Anthropogenic Activities and the Problem of Antibiotic Resistance in Latin America: A Water Issue
Abstract
1. Introduction
2. Methods
- Water sources, where the studied water samples were obtained, comprising rivers, and irrigation channels (surface water), seawater, and wastewater (treated and untreated).
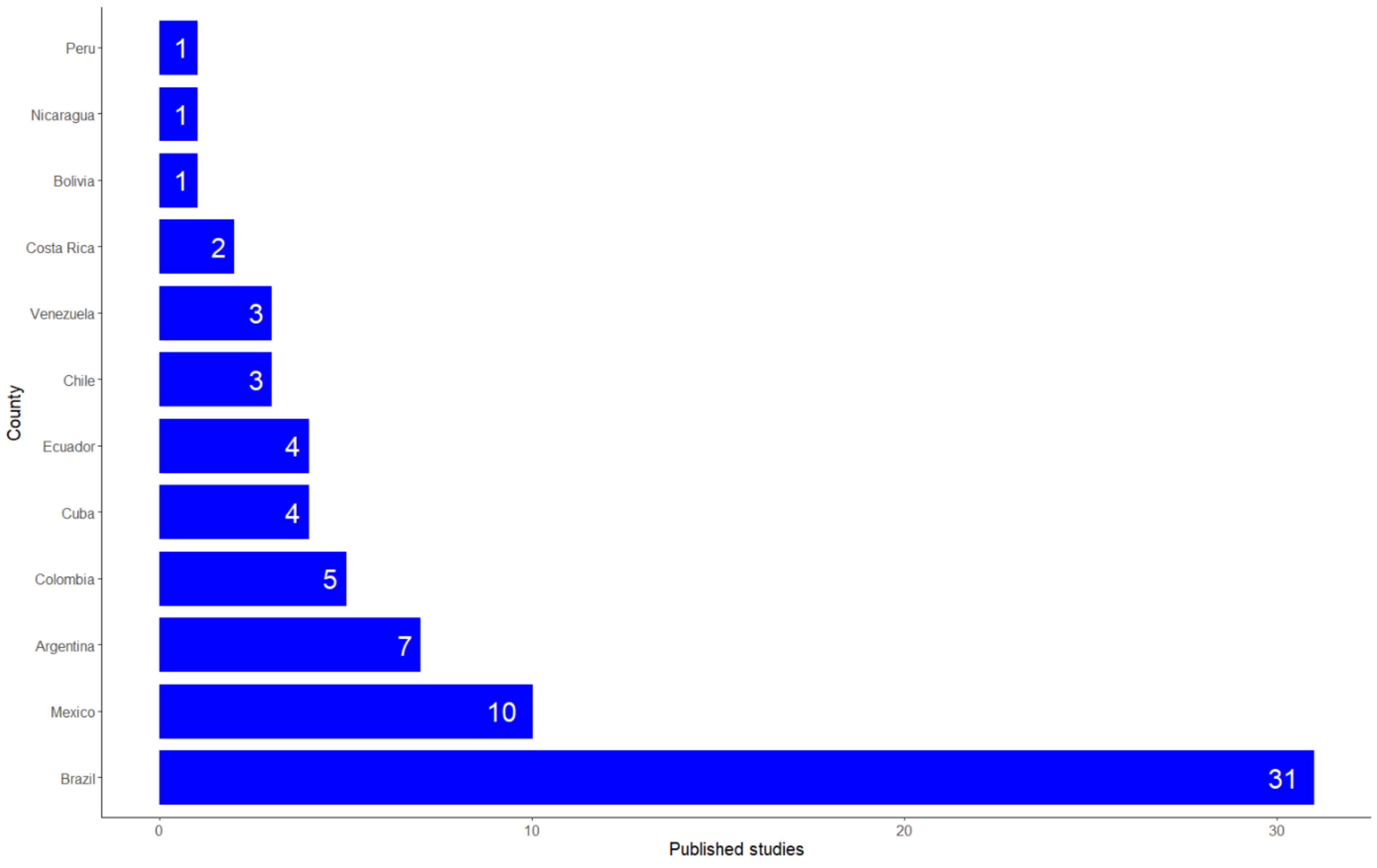
- Country where the studies were performed.
- Anthropogenic activity affecting water bodies: agricultural activities, drinking water production, and wastewater discharges.
- Reported antibiotic susceptibility analysis and antibiotic genetic marker detection methods.
- Antibiotic resistance levels/Antibiotic resistance genes presence.
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMR | Antimicrobial Resistance |
| ARG | Antimicrobial Resistant Genes |
| CDC | Centers for Disease Control and Prevention |
| ESBL | Extended Spectrum Beta Lactamases |
| GARP | Global Antibiotic Resistance Partnership |
| GDP | Gross Domestic Product |
| GHSA | Global Health Security Agenda |
| LMIC | Low- and Middle- Income Countries |
| LIC | Low- Income Countries |
| MALDI-TOF | Matrix Assisted Laser Desorption Ionization Time of Flight |
| MIC | Middle- Income Countries |
| MIC | Minimum Inhibitory concentration |
| MRDO | Multidrug Resistant Organisms |
| PAHO | Pan American Health Organization |
| PCR | Polymerase Chain Reaction |
| TATFAR | Transatlantic Taskforce on Antimicrobial Resistance |
| WHO | World Health Organization |
| WWTP | Wastewater Treatment Plants |
References
- Collignon, P.; McEwen, S. One Health—Its Importance in Helping to Better Control Antimicrobial Resistance. Trop. Med. Infect. Dis. 2019, 4, 22. [Google Scholar] [CrossRef]
- Nahrgang, S.; Nolte, E.; Rechel, B. The role of public health organizations in addressing antimicrobial resistance in Europe. Eur. J. Public Health 2018, 28. [Google Scholar] [CrossRef]
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS). Available online: https://www.who.int/initiatives/glass (accessed on 23 September 2021).
- World Health Organization. Global Action Plan on Antimicrobial Resistance; World Health Organization (WHO), Ed.; World Health Organization: Geneva, Switerland, 2016; ISBN 9789241509763. [Google Scholar]
- Centers of Disease Control and Prevention. The ARM Challenge. Available online: https://www.cdc.gov/drugresistance/intl-activities/amr-challenge.html (accessed on 23 September 2021).
- Centers of Disease Control and Prevention. Antibiotic/Antimicrobial Resistance. Available online: https://www.cdc.gov/drugresistance/index.html (accessed on 23 September 2021).
- Martins, A.F.; Rabinowitz, P. The impact of antimicrobial resistance in the environment on public health. Future Microbiol. 2020, 15, 699–702. [Google Scholar] [CrossRef]
- O’Neil, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Wellcome Trust: London, UK, 2016. [Google Scholar]
- Van Boeckel, T.P.; Gandra, S.; Ashok, A.; Caudron, Q.; Grenfell, B.T.; Levin, S.A.; Laxminarayan, R. Global antibiotic consumption 2000 to 2010: An analysis of national pharmaceutical sales data. Lancet Infect. Dis. 2014, 14, 742–750. [Google Scholar] [CrossRef]
- Center For Disease Dynamics Economics & Policy. The State of the World’s Antibiotics 2015; Cambridge University Press: Cambridge, UK, 2015. [Google Scholar]
- Ahmad, M.; Khan, A.U. Global economic impact of antibiotic resistance: A review. J. Glob. Antimicrob. Resist. 2019, 19, 313–316. [Google Scholar] [CrossRef]
- Pruden, A.; Pei, R.; Storteboom, H.; Carlson, K.H. Antibiotic Resistance Genes as Emerging Contaminants: Studies in Northern Colorado. Environ. Sci. Technol. 2006, 40, 7445–7450. [Google Scholar] [CrossRef]
- Baquero, F.; Martínez, J.-L.; Cantón, R. Antibiotics and antibiotic resistance in water environments. Curr. Opin. Biotechnol. 2008, 19, 260–265. [Google Scholar] [CrossRef]
- Domínguez, D.C.; Meza-Rodriguez, S.M. Development of antimicrobial resistance: Future challenges. In Pharmaceuticals and Personal Care Products: Waste Management and Treatment Technology; Butterworth-Heinemann: Oxford, UK, 2019; pp. 383–408. [Google Scholar]
- Magouras, I.; Carmo, L.P.; Stärk, K.D.C.; Schüpbach-Regula, G. Antimicrobial Usage and -Resistance in Livestock: Where Should We Focus? Front. Vet. Sci. 2017, 4, 148. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.; Ramos, F. Analytical strategies for the detection and quantification of antibiotic residues in aquaculture fishes: A review. Trends Food Sci. Technol. 2016, 52, 16–30. [Google Scholar] [CrossRef]
- Watts, J.; Schreier, H.; Lanska, L.; Hale, M. The Rising Tide of Antimicrobial Resistance in Aquaculture: Sources, Sinks and Solutions. Mar. Drugs 2017, 15, 158. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Wang, W.; Regev-Yochay, G.; Lipsitch, M.; Hanage, W.P. Antibiotics in agriculture and the risk to human health: How worried should we be? Evol. Appl. 2015, 8, 240–247. [Google Scholar] [CrossRef]
- Vikesland, P.; Garner, E.; Gupta, S.; Kang, S.; Maile-Moskowitz, A.; Zhu, N. Differential Drivers of Antimicrobial Resistance across the World. Acc. Chem. Res. 2019, 52, 916–924. [Google Scholar] [CrossRef]
- Uyaguari-Díaz, M.I.; Croxen, M.A.; Luo, Z.; Cronin, K.I.; Chan, M.; Baticados, W.N.; Nesbitt, M.J.; Li, S.; Miller, K.M.; Dooley, D.; et al. Human Activity Determines the Presence of Integron-Associated and Antibiotic Resistance Genes in Southwestern British Columbia. Front. Microbiol. 2018, 9, 852. [Google Scholar] [CrossRef]
- Pokharel, S.; Raut, S.; Adhikari, B. Tackling antimicrobial resistance in low-income and middle-income countries. BMJ Glob. Heal. 2019, 4, e002104. [Google Scholar] [CrossRef]
- Vila, J. Update on Antibacterial Resistance in Low-Income Countries: Factors Favoring the Emergence of Resistance. Open Infect. Dis. J. 2010, 4, 38–54. [Google Scholar] [CrossRef]
- Pierce, J.; Apisarnthanarak, A.; Schellack, N.; Cornistein, W.; Maani, A.A.; Adnan, S.; Stevens, M.P. Global Antimicrobial Stewardship with a Focus on Low- and Middle-Income Countries: A position statement for the international society for infectious diseases. Int. J. Infect. Dis. 2020, 96, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Gandra, S.; Alvarez-Uria, G.; Turner, P.; Joshi, J.; Limmathurotsakul, D.; van Doorn, H.R. Antimicrobial Resistance Surveillance in Low- and Middle-Income Countries: Progress and Challenges in Eight South Asian and Southeast Asian Countries. Clin. Microbiol. Rev. 2020, 33. [Google Scholar] [CrossRef]
- Collignon, P.; Beggs, J.J.; Walsh, T.R.; Gandra, S.; Laxminarayan, R. Anthropological and socioeconomic factors contributing to global antimicrobial resistance: A univariate and multivariable analysis. Lancet Planet. Health 2018, 2, e398–e405. [Google Scholar] [CrossRef]
- Gales, A.C.; Castanheira, M.; Jones, R.N.; Sader, H.S. Antimicrobial resistance among Gram-negative bacilli isolated from Latin America: Results from SENTRY Antimicrobial Surveillance Program (Latin America, 2008–2010). Diagn. Microbiol. Infect. Dis. 2012, 73, 354–360. [Google Scholar] [CrossRef]
- da Silva, J.B., Jr.; Espinal, M.; Ramón-Pardo, P. Antimicrobial resistance: Time for action. Rev. Panam. Salud Pública 2020, 44, 1. [Google Scholar] [CrossRef]
- Pan American Health Organization. Latin American Network for Antimicrobial Resistance Surveillance—ReLAVRA. Available online: https://www.paho.org/en/topics/antimicrobial-resistance/latin-american-network-antimicrobial-resistance-surveillance (accessed on 23 September 2021).
- Wirtz, V.J.; Dreser, A.; Gonzales, R. Trends in antibiotic utilization in eight Latin American countries, 1997–2007. Rev. Panam. Salud Publica/Pan Am. J. Public Health 2010, 27, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Huesca, R.; Lerma, A.; Guzmán-Saldaña, R.M.E.; Lerma, C. Prevalence of Antibiotics Prescription and Assessment of Prescribed Daily Dose in Outpatients from Mexico City. Antibiotics 2020, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, C.; Gomez, C.; Montañez, A.; Ordoñez, K.; Bonilla, D.; Sussmann, O.; Group, A. Antibiotic Consumption from 2013 to 2015 at Colombian Hospitals of Third Level of Complexity. In Open Forum Infectious Diseases; Oxford University Press: Oxford, UK, 2016; Volume 3, p. 993. [Google Scholar] [CrossRef]
- Miranda-Novales, M.G.; Flores-Moreno, K.; López-Vidal, Y.; Rodríguez-Álvarez, M.; Solórzano-Santos, F.; Soto-Hernández, J.L.; Ponce de León-Rosales, S.; UNCAR Network. Antimicrobial resistance and antibiotic consumption in Mexican hospitals. Salud Publica Mex. 2019, 62, 42. [Google Scholar] [CrossRef] [PubMed]
- Neves e Castro, P.B.; da Silva Rodrigues, D.A.; Roeser, H.M.P.; da Fonseca Santiago, A.; de Cássia Franco Afonso, R.J. Antibiotic consumption in developing countries defies global commitments: An overview on Brazilian growth in consumption. Environ. Sci. Pollut. Res. 2020, 27, 21013–21020. [Google Scholar] [CrossRef]
- World Health Organization. WHO Report on Surveillance of Antibiotic Consumption: 2016–2018 Early Implementation; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Peralta, N.; Camou, B.I.; Leszczuk, K.; Arcidiácono, D.; Lerena, R.G.; Herrera, P.; Cornistein, W.; Riera, F.; Cosgrove, S.E.; Fabre, V. Prevalence of hospital antibiotic use in Argentina, 2018. Infect. Control Hosp. Epidemiol. 2019, 40, 1301–1304. [Google Scholar] [CrossRef]
- Bonelli, R.R.; Moreira, B.M.; Picão, R.C. Antimicrobial resistance among Enterobacteriaceae in South America: History, current dissemination status and associated socioeconomic factors. Drug Resist. Updates 2014, 17, 24–36. [Google Scholar] [CrossRef]
- Canizalez-Roman, A.; Velazquez-Roman, J.; Valdez-Flores, M.A.; Flores-Villaseñor, H.; Vidal, J.E.; Muro-Amador, S.; Guadrón-Llanos, A.M.; Gonzalez-Nuñez, E.; Medina-Serrano, J.; Tapia-Pastrana, G.; et al. Detection of antimicrobial-resistance diarrheagenic Escherichia coli strains in surface water used to irrigate food products in the northwest of Mexico. Int. J. Food Microbiol. 2019, 304, 1–10. [Google Scholar] [CrossRef]
- Lozano-Muñoz, I.; Wacyk, J.; Kretschmer, C.; Vásquez-Martínez, Y.; Martin, M.C.-S. Antimicrobial resistance in Chilean marine-farmed salmon: Improving food safety through One Health. One Health 2021, 12, 100219. [Google Scholar] [CrossRef]
- Leite, B.; de Chaves, M.A.; Nunes, A.A.T.; Jank, L.; Corção, G. Antibiotic resistance in surface waters from a coastal lagoon of Southern Brazil under the impact of anthropogenic activities. Rev. Ambient. Agua. 2019, 14, 1. [Google Scholar] [CrossRef]
- Chamosa, L.S.; Álvarez, V.E.; Nardelli, M.; Quiroga, M.P.; Cassini, M.H.; Centrón, D. Lateral Antimicrobial Resistance Genetic Transfer is active in the open environment. Sci. Rep. 2017, 7, 513. [Google Scholar] [CrossRef]
- Coutinho, F.H.; Silveira, C.B.; Pinto, L.H.; Salloto, G.R.B.; Cardoso, A.M.; Martins, O.B.; Vieira, R.P.; Clementino, M.M. Antibiotic Resistance is Widespread in Urban Aquatic Environments of Rio de Janeiro, Brazil. Microb. Ecol. 2014, 68, 441–452. [Google Scholar] [CrossRef]
- Moreno-Switt, A.I.; Rivera, D.; Caipo, M.L.; Nowell, D.C.; Adell, A.D. Antimicrobial resistance in water in Latin America and the Caribbean: Available Research and Gaps. Front. Vet. Sci. 2020, 7, 546. [Google Scholar] [CrossRef]
- Overbey, K.N.; Hatcher, S.M.; Stewart, J.R. Water quality and antibiotic resistance at beaches of the Galápagos Islands. Front. Environ. Sci. 2015, 3, 64. [Google Scholar] [CrossRef]
- The World Bank. GDP (Curren US$). Available online: https://data.worldbank.org/indicator/NY.GDP.MKTP.CD?view=map (accessed on 23 September 2021).
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 23 September 2021).
- Bartley, P.S.; Domitrovic, T.N.; Moretto, V.T.; Santos, C.S.; Ponce-Terashima, R.; Reis, M.G.; Barbosa, L.M.; Blanton, R.E.; Bonomo, R.A.; Perez, F. Antibiotic resistance in enterobacteriaceae from surface waters in Urban Brazil highlights the risks of poor sanitation. Am. J. Trop. Med. Hyg. 2019, 100, 1369–1377. [Google Scholar] [CrossRef]
- De Oliveira, A.J.F.C.; Watanabe Pinhata, J.M. Antimicrobial resistance and species composition of Enterococcus spp. isolated from waters and sands of marine recreational beaches in Southeastern Brazil. Water Res. 2008, 42, 2242–2250. [Google Scholar] [CrossRef]
- Basso, A.P.; Martins, P.D.; Nachtigall, G.; van der Sand, S.; de Moura, T.M.; Frazzon, A.P.G. Antibiotic resistance and enterotoxin genes in staphylococcus sp. Isolates from polluted water in southern Brazil. An. Acad. Bras. Cienc. 2014, 86, 1813–1820. [Google Scholar] [CrossRef][Green Version]
- Turano, H.; Gomes, F.; Medeiros, M.; Oliveira, S.; Fontes, L.C.; Sato, M.I.Z.; Lincopan, N. Presence of high-risk clones of OXA-23-producing Acinetobacter baumannii (ST79) and SPM-1-producing Pseudomonas aeruginosa (ST277) in environmental water samples in Brazil. Diagn. Microbiol. Infect. Dis. 2016, 86, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Chagas, T.P.G.; Seki, L.M.; Cury, J.C.; Oliveira, J.A.L.; Dávila, A.M.R.; Silva, D.M.; Asensi, M.D. Multiresistance, beta-lactamase-encoding genes and bacterial diversity in hospital wastewater in Rio de Janeiro, Brazil. J. Appl. Microbiol. 2011, 111, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Edirsana, M.R.C.; Renata, A.C.; Alberto, J.G.A.; Ftima, C.T.C.; Silvano, P.P.; Oscarina, V.S.; Regine, H.S.F.V. Multiple antibiotic-resistance of Enterococcus isolated from coastal water near an outfall in Brazil. Afr. J. Microbiol. Res. 2014, 8, 1825–1831. [Google Scholar] [CrossRef]
- Miranda, C.C.; de Filippis, I.; Pinto, L.H.; Coelho-Souza, T.; Bianco, K.; Cacci, L.C.; Picão, R.C.; Clementino, M.M. Genotypic characteristics of multidrug-resistant Pseudomonas aeruginosa from hospital wastewater treatment plant in Rio de Janeiro, Brazil. J. Appl. Microbiol. 2015, 118, 1276–1286. [Google Scholar] [CrossRef]
- Dos Rocha, R.S.; de Sousa, O.V.; dos Vieira, R.H.S.F. Multidrug-resistant Vibrio associated with an estuary affected by shrimp farming in Northeastern Brazil. Mar. Pollut. Bull. 2016, 105, 337–340. [Google Scholar] [CrossRef]
- Conte, D.; Palmeiro, J.K.; da Silva Nogueira, K.; de Lima, T.M.R.; Cardoso, M.A.; Pontarolo, R.; Degaut Pontes, F.L.; Dalla-Costa, L.M. Characterization of CTX-M enzymes, quinolone resistance determinants, and antimicrobial residues from hospital sewage, wastewater treatment plant, and river water. Ecotoxicol. Environ. Saf. 2017, 136, 62–69. [Google Scholar] [CrossRef]
- Oliveira, L.G.; Ferreira, L.G.R.; Nascimento, A.M.A.; Reis, M.D.P.; Dias, M.F.; Lima, W.G.; Paiva, M.C. Antibiotic resistance profile and occurrence of Amp C between Pseudomonas aeruginosa isolated from a domestic full-scale WWTP in southeast Brazil. Water Sci. Technol. 2018, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Freitas, D.Y.; Araújo, S.; Folador, A.R.C.; Ramos, R.T.J.; Azevedo, J.S.N.; Tacão, M.; Silva, A.; Henriques, I.; Baraúna, R.A. Extended spectrum beta-lactamase-producing gram-negative bacteria recovered from an amazonian lake near the city of Belém, Brazil. Front. Microbiol. 2019, 10, 364. [Google Scholar] [CrossRef]
- Zagui, G.S.; de Andrade, L.N.; Moreira, N.C.; Silva, T.V.; Machado, G.P.; da Costa Darini, A.L.; Segura-Muñoz, S.I. Gram-negative bacteria carrying β-lactamase encoding genes in hospital and urban wastewater in Brazil. Environ. Monit. Assess. 2020, 192. [Google Scholar] [CrossRef]
- Spindler, A.; Otton, L.M.; Fuentefria, D.B.; Corção, G. Beta-lactams resistance and presence of class 1 integron in Pseudomonas spp. isolated from untreated hospital effluents in Brazil. Antonie Leeuwenhoek 2012, 102, 73–81. [Google Scholar] [CrossRef] [PubMed]
- De Souza, M.; da Pinto, F.G.S.; Fruet, T.K.; Piana, P.A.; de Moura, A.C. Water quality indicators for environmental and resistance profile of Escherichia coli strains isolated in Rio Cascavel, Paraná, Brazil. Eng. Agrícola 2014, 34, 352–362. [Google Scholar] [CrossRef][Green Version]
- Picão, R.C.; Cardoso, J.P.; Campana, E.H.; Nicoletti, A.G.; Petrolini, F.V.B.; Assis, D.M.; Juliano, L.; Gales, A.C. The route of antimicrobial resistance from the hospital effluent to the environment: Focus on the occurrence of KPC-producing Aeromonas spp. and Enterobacteriaceae in sewage. Diagn. Microbiol. Infect. Dis. 2013, 76, 80–85. [Google Scholar] [CrossRef]
- Zanetti, M.O.; Martins, V.V.; Pitondo-Silva, A.; Stehling, E.G. Antimicrobial resistance, plasmids and class 1 and 2 integrons occurring in Pseudomonas aeruginosa isolated from Brazilian aquatic environments. Water Sci. Technol. 2013, 67, 1144–1149. [Google Scholar] [CrossRef]
- Giowanella, M.; Bozza, A.; do Rocio Dalzoto, P.; Dionísio, J.A.; Andraus, S.; Guimarães, E.L.G.; Pimentel, I.C. Microbiological quality of water from the rivers of Curitiba, Paraná State, Brazil, and the susceptibility to antimicrobial drugs and pathogenicity of Escherichia coli. Environ. Monit. Assess. 2015, 187. [Google Scholar] [CrossRef]
- Falcão, J.P.; Brocchi, M.; Proença-Módena, J.L.; Acrani, G.O.; Corrêa, E.F.; Falcão, D.P. Virulence characteristics and epidemiology of Yersinia enterocolitica and Yersiniae other than Y. pseudotuberculosis and Y. pestis isolated from water and sewage. J. Appl. Microbiol. 2004, 96, 1230–1236. [Google Scholar] [CrossRef]
- Gomes Freitas, D.; Silva, R.D.R.; Bataus, L.A.M.; Barbosa, M.S.; da Silva Bitencourt Braga, C.A.; Carneiro, L.C. Bacteriological water quality in school’s drinking fountains and detection antibiotic resistance genes. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 1–5. [Google Scholar] [CrossRef]
- Lima-Bittencourt, C.I.; Cursino, L.; Gonçalves-Dornelas, H.; Pontes, D.S.; Nardi, R.M.D.; Callisto, M.; Chartone-Souza, E.; Nascimento, A.M.A. Multiple antimicrobial resistance in Enterobacteriaceae isolates from pristine freshwater. Genet. Mol. Res. 2007, 6, 510–521. [Google Scholar]
- Abraham, W.R.; Macedo, A.J.; Gomes, L.H.; Tavares, F.C.A. Occurrence and resistance of pathogenic bacteria along the Tietê River downstream of São Paulo in Brazil. Clean-Soil Air Water 2007, 35, 339–347. [Google Scholar] [CrossRef]
- Canal, N.; Meneghetti, K.L.; De Almeida, C.P.; Da Rosa Bastos, M.; Otton, L.M.; Corção, G. Characterization of the variable region in the class 1 integron of antimicrobial-resistant Escherichia coli isolated from surface water. Braz. J. Microbiol. 2016, 47, 337–344. [Google Scholar] [CrossRef]
- De Araujo, C.F.M.; Silva, D.M.; Carneiro, M.T.; Ribeiro, S.; Fontana-Maurell, M.; Alvarez, P.; Asensi, M.D.; Zahner, V.; Carvalho-Assef, A.P.D. Detection of Carbapenemase Genes in Aquatic Environments in Rio de Janeiro, Brazil. Antimicrob. Agents Chemother. 2016, 60, 4380–4383. [Google Scholar] [CrossRef] [PubMed]
- Furlan, J.P.R.; Stehling, E.G. Detection of β-lactamase encoding genes in feces, soil and water from a Brazilian pig farm. Environ. Monit. Assess. 2018, 190. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, D.G.; de Melo, F.M.; Savazzi, E.A.; Stehling, E.G. Detection of different β-lactamases encoding genes, including bla NDM, and plasmid-mediated quinolone resistance genes in different water sources from Brazil. Environ. Monit. Assess. 2018, 190. [Google Scholar] [CrossRef]
- Gonçalves, V.D.; Meirelles-Pereira, F.; Cataldo, M.; de Fonseca, B.O.; Nogueira, B.A.; Olivella, J.G.B.; de Esteves, F.A.; Mattos-Guaraldi, A.L.; de Andrade, A.F.B.; Bello, A.R.; et al. Detection of multidrug-resistant Enterobacteria isolated from river waters flowing to Guanabara Bay (Rio de Janeiro, Brazil) and from clinical samples of hospital origin. Biomedica 2019, 39, 135–149. [Google Scholar] [CrossRef]
- Arsand, J.B.; Hoff, R.B.; Jank, L.; Bussamara, R.; Dallegrave, A.; Bento, F.M.; Kmetzsch, L.; Falção, D.A.; do Carmo Ruaro Peralba, M.; de Araujo Gomes, A.; et al. Presence of antibiotic resistance genes and its association with antibiotic occurrence in Dilúvio River in southern Brazil. Sci. Total Environ. 2020, 738, 139781. [Google Scholar] [CrossRef]
- Fernandes, M.R.; Sellera, F.P.; Moura, Q.; Esposito, F.; Sabino, C.P.; Lincopan, N. Identification and genomic features of halotolerant extended-spectrum-β-lactamase (CTX-M)-producing Escherichia coli in urban-impacted coastal waters, Southeast Brazil. Mar. Pollut. Bull. 2020, 150, 110689. [Google Scholar] [CrossRef]
- Fuentefria, D.B.; Ferreira, A.E.; Gräf, T.; Corção, G. Spread of metallo-β-lactamases: Screening reveals the presence of a blaspm-1 gene in hospital sewage in southern Brazil. Braz. J. Microbiol. 2009, 40, 82–85. [Google Scholar] [CrossRef][Green Version]
- Gutierrez, O.; Navarro, L.; Loeza, P.; Del Río, O.; Jiménez, R. Perfiles de resistencia a antibióticos y metales pesados en Pseudomonas aeruginosa potencialmente patógenas aisladas de agua de uso agrícola Antibiotic and heavy metal resistance profiles in potentially pathogenic Pseudom. Rev. Electrónica Nov. Sci. 2017, 9, 97–112. [Google Scholar]
- Correa-Basurto, A.; Peña-Betancourt, M.T.N.-C.S.D. Bacterias heterótrofas aisladas del lago de los Reyes Aztecas ( Tlahuac ) y su resistencia a diferentes antibióticos. Rev. Latinoam. Recur. Nat. 2007, 3, 84–89. [Google Scholar]
- Cruz-Leyva, M.C.; González-de la Cruz, J.U.; Debelis Urbina, V.C.; Álvarez González, C.A.; Cuenca-Soria, C.A. Resistencia De Salmonella Spp A Antibióticos Y Fluctuación Fisicoquímica Del Agua A Nivel Espacial En La Cuenca Media Del Río Usumacinta, México. Eur. Sci. J. ESJ 2020, 16, 43–56. [Google Scholar] [CrossRef]
- Martínez-Orgániz, A.; Garza-Ramos, U.; Sampedro-Rosas, M.L.; González-González, J.; Nava-Faustino, G.; Toribio Jiménez, J. Patotipos y resistencia aantibióticos de Escherichia coli en agua residual. Rev. Int. Contam. Ambient. 2020, 36, 957–966. [Google Scholar] [CrossRef]
- Delgado-Gardea, M.C.E.; Tamez-Guerra, P.; Gomez-Flores, R.; de la Serna, F.J.Z.D.; Eroza-de la Vega, G.; Nevárez-Moorillón, G.V.; Pérez-Recoder, M.C.; Sánchez-Ramírez, B.; González-Horta, M.D.C.; Infante-Ramírez, R. Multidrug-resistant bacteria isolated from surface water in Bassaseachic Falls National Park, Mexico. Int. J. Environ. Res. Public Health 2016, 13, 597. [Google Scholar] [CrossRef]
- Mondragón, V.A.; Llamas-Pérez, D.F.; González-Guzmán, G.E.; Márquez-González, A.R.; Padilla-Noriega, R.; Durán-Avelar, M.d.J.; Franco, B. Identification of Enterococcus faecalis bacteria resistant to heavy metals and antibiotics in surface waters of the Mololoa River in Tepic, Nayarit, Mexico. Environ. Monit. Assess. 2011, 183, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Ramírez Castillo, F.Y.; Avelar González, F.J.; Garneau, P.; Díaz, F.M.; Guerrero Barrera, A.L.; Harel, J. Presence of multi-drug resistant pathogenic Escherichia coli in the San Pedro River located in the State of Aguascalientes, Mexico. Front. Microbiol. 2013, 4, 147. [Google Scholar] [CrossRef] [PubMed]
- Rosas, I.; Salinas, E.; Martínez, L.; Cruz-Còrdova, A.; Gonzàlez-Pedrajo, B.; Espinosa, N.; Amábile-Cuevas, C.F. Characterization of Escherichia coli isolates from an urban lake receiving water from a wastewater treatment plant in Mexico city: Fecal pollution and antibiotic resistance. Curr. Microbiol. 2015, 71, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Lugo-Melchor, Y.; Quiñones, B.; Amézquita-López, B.A.; León-Félix, J.; García-Estrada, R.; Chaidez, C. Characterization of tetracycline resistance in salmonella enterica strains recovered from irrigation water in the culiacan valley, Mexico. Microb. Drug Resist. 2010, 16, 185–190. [Google Scholar] [CrossRef]
- Nuñez, L.; Tornello, C.; Puentes, N.; Moretton, J. Bacterias resistentes a antibióticos en aguas grises como agentes de riesgo sanitario. Rev. Ambient. Agua 2012, 7, 235–243. [Google Scholar] [CrossRef]
- Muñoz, A.V.; Pucci, O.H.; Pucci, G.N. Cepas bacterianas resistentes a ampicilina, cefotaxima, ceftazidima, clindamicina, gentamicina, imipenem, meropenem y vancomicina, aisladas de agua de mar en la ciudad de Comodoro Rivadavia (Argentina). Hig. Sanid. Ambient. 2014, 14, 1253–1257. [Google Scholar]
- Baldini, M.; Selzer, P. Patrones de resistencia a antibióticos de enterococos aislados de aguas estuarinas. Rev. Argent. Microbiol. 2008, 40, 48–51. [Google Scholar]
- Balcaza, J.A.; Kurz, I.; Macin, C.I.; Mosquera, M.S.; Sandi, A.; López, D.; Leyes, S.R.; Lösch, L.S.; Merino, L.A. Resistencia a fosfomicina, tigeciclina y colistina en enterobacterias provenientes de ambientes acuáticos del Chaco, Argentina. Rev. Cienc. Tecnol. 2020, 96–100. [Google Scholar] [CrossRef]
- Bianchi, V.; Varela, P.; Flores, D.; Ruta, N.; Andes, J.D.L.; Bianchi, V.; Varela, P.; Flores, D.; Durando, P. Evaluation of Escherichia coli resistant to antibiotics as a bioindicator species of fecal pollution in water and fishes in the lower basin of the San Juan River. Nat. Neotrop. 2014, 45, 45–69. [Google Scholar]
- Lösch, L.S.; Merino, L.A. Susceptibilidad antimicrobiana de aislamientos de Escherichia coli provenientes de diversas fuentes de agua del Chaco (Argentina). Hig. Sanid. Ambient. 2012, 12, 913–917. [Google Scholar]
- Lösch, L. Occurrence of antimicrobial-resistant Enterobacteriaceae in water from different sources in a subtropical region of Argentina. Ambient. Agua 2008, 3, 28–36. [Google Scholar] [CrossRef]
- Pulido Beltrán, J.A.; Rodríguez, X.A.; Méndez, I.A. Perfil de resistencia antimicrobiana en bacilos Gram negativos no fermentadores aislados en fuentes hídricas. Rev. Médica Risaralda 2017, 23, 38–42. [Google Scholar] [CrossRef]
- Tamayo Morales, L.; Husserl Orjuela, J. Cuantificación de cepas de Salmonella sp. resistentes a antibióticos en muestras de agua del Río Bogotá. In Proceedings of the IAMB201320, Los Andes, Colombia, 26–28 September 2013. [Google Scholar]
- Vivas, M.C.; Caicedo, L.D.; Castillo, J.E. Occurrence of β-Lactamase-Producing Gram-Negative Bacterial Isolates in Water Sources in Cali City, Colombia. Int. J. Microbiol. 2019, 2019, 1375060. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, E.A.; Garzón, L.M.; Gómez, I.D.; Jiménez, J.N. Multidrug resistance and diversity of resistance profiles in carbapenem-resistant Gram-negative bacilli throughout a wastewater treatment plant in Colombia. J. Glob. Antimicrob. Resist. 2020, 22, 358–366. [Google Scholar] [CrossRef]
- Díaz, R.D.L.A.J.; Pita, M.T.S.; Weng, Z.; Rubalcaba, S.C.; González, M.I.; Rosa, O.E.D.; Caridad, M.; Salazar, R. Sensibilidad antimicrobiana en bacterias de origen ambiental. Hig. Sanid. Ambient. 2006, 6, 150–159. [Google Scholar]
- Martínez, A.; Cruz, M.; Veranes, O.; Carballo, M.E.; Salgado, I.; Olivares, S.; Lima, L.; Rodríguez, D. Resistencia a antibióticos y a metales pesados en bacterias aisladas del río Almendares. Rev. CENIC Cienc. Biológicas 2010, 41, 1–10. [Google Scholar]
- Romeu Álvarez, B.; Salazar Jiménez, P.; Lugo Moya, D.; Rojas Hernández, N.M.; Eslava Campos, C.A. Susceptibilidad antimicrobiana de aislamientos de Escherichia coli procedentes de ecosistemas dulceacuícolas. Rev. Cubana Med. Trop. 2012, 64, 132–141. (In Spanish) [Google Scholar]
- Arpajón-Peña, Y.; Doval-García, R.; Hernández-Carretero, J.G.; Pérez-Cosme, M.; Llano-González, Y. La antibiótico-resistencia de bacterias de importancia clínica aisladas del río Almendares, Cuba, abordada como problema de salud ecosistémica. Univ. Salud 2014, 16, 58–66. [Google Scholar]
- Vargas, E.; Escobar, S.; Espinoza, C.; Fierro, A.; Araque, J.; Andueza, F. Perfiles de susceptibilidad antimicrobiana en bacterias aisladas del agua de consumo en la ciudad de Riobamba, provincia de Chimborazo, Ecuador. Vitae 2016, 23, S25–S26. [Google Scholar]
- González, M.; Viteri, F.; Villacís, L.; Escobar, J.; Araujo, L.; González, A.; Medina, G.; Araque, J. Perfiles de susceptibilidad a los antibioticos en cepas del genero Bacillus aisladas de ambientes acuáticos estremos del Ecuador. An. RANF 2021, 87, 27–34. [Google Scholar] [CrossRef]
- Andueza, F.; Albuja, A.; Arguelles, P.; Escobar, S.; Espinoza, C.; Araque, J.; Medina, G. Resistencia antimicrobiana en cepas de Pseudomonas aeruginosa aisladas de aguas termales de la provincia del Chimborazo, Ecuador. An. RANF 2015, 81, 158–163. [Google Scholar]
- Ortega-Paredes, D.; Barba, P.; Mena-López, S.; Espinel, N.; Crespo, V.; Zurita, J. High quantities of multidrug-resistant Escherichia coli are present in the Machángara urban river in Quito, Ecuador. J. Water Health 2020, 18, 67–76. [Google Scholar] [CrossRef]
- Silva, J.; Castillo, G.; Callejas, L.; López, H.; Olmos, J. Frequency of transferable multiple antibiotic resistance amongst coliform bacteria isolated from a treated sewage effluent in Antofagasta, Chile. Electron. J. Biotechnol. 2006, 9, 533–540. [Google Scholar] [CrossRef]
- Martínez, M.C.; Retamal, P.; Rojas-Aedo, J.F.; Fernández, J.; Fernández, A.; Lapierre, L. Multidrug-Resistant Outbreak-Associated Salmonella Strains in Irrigation Water from the Metropolitan Region, Chile. Zoonoses Public Health 2017, 64, 299–304. [Google Scholar] [CrossRef]
- Tomova, A.; Ivanova, L.; Buschmann, A.H.; Rioseco, M.L.; Kalsi, R.K.; Godfrey, H.P.; Cabello, F.C. Antimicrobial resistance genes in marine bacteria and human uropathogenic Escherichia coli from a region of intensive aquaculture. Environ. Microbiol. Rep. 2015, 7, 803–809. [Google Scholar] [CrossRef]
- Rojas Pirela, M.L.; Botello Suárez, W.A.; Ball Vargas, M.M. Antibiotic- and heavy-metal resistance in bacteria isolated from deep subsurface in El Callao region, Venezuela. Rev. Colomb. Biotechnol. 2014, 16, 141–149. [Google Scholar] [CrossRef]
- Correa Rivas, K.; Bravo Torrealba, M.V.; Silva Alvarado, R.A.; Montiel, M. Susceptibilidad a antibióticos de Pseudomonas aeruginosa aislada de agua de consumo humano de la comunidad Santa Rosa de Agua, Maracaibo, estado Zulia. Rev. Soc. Venez. Microbiol. 2015, 35, 83–88. [Google Scholar]
- Fernández-Delgado, M.; Suárez, P. Multiple antibiotic resistances of enteric bacteria isolated from recreational coastal waters and oysters of the Caribbean Sea. Ann. Microbiol. 2009, 59, 409–414. [Google Scholar] [CrossRef]
- Rojas-Alfaro, R.; Umaña-Castro, R.; Rojas-Campos, N.; Vargas-Montero, M. Primer reporte de bacterias y dinoflagelados marinos luminiscentes del Parque Nacional Isla del Coco, Costa Rica. Rev. Biol. Trop. 2020, 68, S213–S224. [Google Scholar] [CrossRef]
- Tzoc, E.; Arias, M.L.; Valiente, C. Efecto de las aguas residuales hospitalarias sobre los patrones de resistencia a los antibióticos de Escherichia coli y Aeromonas sp. Rev. Biomédica 2004, 15, 165–172. [Google Scholar] [CrossRef]
- Guzman-Otazo, J.; Gonzales-Siles, L.; Poma, V.; Bengtsson-Palme, J.; Thorell, K.; Flach, C.-F.; Iñiguez, V.; Sjöling, Å. Diarrheal bacterial pathogens and multi-resistant enterobacteria in the Choqueyapu River in La Paz, Bolivia. PLoS ONE 2019, 14, e0210735. [Google Scholar] [CrossRef]
- Amaya, E.; Reyes, D.; Paniagua, M.; Calderón, S.; Rashid, M.U.; Colque, P.; Kühn, I.; Möllby, R.; Weintraub, A.; Nord, C.E. Antibiotic resistance patterns of Escherichia coli isolates from different aquatic environmental sources in León, Nicaragua. Clin. Microbiol. Infect. 2012, 18. [Google Scholar] [CrossRef] [PubMed]
- Larson, A.; Hartinger, S.M.; Riveros, M.; Salmon-Mulanovich, G.; Hattendorf, J.; Verastegui, H.; Huaylinos, M.L.; Mäusezahl, D. Antibiotic-resistant Escherichia coli in drinking water samples from rural andean households in Cajamarca, Peru. Am. J. Trop. Med. Hyg. 2019, 100, 1363–1368. [Google Scholar] [CrossRef]
- International Monetary Fund. Report for Selected Countries and Subjects. Available online: https://www.imf.org/en/Publications/SPROLLs/world-economic-outlook-databases#sort=%40imfdatedescending (accessed on 23 September 2021).
- Pan American Health Organization. Health Situation in the Americas. Core Indicators 2018; Pan American Health Organization: Washington, DC, USA, 2018. [Google Scholar]
- Ramírez-Castillo, F.Y.; Harel, J.; Moreno-Flores, A.C.; Loera-Muro, A.; Guerrero-Barrera, A.L.; Avelar-González, F.J. Antimicrobial resistance: The role of aquatic environments. Int. J. Curr. Res. Acad. Rev. 2014, 2, 231–243. [Google Scholar]
- World Health Organization. WHO Guidelines for Safe Use of Wasteater, Excreta and Greywater; World Health Organization: Geneva, Switzerland, 2006; Available online: https://www.who.int/water_sanitation_health/publications/gsuweg4/en/ (accessed on 23 September 2021).
- Rodríguez, D. Investing in Wastewater in Latin America Can Pay Off. 2017. Available online: https://blogs.worldbank.org/water/how-can-we-make-wastewater-investments-sustainable-latin-america (accessed on 23 September 2021).
- Deser, A.; Vázquez-Vélez, E.; Treviño, S.; Wirtz, V. Regulation of antibiotic sales in Mexico: An analysis of printed media coverage and stakeholder participation. BMC Public Health 2012, 12, 1051. [Google Scholar] [CrossRef]
- Pan American Health Organization. Latin American Countries Advance in the Surveillance of Antimicrobial Consumption; Pan American Health Organization: Washington, DC, USA, 2021; Available online: https://www.paho.org/en/news/21-4-2021-latin-american-countries-advance-surveillance-antimicrobial-consumption (accessed on 23 September 2021).
- Zaidi, M.; Dreser, A.; Figueroa, I.M. A collaborative initiative for the containment of antimicrobial resistance in Mexico. Zoonoses Public Health. 2015, 62 (Suppl. 1), 52–57. [Google Scholar] [CrossRef]
- Rabello, R.; Bonelli, R.; Penna, B.; Alburquerque, J.; Souza, R.; Cerqueira, A. Antimicrobial resistance in farm animals in Brazil: An update overview. Animals 2020, 10, 552. [Google Scholar] [CrossRef]
- Cabrera-Pardo, J.; Lood, R.; Udekwu, K.; Gonzalez-Rocha, G.; Munita, J.; Järhult, J.; Opazo-Capurro, A. A One Health—One World initiative to control antibiotic resistance: A Chile–Sweden collaboration. One Health 2019, 8, 100100. [Google Scholar] [CrossRef]


| Antibiotic Group | NCC | SA |
|---|---|---|
| Aminoglucoside | 93 | 89 |
| Aminocumarine | 100 | NT |
| Amphenichol | 92 | 83 |
| Ansamycin | NT | 100 |
| Carbapenem | 40 | 84 |
| Carboxipenicillin | 100 | 100 |
| Cephalosporin (1st generation) | 100 | 100 |
| Cephalosporin (2nd generation) | 100 | 90 |
| Cephalosporin (3rd generation) | 79 | 96 |
| Cephalosporin (4th generation) | NT | 100 |
| Fluoroquinolone | 100 | 83 |
| Glycopeptide | 100 | 71 |
| Glycylcycline | NT | 75 |
| Inhibition nucleic acid synthesis | 100 | 87 |
| Lincomycin | NT | 100 |
| Macrolide | 100 | 100 |
| Nitrofurane | 100 | 100 |
| Penicillin | 100 | 94 |
| Penicillin + betalactamase inhibitor | 80 | 90 |
| Phosphonic | NT | 100 |
| Polymyxin | NT | 75 |
| Quinolone | 87.5 | 83 |
| Tetracycline | 100 | 97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domínguez, D.C.; Chacón, L.M.; Wallace, D. Anthropogenic Activities and the Problem of Antibiotic Resistance in Latin America: A Water Issue. Water 2021, 13, 2693. https://doi.org/10.3390/w13192693
Domínguez DC, Chacón LM, Wallace D. Anthropogenic Activities and the Problem of Antibiotic Resistance in Latin America: A Water Issue. Water. 2021; 13(19):2693. https://doi.org/10.3390/w13192693
Chicago/Turabian StyleDomínguez, Delfina C., Luz María Chacón, and D’Janique Wallace. 2021. "Anthropogenic Activities and the Problem of Antibiotic Resistance in Latin America: A Water Issue" Water 13, no. 19: 2693. https://doi.org/10.3390/w13192693
APA StyleDomínguez, D. C., Chacón, L. M., & Wallace, D. (2021). Anthropogenic Activities and the Problem of Antibiotic Resistance in Latin America: A Water Issue. Water, 13(19), 2693. https://doi.org/10.3390/w13192693






