the laboratory. Crustaceana 1989, 56, 141–161. [Google Scholar] [CrossRef]
- Turki, S.; Turki, B. Copepoda and Branchiopoda from Tunisian temporary waters. Int. J. Biodivers. Conserv. 2010, 2, 86–97. [Google Scholar]
- Kulkarni, M.R.; Pai, K. The freshwater diaptomid copepod fauna (Crustacea: Copepoda: Diaptomidae) of the Western Ghats of Maharashtra with notes on distribution, species richness and ecology. J. Limnol. 2016, 75, 135–143. [Google Scholar] [CrossRef]
- Burns, C.W. New records of distribution and co-occurrence of freshwater calanoid copepods in New Zealand (Note). N. Z. J. Mar. Freshw. Res. 1987, 21, 149–150. [Google Scholar] [CrossRef]







| No. | Sampling Habitats | Regions in Thailand | Sampling Years | Number of Localities | Number of Samples |
|---|---|---|---|---|---|
| 1 | Various habitats in Northeast Thailand | NE | 1993 | 93 | 200 |
| 2 | Various habitats in Nakhon Ratchasima and Surin Provinces | NE | 1996–1999 | 138 | 412 |
| 3 | Man-made reservoirs in the Phuphan National Park | NE | 1997–1998 | 16 | 336 |
| 4 | Major rivers in Thailand | N,NE,C,E,W,S | 1998–1999 | 20 | 60 |
| 5 | Various habitats throughout Thailand | N,NE,C,E,W,S | 1998–2004 | 294 | 508 |
| 6 | Temporary-water habitats in seven provinces of NE Thailand | NE | 1999–2003 | 521 | 844 |
| 7 | Various habitats in the floodplain of the Rivers Nan, Chi, Mun and Song Khram | N,NE,C | 2000–2004 | 186 | 372 |
| 8 | Various habitats in Central and Northern Thailand | N,C | 2001–2002 | 120 | 360 |
| 9 | Various habitats in Central and Northern Thailand | N,C | 2003 | 55 | 110 |
| 10 | Three natural lakes (Buemg Boraphet, Bueng Khong Long, and Lake Kud-Thing) | NE,C | 2002–2004 | 30 | 60 |
| 11 | Various habitats in Eastern Thailand | E | 2003–2004 | 55 | 110 |
| 12 | Various habitats in Sisaket Province | NE | 2006–2007 | 99 | 190 |
| 13 | Various habitats in Suphan Buri, Kanchanaburi, Ratchaburi and Phetchaburi Provinces | W | 2007 | 200 | 218 |
| 14 | Various habitats in Nam Nao National Park, Phetchabun Province | C | 2007–2009 | 10 | 75 |
| 15 | Lake Kud-Thing and various habitats in Bueng Kan and Udon Thani Provinces | NE | 2012–2014 | 97 | 408 |
| 16 | Various habitats in Chiang Rai, Phayao and Nan Provinces | N | 2014–2016 | 67 | 154 |
| 17 | Various habitats in Khon Kaen, Kalasin and Buriram Provinces | NE | 2014–2018 | 128 | 344 |
| 18 | Various habitats in Saraburi and Lopburi Provinces | C | 2019 | 21 | 201 |
| Total | 2150 | 4962 | |||
| No | Species | Habitat Types | Distributional Regions | Relative Occurrences | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Permanent Waters | Temporary Waters | |||||||||||||||
| C | L | Pp/Sw | Re | Ri | Rf | Rc | Tp | N | NE | C | E | W | S | |||
| Subfamily Diaptominae Kiefer, 1932 | ||||||||||||||||
| 1 | Allodiaptomus raoi Kiefer, 1936 | ✓ | ✓ | ✓ | ✓ | ✓ | uncommon | |||||||||
| 2 | Arctodiaptomus sp. | ✓ | ✓ | extremely rare | ||||||||||||
| 3 | Dentodiaptomus javanus (Grochmalicki, 1915) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | fairly common | ||||||
| 4 | Dentodiaptomus orientalis Sanoamuang & Watiroyram, 2021 | ✓ | ✓ | ✓ | ✓ | uncommon | ||||||||||
| 5 | Eodiaptomus draconisignivomi Brehm, 1952 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | fairly common | |||||
| 6 | Eodiaptomus phuphanensis Sanoamuang, 2001 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | fairly common | ||||||||
| 7 | Eodiaptomus phuvongi Sanoamuang & Sivongxay, 2004 | ✓ | ✓ | ✓ | ✓ | fairly common | ||||||||||
| 8 | Eodiaptomus sanoamuangae Ranga Reddy & Dumont, 1998 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | common | |||
| 9 | Heliodiaptomus elegans Kiefer, 1935 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | fairly common | ||||||
| 10 | Heliodiaptomus phuthaiorum Sanoamuang, 2004 | ✓ | ✓ | ✓ | rare | |||||||||||
| 11 | Heliodiaptomus viduus (Gurney, 1916) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | uncommon | ||||||
| 12 | Mongolodiaptomus botulifer (Kiefer, 1974) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | common |
| 13 | Mongolodiaptomus calcarus (Shen & Tai, 1965) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | common | |
| 14 | Mongolodiaptomus dumonti Sanoamuang, 2001 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | common | |
| 15 | Mongolodiaptomus loeiensis Watiroyram & Sanoamuang, 2017 | ✓ | ✓ | extremely rare | ||||||||||||
| 16 | Mongolodiaptomus malaindosinensis (Lai & Fernando, 1978) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | common | ||||
| 17 | Mongolodiaptomus mekongensis Sanoamuang & Watiroyram, 2018 | ✓ | ✓ | ✓ | ✓ | ✓ | uncommon | |||||||||
| 18 | Mongolodiaptomus pectinidactylus (Shen & Tai, 1964) * | ✓ | ✓ | ✓ | rare | |||||||||||
| 19 | Mongolodiaptomus rarus (Ranga Reddy, Sanoamuang & Dumont, 1998) | ✓ | ✓ | ✓ | ✓ | uncommon | ||||||||||
| 20 | Mongolodiaptomus uenoi (Kikuchi, 1936) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | fairly common | |||||||
| 21 | Mongolodiaptomus sp. | ✓ | ✓ | ✓ | extremely rare | |||||||||||
| 22 | Neodiaptomus laii Kiefer, 1974 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | uncommon | ||||||||
| 23 | Neodiaptomus meggitti Kiefer, 1932 * | ✓ | ✓ | extremely rare | ||||||||||||
| 24 | Neodiaptomus schmackeri (Poppe & Richard, 1892) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | fairly common | ||||||
| 25 | Neodiaptomus siamensis Proogkiat & Sanoamuang, 2008 | ✓ | ✓ | extremely rare | ||||||||||||
| 26 | Neodiaptomus songkhramensis Sanoamuang & Athibai, 2002 | ✓ | ✓ | ✓ | ✓ | uncommon | ||||||||||
| 27 | Neodiaptomus yangtsekiangensis Mashiko, 1951 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | common | ||||
| 28 | Phyllodiaptomus(Ctenodiaptomus) praedictus Dumont & Ranga Reddy, 1994 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | common | |
| 29 | Phyllodiaptomus(Ctenodiaptomus) surinensis Sanoamuang & Yindee, 2001 | ✓ | ✓ | extremely rare | ||||||||||||
| 30 | Phyllodiaptomus(Phyllodiaptomus) christineae Dumont, Ranga Reddy & Sanoamuang, 1996 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | common | |||
| 31 | Phyllodiaptomus(Phyllodiaptomus) roietensis Sanoamuang & Watiroyram, 2020 | ✓ | ✓ | ✓ | ✓ | rare | ||||||||||
| 32 | Phyllodiaptomus(Phyllodiaptomus) thailandicus Sanoamuang & Teeramaethee, 2006 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | rare | ||||||||
| 33 | Phyllodiaptomus (Phyllodiaptomus) sp. | ✓ | ✓ | ✓ | rare | |||||||||||
| 34 | Tropodiaptomushebereri (Kiefer, 1930) * | ✓ | ✓ | extremely rare | ||||||||||||
| 35 | Tropodiaptomus lanaonus Kiefer, 1982 * | ✓ | ✓ | ✓ | rare | |||||||||||
| 36 | Tropodiaptomus megahyaline Saetang, Sanoamuang & Maiphae, 2020 | ✓ | ✓ | ✓ | ✓ | rare | ||||||||||
| 37 | Tropodiaptomus oryzanus Kiefer, 1937 * | ✓ | ✓ | ✓ | ✓ | ✓ | uncommon | |||||||||
| 38 | Tropodiaptomusruttneri (Brehm, 1923) * | ✓ | ✓ | ✓ | ✓ | uncommon | ||||||||||
| 39 | Tropodiaptomus vicinus (Kiefer, 1930) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | fairly common | |||||
| 40 | Tropodiaptomus sp. | ✓ | ✓ | extremely rare | ||||||||||||
| 41 | Vietodiaptomus blachei (Brehm, 1951) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | common | ||
| Subfamily Paradiaptominae Kiefer, 1932 | ||||||||||||||||
| 42 | Paradiaptomus greeni (Gurney, 1906) * | ✓ | ✓ | extremely rare | ||||||||||||
| Total | 17 | 35 | 14 | 11 | 15 | 8 | ||||||||||
| Localities | Sampling Dates | Co-occurrence of Diaptomid Species |
|---|---|---|
| Canal at Ban Paiklao, Chumphon Buri District, Surin Province | 2 April 1999 | 1. Dentodiaptomus javanus 2. Mongolodiaptomus dumonti 3. Mongolodiaptomus malaindosinensis 4. Mongolodiaptomus uenoi 5. Neodiaptomus yangtsekiangensis 6. Phyllodiaptomus (P.) christineae |
| Irrigation Canal at Ban Nayom, Sanom District, Surin Province | 29 July 1999 | 1. Dentodiaptomus javanus 2. Mongolodiaptomus dumonti 3. Neodiaptomus laii 4. Phyllodiaptomus (P.) christineae 5. Phyllodiaptomus (C.) surinensis 6. Tropodiaptomus oryzanus 7. Tropodiaptomus vicinus |
| Temporary pond at Ban Chaiya Buri, Chaiya Buri Sub-district, Tha Uthen District, Nakhon Phanom Province | 18 May 2000 | 1. Dentodiaptomus javanus 2. Eodiaptomus phuphanensis 3. Heliodiaptomus phuthaiorum 4. Mongolodiaptomus malaindosinensis 5. Neodiaptomus yangtsekiangensis 6. Vietodiaptomus blachei |
| Roadside canal (temporary-water habitat), KM 24, Road #2050, Lao Suekok Sub-district, Ubon Rachathani Province | 8 June 2002 | 1. Eodiaptomus phuphanensis 2. Mongolodiaptomus pectinidactylus 3. Mongolodiaptomus uenoi 4. Neodiaptomus yangtsekiangensis 5. Tropodiaptomus oryzanus, 6. Vietodiaptomus blachei |
| Lake Bueng Khong Long, Bueng Khong Long District, Bueng Kan Province | 13 August 2002 | 1. Allodiaptomus raoi 2. Heliodiaptomus elegans 3. Mongolodiaptomus pectinidactylus 4. Neodiaptomus yangtsekiangensis 5. Tropodiaptomus megahyaline |
| Roadside canal (temporary-water habitat), Ban Dirkai, Muangsamsip District, Ubon Rachathani Province | 19 October 2002 | 1. Eodiaptomus phuphanensis 2. Heliodiaptomus elegans 3. Mongolodiaptomus malaindosinensis 4. Neodiaptomus yangtsekiangensis 5. Phyllodiaptomus (P.) christineae 6. Vietodiaptomus blachei |
| Temporary pond at Ban Thasongcorn, Panna Nikom District, Sakon Nakhon Province | 17 May 2004 | 1. Eodiaptomus phuphanensis 2. Mongolodiaptomus malaindosinensis 3. Mongolodiaptomus uenoi 4. Neoodiaptomus songkhramensis 5. Neodiaptomus yangtsekiangensis 6. Tropodiaptomus oryzanus, 7. Vietodiaptomus blachei |
| Distributional Regions | Number of Species | % of Species Recorded | List of Species |
|---|---|---|---|
| Thailand only (Endemic species) | 12 | 28.6 | Arctodiaptomus sp., Heliodiaptomus phuthaiorum, Mongolodiaptomus dumonti, M. loeiensis, M. rarus, Mongolodiaptomus sp. Neodiaptomus siamensis, N. songkhramensis, Phyllodiaptomus (C.) surinensis, P. (P.) thailandicus, Tropodiaptomus megahyaline, Tropodiaptomus. sp. |
| Lower Mekong River Basin | 8 | 19.0 | Dentodiaptomus orientalis, Eodiaptomus draconisignivomi, E. phuphanensis, E. phuvongi, Mongolodiaptomus mekongensis, Phyllodiaptomus(P.)christineae, P. (P.) roietensis, Phyllodiaptomus sp. |
| Southeast Asia | 8 | 19.0 | Mongolodiaptomus botulifer, M.malaindosinensis, Neodiaptomus laii, N.meggitti, Phyllodiaptomus(C.)praedictus, Tropodiaptomus lanaonus, T.vicinus, Vietodiaptomus blachei |
| Southeast Asia and East Asia | 10 | 23.8 | Allodiaptomus raoi, Dentodiaptomus javanus, Eodiaptomus sanoamuangae, Mongolodiaptomus calcarus, M.pectinidactylus, M.uenoi, Neodiaptomus yangtsekiangensis, Tropodiaptomus hebereri, T.oryzanus, T.ruttneri |
| Southeast Asia, East Asia and South Asia | 3 | 7.2 | Heliodiaptomus elegans, Heliodiaptomus viduus, Neodiaptomus schmackeri |
| Southeast Asia, South Asia and Africa | 1 | 2.4 | Paradiaptomus greeni |
| Total | 42 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanoamuang, L.; Dabseepai, P. Diversity, Distribution, and Habitat Occurrence of the Diaptomid Copepods (Crustacea: Copepoda: Diaptomidae) in Freshwater Ecosystems of Thailand. Water 2021, 13, 2381. https://doi.org/10.3390/w13172381
Sanoamuang L, Dabseepai P. Diversity, Distribution, and Habitat Occurrence of the Diaptomid Copepods (Crustacea: Copepoda: Diaptomidae) in Freshwater Ecosystems of Thailand. Water. 2021; 13(17):2381. https://doi.org/10.3390/w13172381
Chicago/Turabian StyleSanoamuang, Laorsri, and Prapatsorn Dabseepai. 2021. "Diversity, Distribution, and Habitat Occurrence of the Diaptomid Copepods (Crustacea: Copepoda: Diaptomidae) in Freshwater Ecosystems of Thailand" Water 13, no. 17: 2381. https://doi.org/10.3390/w13172381
APA StyleSanoamuang, L., & Dabseepai, P. (2021). Diversity, Distribution, and Habitat Occurrence of the Diaptomid Copepods (Crustacea: Copepoda: Diaptomidae) in Freshwater Ecosystems of Thailand. Water, 13(17), 2381. https://doi.org/10.3390/w13172381






