Structural Characteristics of Aerobic Granular Sludge and Factors That Influence Its Stability: A Mini Review
Abstract
:1. Introduction
2. Morphology Classification of AGS
2.1. Zoogloea Type AGS
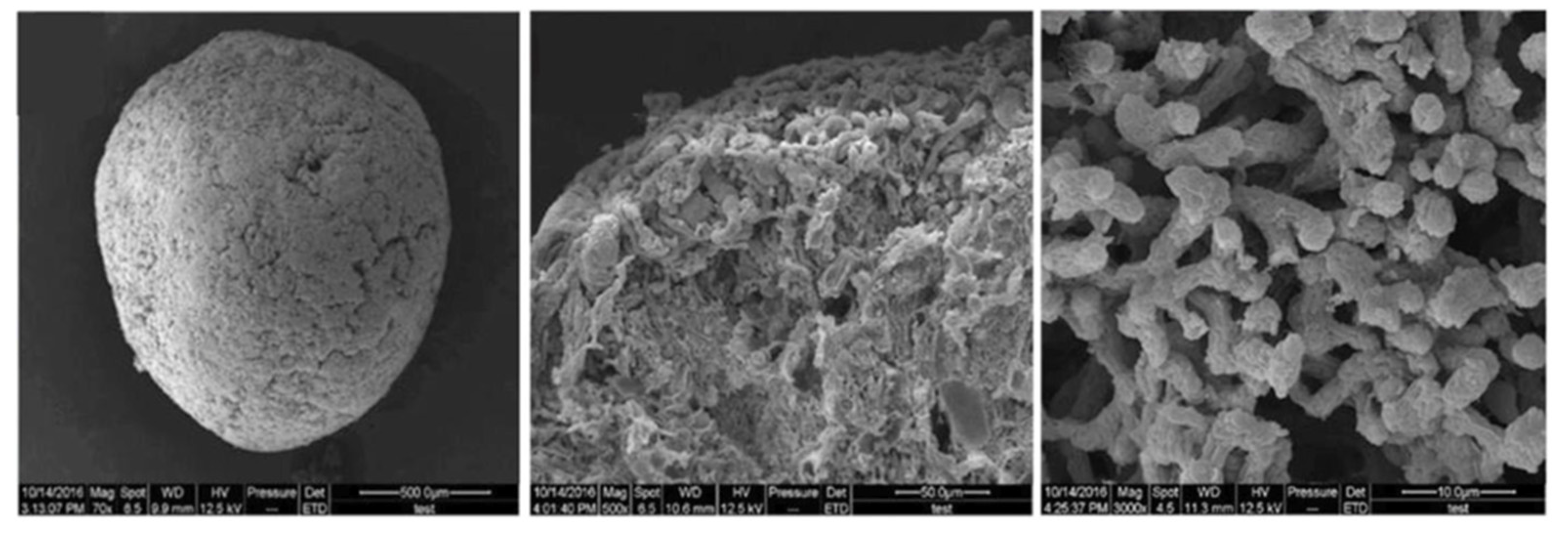
2.2. Filamentous Type AGS
2.3. Integrated Type AGS
3. Microbial Characteristics of AGS
3.1. Dominant Bacterial Group
3.2. Spatial Distribution Characteristics of Microflora
4. Macroscale Influencing Factors on AGS Structural Stability
4.1. Reactor
4.1.1. Operating Mode and Aspect Ratio (H/D)
4.1.2. Hydraulic Shear Stress
4.1.3. Organic Loading Rate (OLR)
4.1.4. Other Factors
4.2. Reaction System
4.2.1. Substrate Conditions
4.2.2. C/N Ratio
4.2.3. Inoculated Sludge
4.2.4. pH
4.2.5. Temperature
5. Microscale Influencing Factors on AGS Structural Stability
5.1. Microorganisms
5.1.1. Microbial Community Structure
5.1.2. Microbial Quorum Sensing Effect
5.2. Physicochemical Properties of Sludge
5.2.1. PN/PS
5.2.2. Particle Size
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bengtsson, S.; de Blois, M.; Wilén, B.-M.; Gustavsson, D. A comparison of aerobic granular sludge with conventional and compact biological treatment technologies. Environ. Technol. 2018, 40, 2769–2778. [Google Scholar] [CrossRef] [PubMed]
- Winkler, M.-K.H.; Meunier, C.; Henriet, O.; Mahillon, J.; Suárez-Ojeda, M.E.; Moro, G.D.; De Sanctis, M.; Di laconi, C.; Weissbrodt, D.G. An integrative review of granular sludge for the biological removal of nutrients and recalcitrant organic matter from wastewater. Chem. Eng. J. 2018, 336, 489–502. [Google Scholar] [CrossRef]
- Jeong, K.; Kim, D.G.; Ko, S.O. Adsorption characteristics of effluent organic matter and natural organic matter by carbon based nanomaterials. KSCE J. Civ. Eng. 2017, 21, 119–126. [Google Scholar] [CrossRef]
- Yu, Y.; Yu, H.J.; Ma, C.C. Study on the impact of do and organic matter on aerobic granular sludge treating municipal sewage. Adv. Mater. Res. 2014, 989–994, 603–606. [Google Scholar] [CrossRef]
- Sarma, S.J.; Tay, J.H.; Chu, A. Finding knowledge gaps in aerobic granulation technology. Trends Biotechnol. 2017, 35, 66–78. [Google Scholar] [CrossRef]
- Ou, D.; Li, H.; Li, W.; Wu, X.; Wang, Y.; Liu, Y. Salt-tolerance aerobic granular sludge: Formation and microbial community characteristics. Bioresour. Technol. 2018, 249, 132–138. [Google Scholar] [CrossRef]
- Purnell, S.; Ebdon, J.; Buck, A.; Tupper, M.; Taylor, H. Removal of phages and viral pathogens in a full-scale MBR: Implications for wastewater reuse and potable water. Water Res. 2016, 100, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Mishima, K.; Nakamura, M. Self-immobilization of aerobic activated sludge—A pilot study of the aerobic upflow sludge blanket process in municipal sewage treatment. Water Sci. Technol. 1991, 23, 981–990. [Google Scholar] [CrossRef]
- Pronk, M.; de Kreuk, M.K.; de Bruin, B.; Kamminga, P.; Kleerebezem, R.; van Loosdrecht, M.C.M. Full scale performance of the aerobic granular sludge process for sewage treatment. Water Res. 2015, 84, 207–217. [Google Scholar] [CrossRef]
- Sarma, S.J.; Tay, J.H. Aerobic granulation for future wastewater treatment technology: Challenges ahead. Environ. Sci. Water Res. Technol. 2018, 4, 9–15. [Google Scholar] [CrossRef]
- Corsino, S.F.; Trapani, D.D.; Torregrossa, M.; Viviani, G. Aerobic granular sludge treating high strength citrus wastewater: Analysis of pH and organic loading rate effect on kinetics, performance and stability. J. Environ. Manag. 2018, 214, 23–35. [Google Scholar] [CrossRef]
- Gao, M.; Yuan, S.; Zhu, F. Disintegration of aerobic granules during prolonged operation. Environ. Sci. Water Res. Technol. 2017, 3, 757–766. [Google Scholar]
- Yuan, Q.; Gong, H.; Xi, H.; Xu, H.; Jin, Z.; Ali, N.; Wang, K. Strategies to improve aerobic granular sludge stability and nitrogen removal based on feeding mode and substrate. J. Environ. Manag. 2019, 84, 144–154. [Google Scholar] [CrossRef]
- Lin, H.; Ma, R.; Hu, Y.; Lin, J.; Sun, S.; Jiang, J.; Li, T.; Liao, Q.; Luo, J. Reviewing bottlenecks in aerobic granular sludge technology: Slow granulation and low granular stability. Environ. Pollut. 2020, 263, 114638. [Google Scholar] [CrossRef]
- Liu, Y.; Tay, J.H. State of the art of biogranulation technology for wastewater treatment. Biotechnol. Adv. 2004, 22, 533–563. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhang, N.; Yang, J.; Fu, Q.; Wang, Y.; Wang, D.; Tang, L.; Xia, J.; Liu, X.; Li, X.; et al. Interaction between perfluorooctanoic acid and aerobic granular sludge. Water Res. 2020, 169, 115249. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Lu, H.; Yao, L.; Leng, L.; Guan, L. Rapid formation of aerobic granular sludge and its mechanism in a continuous-flow bioreactor. Appl. Biochem. Biotechnol. 2016, 181, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Li, W.; Yang, S.; Du, P. Extraction and structural characteristics of extracellular polymeric substances (EPS), pellets in autotrophic nitrifying biofilm and activated sludge. Chemosphere 2010, 81, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; An, Q.; Zhao, B.; Guo, J.S.; Huang, Y.S.; Tian, M. Auto-aggregation properties of a novel aerobic denitrifier Enterobacter sp. strain FL. Appl. Microbiol. Biotechnol. 2018, 102, 2019–2030. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, N.; Gao, M.; Wang, J.; Zhang, A.; Liu, Y. Synergistic strengthening mechanism of hydraulic selection pressure and poly aluminum chloride (PAC) regulation on the aerobic sludge granulation. Sci. Total. Environ. 2019, 650, 941–950. [Google Scholar] [CrossRef]
- Zhang, B.; Lens, P.N.L.; Shi, W.; Zhang, R.; Zhang, Z.; Guo, Y.; Bao, X.; Cui, F. Enhancement of aerobic granulation and nutrient removal by an algal-bacterial consortium in a lab-scale photobioreactor. Chem. Eng. J. 2018, 334, 2373–2382. [Google Scholar] [CrossRef]
- Li, Z.H.; Zhang, T.; Li, N.; Wang, X.C. Granulation of filamentous microorganisms in a sequencing batch reactor with saline wastewater. J. Environ. Sci. 2010, 22, 62–67. [Google Scholar] [CrossRef]
- Li, A.; Zhang, T.; Li, X. Fate of aerobic bacterial granules with fungal contamination under different organic loading conditions. Chemosphere 2010, 78, 500–509. [Google Scholar] [CrossRef] [Green Version]
- Du, S.; Zhang, Z.H.; Zhang, Y.F.; Jian, P.C. Characteristics of aerobic granular sludge cultivated by promoting and inhibiting filamentous bacteria. Adv. Mater. Res. 2011, 183–185, 1075–1079. [Google Scholar] [CrossRef]
- Chen, M.Y.; Lee, D.J.; Tay, J.H. Distribution of extracellular polymeric substances in aerobic granules. Appl. Microbiol. Biotechnol. 2007, 73, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Wang, L.; Su, H. Role and influence of extracellular polymeric substances on the preparation of aerobic granular sludge. J. Environ. Manag. 2016, 173, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Adav, S.S.; Lee, D.-J.; Show, K.-Y.; Tay, J.-H. Aerobic granular sludge: Recent advances. Biotechnol. Adv. 2008, 26, 411–423. [Google Scholar] [CrossRef]
- Yang, X.; Xu, T.; Cao, P.; Qiao, K.; Wang, L.; Zhao, T.; Zhu, J. The viscosity behaviors of bacterial suspensions or extracellular polymeric substances and their effects on aerobic granular sludge. Environ. Sci. Pollut. Res. 2019, 26, 30087–30097. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Zhang, T.; Ai, C.; Liu, D.; Chen, Q. Rapid granulation of sludge in anaerobic sequencing batch reactor: Strategy, mechanism and population structure. China Water Wastewater 2018, 34, 30–36. (In Chinese) [Google Scholar]
- Chen, Y.; Zhu, Z.; Yu, W.; Zhang, C. Granulation process and mechanism of aerobic granular sludge under salt stress in a sequencing batch reactor. Tecnol. Cienc. Agua 2019, 10, 156–181. [Google Scholar] [CrossRef]
- Zhao, L.; Su, C.; Wang, A.; Fan, C.; Huang, X.; Li, F.; Li, R. Comparative study of aerobic granular sludge with different carbon sources: Effluent nitrogen forms and microbial community. J. Water Process Eng. 2021, 43, 102211. [Google Scholar] [CrossRef]
- Winkler, M.-K.H.; Kleerebezem, R.; de Bruin, L.M.M.; Verheijen, P.J.T.; Abbas, B.; Habermacher, J.; van Loosdrecht, M.C.M. Microbial diversity differences within aerobic granular sludge and activated sludge flocs. Appl. Microbiol. Biotechnol. 2013, 97, 7447–7458. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Ye, L.; Ren, H. Microbial community structure and function in aerobic granular sludge. Appl. Microbiol. Biotechnol. 2018, 102, 3967–3979. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Tu, Q.; Su, X.; Yang, X.; Chen, J.; Chen, Y.; Li, H.; Liu, C.; He, Q. Structural characteristics of different enhanced aerobic granules. J. Civ. Environ. Eng. 2019, 41, 167–173. [Google Scholar]
- He, Q.; Song, J.; Zhang, W.; Gao, S.; Wang, H.; Yu, J. Enhanced simultaneous nitrification, denitrification and phosphorus removal through mixed carbon source by aerobic granular sludge. J. Hazard. Mater. 2020, 382, 121043. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yu, P.; Li, Q.; Ren, H.; Liu, B.; Ye, L.; Zhang, X.-X. Transformation of anaerobic granules into aerobic granules and the succession of bacterial community. Appl. Microbiol. Biotechnol. 2017, 101, 7703–7713. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Dai, X.; Xu, X.; Lv, M.; Cao, D. Microbial community analysis for aerobic granular sludge reactor treating high-level 4-chloroaniline wastewater. Int. J. Environ. Sci. Technol. 2014, 11, 1845–1854. [Google Scholar] [CrossRef] [Green Version]
- Geng, M.; Ma, F.; Guo, H.; Su, D. Enhanced aerobic sludge granulation in a Sequencing Batch Reactor (SBR) by applying mycelial pellets. J. Clean. Prod. 2020, 274, 123037. [Google Scholar] [CrossRef]
- Wang, Q.; Yuan, Q.; Gong, H.; Yao, R.; Qin, Y.; Liu, X.; Xu, H.; Wang, K. Characteristics and microorganism analysis of aerobic granular sludge cultivated by SBR systems with low-strength sewage. Chin. J. Environ. Eng. 2018, 12, 47–56. [Google Scholar]
- Carrera, P.; Campo, R.; Méndez, R.; Bella, D.G.; Campos, J.L.; Corral, A.M.; Rio, A.V. Does the feeding strategy enhance the aerobic granular sludge stability treating saline effluents. Chemosphere 2019, 226, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Yarlagadda, V.N.; Manjunath, S. Aerobic granular sludge process: A fast growing biological treatment for sustainable wastewater treatment. Curr. Opin. Environ. Sci. Health 2019, 12, 57–65. [Google Scholar]
- Santo, F.C.; Alessandro, B.; Tanner, R.D.; Giulio, M.; Michele, T.; Jan, A.O. Effect of extended famine conditions on aerobic granular sludge stability in the treatment of brewery wastewater. Bioresour. Technol. 2017, 226, 150–157. [Google Scholar]
- Hamiruddin, N.A.; Awang, N.A. The Relationship between the biokinetic parameters of an aerobic granular sludge system and the applied operating conditions. Civ. Environ. Eng. Rep. 2021, 31, 161–171. [Google Scholar] [CrossRef]
- Hamiruddin, N.A.; Awang, N.A.; Shaaban, M.G. The performance of extracellular polymeric substance (EPS) on stability of aerobic granular sludge (AGS). Civ. Environ. Eng. Rep. 2019, 29, 60–69. [Google Scholar] [CrossRef] [Green Version]
- Tay, J.-H.; Liu, Q.-S.; Liu, Y. The effects of shear force on the formation, structure and metabolism of aerobic granules. Appl. Microbiol. Biotechnol. 2001, 57, 227–233. [Google Scholar]
- Zhou, J.-H.; Zhang, Z.-M.; Zhao, H.; Yu, H.-T.; Alvarez, P.J.J.; Xu, X.-Y.; Zhu, L. Optimizing granules size distribution for aerobic granular sludge stability: Effect of a novel funnel-shaped internals on hydraulic shear stress. Bioresour. Technol. 2016, 216, 562–570. [Google Scholar] [CrossRef]
- Devlin, T.R.; di Biase, A.; Kowalski, M.S.; Oleszkiewicz, J.A. Granulation of activated sludge under low hydrodynamic shear and different wastewater characteristics. Bioresour. Technol. 2017, 224, 229–235. [Google Scholar] [CrossRef]
- Long, B.; Yang, C.-Z.; Pu, W.-H.; Yang, J.-K.; Liu, F.-B.; Zhang, L.; Zhang, J.; Cheng, K. Tolerance to organic loading rate by aerobic granular sludge in a cyclic aerobic granular reactor. Bioresour. Technol. 2015, 182, 314–322. [Google Scholar] [CrossRef]
- Liu, Y.-Q.; Tay, J.-H. Fast formation of aerobic granules by combining strong hydraulic selection pressure with overstressed organic loading rate. Water Res. 2015, 80, 256–266. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Qiu, J.; Xiang, R.; Yu, H.; Xu, X.; Zhu, L. Organic loading rate (OLR) regulation for enhancement of aerobic sludge granulation: Role of key microorganism and their function. Sci. Total. Environ. 2019, 653, 630–637. [Google Scholar] [CrossRef]
- Chen, H.; Li, A.; Cui, D.; Cui, C.; Ma, F. Evolution of microbial community and key genera in the formation and stability of aerobic granular sludge under a high organic loading rate. Bioresour. Technol. Rep. 2019, 7, 100280. [Google Scholar] [CrossRef]
- de Rollemberg, S.L.S.; Barros, A.R.M.; Firmino, P.I.M.; dos Santos, A. Aerobic granular sludge: Cultivation parameters and removal mechanisms. Bioresour. Technol. 2018, 270, 678–688. [Google Scholar] [CrossRef]
- Zhu, L.; Yu, Y.; Dai, X.; Xu, X.; Qi, H. Optimization of selective sludge discharge mode for enhancing the stability of aerobic granular sludge process. Chem. Eng. J. 2013, 217, 442–446. [Google Scholar] [CrossRef]
- He, Q.; Chen, L.; Zhang, S.; Wang, L.; Liang, J.; Xia, W.; Wang, H.; Zhou, J. Simultaneous nitrification, denitrification and phosphorus removal in aerobic granular sequencing batch reactors with high aeration intensity: Impact of aeration time. Bioresour. Technol. 2018, 263, 214–222. [Google Scholar] [CrossRef]
- Yilmaz, G.; Bozkurt, U.; Magden, K.A. Effect of iron ions (Fe2+, Fe3+) on the formation and structure of aerobic granular sludge. Biodegradation 2017, 28, 53–68. [Google Scholar] [CrossRef]
- Sajjad, M.; Kim, K.S. Studies on the interactions of Ca2+ and Mg2+ with EPS and their role in determining the physicochemical characteristics of granular sludges in SBR system. Process Biochem. 2015, 50, 966–972. [Google Scholar] [CrossRef]
- Yang, X.-P.; Han, J.; Zhou, L.-X. Role of Ca2 in the formation of glucose-fed aerobic granular sludge in sequencing batch reactor. Environ. Sci. 2010, 31, 1269–1273. [Google Scholar]
- Felz, S.; Kleikamp, H.; Zlopasa, J.; van Loosdrecht, M.C.M.; Lin, Y. Impact of metal ions on structural EPS hydrogels from aerobic granular sludge. Biofilm 2020, 2, 100011. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Feng, H.X.; Jiang, F.; Chen, N.L.; Wang, X.C. Research of carbon and nitrogen ratio and sludge stability in aerobic granular sludge bioreactor. Adv. Mater. Res. 2012, 518–523, 473–477. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, Z.; Dong, J.; Wang, Z.; Ma, K.; Xu, X.; Alvarezc, P.J.J.; Zhu, L. Stability of aerobic granular sludge under condition of low influent C/N ratio: Correlation of sludge property and functional microorganism. Bioresour. Technol. 2018, 270, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Sarvajith, M.; Kiran Kumar Reddy, G.; Nancharaiah, Y.V. Aerobic granular sludge for high-strength ammonium wastewater treatment: Effect of COD/N ratios, long-term stability and nitrogen removal pathways. Bioresour. Technol. 2020, 306, 123150. [Google Scholar] [CrossRef]
- Song, Z.; Pan, Y.; Zhang, K. Effect of seed sludge on characteristics and microbial community of aerobic granular sludge. J. Environ. Sci. 2010, 22, 1312–1318. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, X.; Nuramkhaan, M.; Lei, Z.; Shimizu, K.; Zhang, Z.; Adachi, Y.; Lee, D.-J.; Tay, J.H. Rapid granulation of aerobic granular sludge: A mini review on operation strategies and comparative analysis. Bioresour. Technol. Rep. 2019, 7, 100206. [Google Scholar] [CrossRef]
- Lei, L.; Yao, J.; Liu, Y.; Li, W. Performance, sludge characteristics and microbial community in a salt-tolerant aerobic granular SBR by seeding anaerobic granular sludge. Int. Biodeterior. Biodegrad. 2021, 163, 105258. [Google Scholar] [CrossRef]
- Wang, X.-C.; Chen, Z.-L.; Kang, J.; Zhao, X.; Shen, J.-M.; Yang, L. The key role of inoculated sludge in fast start-up of sequencing batch reactor for the cultivation of aerobic granular sludge. J. Environ. Sci. 2018, 78, 127–136. [Google Scholar] [CrossRef]
- Zou, J.; Pan, J.; Wu, S.; Qian, M.; He, Z.; Wang, B.; Li, J. Rapid control of activated sludge bulking and simultaneous acceleration of aerobic granulation by adding intact aerobic granular sludge. Sci. Total. Environ. 2019, 674, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, M.; Mirbagheri, S.A.; Bagheri, Z.; Kamarkhani, A.M. Modeling and optimization of activated sludge bulking for a real wastewater treatment plant using hybrid artificial neural networks-genetic algorithm approach. Process Saf. Environ. Prot. 2015, 95, 12–25. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, K.; Shang, Y.; Zhang, H.; Wei, L.; Wang, H. Response and recovery of aerobic granular sludge to pH shock for simultaneous removal of aniline and nitrogen. Chemosphere 2019, 221, 366–374. [Google Scholar] [CrossRef]
- Muñoz-Palazón, B.; Rodriguez-Sanchez, A.; Hurtado-Martínez, M.; Santana, F.; Gonzalez-Lopez, J.; Mack, L.; Gonzalez-Martinez, A. Polar Arctic Circle biomass enhances performance and stability of aerobic granular sludge systems operated under different temperatures. Bioresour. Technol. 2019, 300, 122650. [Google Scholar] [CrossRef] [PubMed]
- González-Martínez, A.; Muñoz-Palazón, B.; Rodriguez-Sanchez, A.; Maza-Márquez, P.; Mikola, A.; Gonzalez-Lopez, J.; Vahala, R. Start-up and operation of an aerobic granular sludge system under low working temperature inoculated with cold-adapted activated sludge from Finland. Bioresour. Technol. 2017, 239, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Ab Halim, M.H.; Anuar, A.N.; Jamal, N.S.A.; Siti, I.A.; Ujang, Z.; Mustafa, M.B. Influence of high temperature on the performance of aerobic granular sludge in biological treatment of wastewater. J. Environ. Manag. 2016, 184, 271–280. [Google Scholar] [CrossRef]
- Sharaf, A.; Guo, B.; Liu, Y. Impact of the filamentous fungi overgrowth on the aerobic granular sludge process. Bioresour. Technol. Rep. 2019, 7, 100272. [Google Scholar] [CrossRef]
- Maddela, N.R.; Sheng, B.B.; Yuan, S.S.; Zhou, Z.B. Roles of quorum sensing in biological wastewater treatment: A critical review. Chemosphere 2019, 221, 616–629. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, Z.; Wang, Z.; Ma, K.; Xu, X.; Alvarezc, P.J.J.; Zhu, L. Understanding of aerobic sludge granulation enhanced by sludge retention time in the aspect of quorum sensing. Bioresour. Technol. 2019, 272, 226–234. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, R.; Jin, L.; Zhu, W.; Ji, Y.; Xu, X.; Zhu, L. The regulation of N-acyl-homoserine lactones (AHLs)-based quorum sensing on EPS secretion via ATP synthetic for the stability of aerobic granular sludge. Sci. Total. Environ. 2019, 673, 83–91. [Google Scholar] [CrossRef]
- Chen, H.; Li, A.; Cui, C.W.; Ma, F.; Cui, D.; Zhao, H.P.; Wang, Q.L.; Ni, B.J.; Yang, J.X. AHL-mediated quorum sensing regulates the variations of microbial community and sludge properties of aerobic granular sludge under low organic loading. Environ. Int. 2019, 130, 104946. [Google Scholar] [CrossRef]
- Wang, S.; Shi, W.; Tang, T.; Wang, Y.; Zhi, L.; Lv, J.; Li, J. Function of quorum sensing and cell signaling in the formation of aerobic granular sludge. Rev. Environ. Sci. Biotechnol. 2017, 16, 1–13. [Google Scholar] [CrossRef]
- He, Q.; Wang, H.; Chen, L.; Gao, S.; Zhang, W.; Song, J.; Yu, J. Robustness of an aerobic granular sludge sequencing batch reactor for low strength and salinity wastewater treatment at ambient to winter temperatures. J. Hazard. Mater. 2020, 384, 121454. [Google Scholar] [CrossRef]
- He, H.; Chen, Y.; Li, X.; Cheng, Y.; Yang, C.; Zeng, G. Influence of salinity on microorganisms in activated sludge processes: A review. Int. Biodeterior. Biodegrad. 2017, 119, 520–527. [Google Scholar] [CrossRef]
- Wang, Y.; Zhi, L.; Ma, X.; Wang, S.; Li, J. Characterization of extracellular polymeric substances from aerobic granular sludge. J. Harbin Inst. Technol. 2019, 52, 153–160. [Google Scholar]
- Wang, Y.; Wang, W.; Feng, D.; Cheng, L. Aerobic granulation of activated sludge to treat the comprehensive municipal wastewater. J. Arid Land Resour. Environ. 2018, 32, 150–155. [Google Scholar]
- Long, B.; Xuan, X.; Yang, C.; Zhang, L.; Cheng, Y.; Wang, J. Stability of aerobic granular sludge in a pilot scale sequencing batch reactor enhanced by granular particle size control. Chemosphere 2019, 225, 460–469. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Y.; Gan, C.; Chen, R.; Chen, Y.; Yuan, S.; Chen, Y. Structural Characteristics of Aerobic Granular Sludge and Factors That Influence Its Stability: A Mini Review. Water 2021, 13, 2726. https://doi.org/10.3390/w13192726
Hou Y, Gan C, Chen R, Chen Y, Yuan S, Chen Y. Structural Characteristics of Aerobic Granular Sludge and Factors That Influence Its Stability: A Mini Review. Water. 2021; 13(19):2726. https://doi.org/10.3390/w13192726
Chicago/Turabian StyleHou, Yizhi, Chunjuan Gan, Renyu Chen, Ying Chen, Shaochun Yuan, and Yao Chen. 2021. "Structural Characteristics of Aerobic Granular Sludge and Factors That Influence Its Stability: A Mini Review" Water 13, no. 19: 2726. https://doi.org/10.3390/w13192726






