Effects of Desiccation, Water Velocity, and Nitrogen Limitation on the Growth and Nutrient Removal of Neoporphyra haitanensis and Neoporphyra dentata (Bangiales, Rhodophyta)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Maintenance
2.2. Desiccation Experiment
2.3. Water Velocity Experiment
2.4. Nitrogen Limitation Experiment
2.5. Statistical Analysis
3. Results
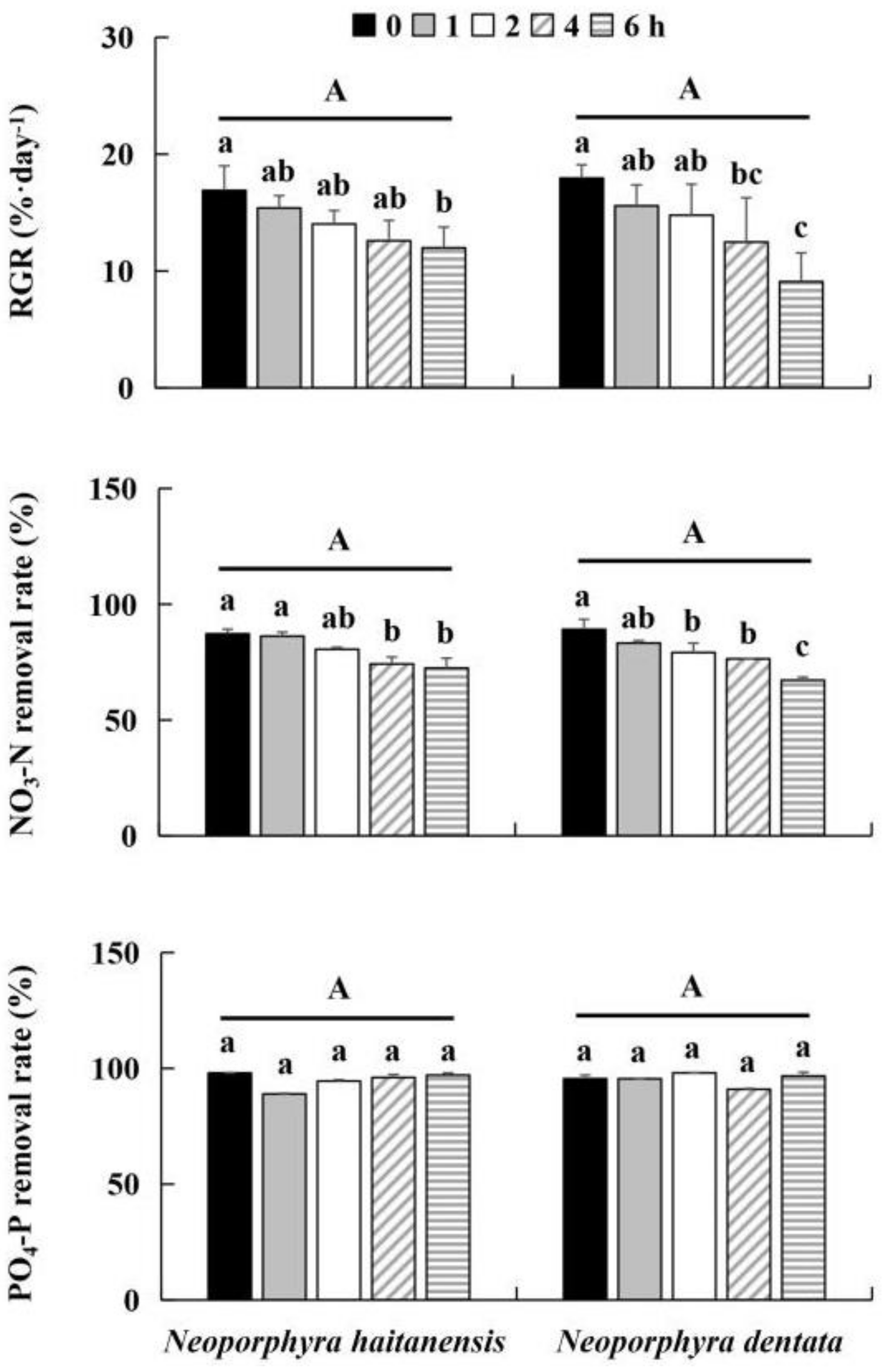
3.1. Effect of Desiccation on Growth and Nutrient Removal
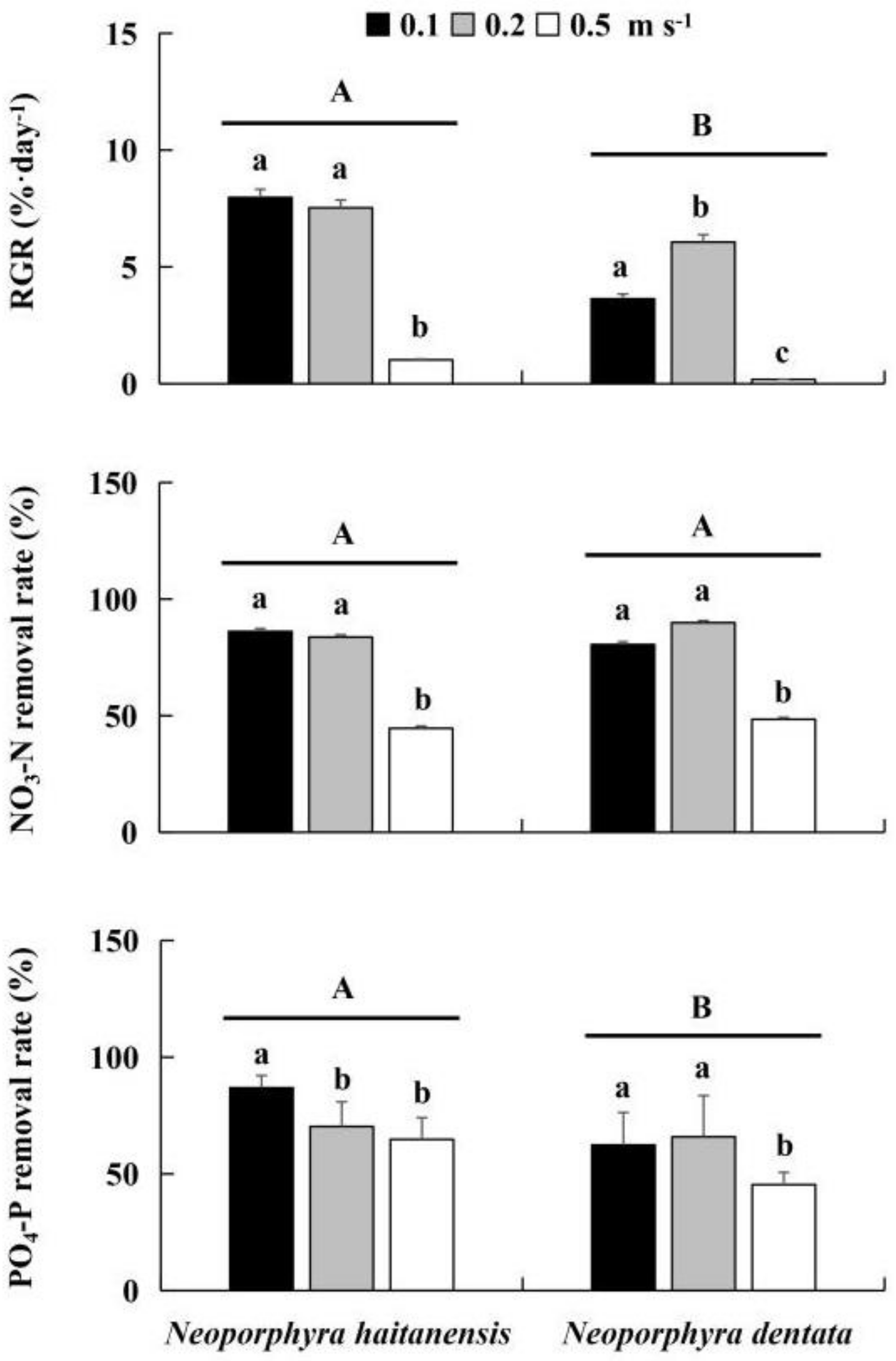
3.2. Effect of the Water Velocity on Growth and Nutrient Removal
3.3. Effect of Nitrogen Limitation on Growth and Nutrient Removal
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2020; FAO: Rome, Italy, 2020. [Google Scholar]
- Martins, C.; Eding, E.; Verdegem, M.; Heinsbroek, L.; Schneider, O.; Blancheton, J.; Roque d’Orbcastel, E.; Verreth, J. New developments in recirculating aquaculture systems in Europe: A perspective on environmental sustainability. Aquac. Eng. 2010, 43, 83–93. [Google Scholar] [CrossRef] [Green Version]
- Baloo, L.; Azman, S.; Said, M.; Ahmad, F.; Mohamad, M. Biofiltration potential of macroalgae for ammonium removal in outdoor tank shrimp wastewater recirculation system. Biomass Bioenerg. 2014, 66, 103–109. [Google Scholar] [CrossRef]
- Badiola, M.; Basurko, O.; Piedrahita, R.; Hundley, P.; Mendiola, D. Energy use in recirculating aquaculture systems (RAS): A review. Aquac. Eng. 2018, S0144860917302327. [Google Scholar] [CrossRef]
- Marinho-Soriano, E.; Azevedo, C.; Trigueiro, T.; Pereira, D.; Carneiro, M.; Camara, M. Bioremediation of aquaculture wastewater using macroalgae and Artemia. Int. Biodeterior Biodegrad. 2011, 65, 253–257. [Google Scholar] [CrossRef]
- Liu, K.; Fang, T.; Feng, Z.; Zong, S. Research progress on algae treatment to aquaculture wastewater. J. Huaihai Inst. Technol. 2016, 25, 74–79, (In Chinese with English abstract). [Google Scholar]
- Lin, Y.F.; Jing, S.; Lee, D. The potential use of constructed wetlands in a recirculating aquaculture system for shrimp culture. Environ. Pollut. 2003, 123, 107–113. [Google Scholar] [CrossRef]
- Burgin, A.; Yang, W.; Hamilton, S.; Silver, W. Beyond carbon and nitrogen: How the microbial energy economy couples elemental cycles in diverse ecosystems. Front. Ecol. Environ. 2011, 9, 44–52. [Google Scholar] [CrossRef] [Green Version]
- Castine, S.; McKinnon, A.; Paul, N.; Trott, L.; de Nys, R. Wastewater treatment for land-based aquaculture: Improvements and value-adding alternatives in model systems from Australia. Aquac. Environ. Interact. 2013, 4, 285–300. [Google Scholar] [CrossRef] [Green Version]
- Brito, L.; Cardoso Junior, L.; Lavander, H. Bioremediation of shrimp biofloc wastewater using clam, seaweed and fish. Chem. Ecol. 2018, 34, 901–913. [Google Scholar] [CrossRef]
- Marinho-Soriano, E. Historical context of commercial exploitation of seaweeds in Brazil. J. Appl. Phycol. 2017, 29, 665–671. [Google Scholar] [CrossRef]
- Shin, S.; Kim, S.; Kim, J.; Han, T.; Yarish, C.; Kim, J. Effects of stocking density on the productivity and nutrient removal of Agarophyton vermiculophyllum in Paralichthys olivaceus biofloc effluent. J. Appl. Phycol. 2020, 32, 2605–2614. [Google Scholar] [CrossRef]
- Burritt, D.; Larkindale, J.; Hurd, K. Antioxidant metabolism in the intertidal red seaweed Stictosiphonia arbuscula following desiccation. Planta 2002, 215, 829–838. [Google Scholar] [CrossRef]
- Kumar, M.; Gupta, V.; Trivedi, N.; Kumari, P.; Bijo, A.; Reddy, C.; Jha, B. Desiccation induced oxidative stress and its biochemical responses in intertidal red alga Gracilaria corticata (Gracilariales, Rhodophyta) Environ. Exp. Bot. 2011, 72, 194–201. [Google Scholar] [CrossRef]
- Hurd, C.L.; Harrison, P.; Bischof, K.; Lobban, C. Seaweed Ecology and Physiology; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Kim, J.; Kraemer, G.; Yarish, C. Comparison of growth and nitrate uptake by New England Porphyra species from different tidal elevations in relation to desiccation. Phycol. Res. 2009, 57, 152–157. [Google Scholar] [CrossRef]
- Wang, W.; Sun, X.; Liu, F.; Liang, R.; Zhang, J.; Wang, F. Effect of abiotic stress on the gameophyte of Pyropia katadae var. hemiphylla (Bangiales, Rhodophyta). J. Appl. Phycol. 2016, 28, 469–479. [Google Scholar] [CrossRef]
- Li, X.; Sun, X.; Gao, L.; Xu, J.; Gao, G. Effects of periodical dehydration on biomass yield and biochemical composition of the edible red alga Pyropia yezoensis grown at different salinities. Algal Res. 2021, 56, 102315. [Google Scholar] [CrossRef]
- Roleda, M.Y.; Hurd, C. Seaweed nutrient physiology: Application of concepts to aquaculture and bioremediation. Phycologia 2019, 58, 552–562. [Google Scholar] [CrossRef] [Green Version]
- Peteiro, C.; Freire, Ó. Biomass yield and morphological features of the seaweed Saccharina latissima cultivated at two different sites in a coastal bay in the Atlantic coast of Spain. J. Appl. Phycol. 2013, 25, 205–213. [Google Scholar] [CrossRef]
- Kregting, L.; Blight, A.; Elsäßer, B.; Savidge, G. The influence of water motion on the growth rate of the kelp Laminaria digitata. J. Exp. Mar. Biol. Ecol. 2016, 478, 86–95. [Google Scholar] [CrossRef] [Green Version]
- Mols-Mortensen, A.; Jacobsen, C.; Holdt, S. Variation in growth, yield and protein concentration in Saccharina latissima (Laminariales, Phaeophyceae) cultivated with different wave and current exposures in the Faroe Islands. J. Appl. Phycol. 2017, 29, 2277–2286. [Google Scholar] [CrossRef] [Green Version]
- Visch, W.; Nylund, G.; Pavia, H. Growth and biofouling in kelp aquaculture (Saccharina latissima): The effect of location and wave exposure. J. Appl. Phycol. 2020, 32, 3199–3209. [Google Scholar] [CrossRef]
- Msuya, F.; Neori, A. Effect of water aeration and nutrient load level on biomass yield, N uptake and protein content of the seaweed Ulva lactuca cultured in seawater tanks. J. Appl. Phycol. 2008, 20, 1021–1031. [Google Scholar] [CrossRef]
- Rula, N.; Ganzon-Fortes, E.T.; Pante, M.; Trono, J. Influence of light, water motion, and stocking density on the growth and pigment content of Halymenia durvillei (Rhodophyceae) under laboratory conditions. J. Appl. Phycol. 2021, 33, 2367–2377. [Google Scholar] [CrossRef]
- Liu, H.; Wang, F.; Wang, Q.; Dong, S.; Tian, X. A comparative study of the nutrient uptake and growth capacities of seaweeds Caulerpa lentillifera and Gracilaria lichenoides. J. Appl. Phycol. 2016, 28, 3083–3089. [Google Scholar] [CrossRef]
- Gao, X.; Endo, H.; Nagaki, M.; Agatsuma, Y. Interactive effects of nutrient availability and temperature on growth and survival of different size classes of Saccharina japonica (Laminariales, Phaeophyceae). Phycologia 2017, 56, 253–260. [Google Scholar] [CrossRef]
- Mouritsen, O.; Mouritsen, J.; Johansen, M. Seaweeds: Edible, Available, and Sustainable; University of Chicago Press: Chicago, IL, USA, 2013. [Google Scholar]
- Du, R.; Liu, L.; Wang, A.; Wang, Y. Effects of temperature, algae biomass and ambient nutrient on the absorption of dissolved nitrogen and phosphate by Rhodophyte Gracilaria asiatica. Chin. J. Oceanol. Limnol. 2013, 31, 353–365. [Google Scholar] [CrossRef]
- Gao, X.; Endo, H.; Taniguchi, K.; Agatsuma, Y. Combined effects of seawater temperature and nutrient condition on growth and survival of juvenile sporophytes of the kelp Undaria pinnatifida (Laminariales; Phaeophyta) cultivated in northern Honshu, Japan. J. Appl. Phycol. 2013, 25, 269–275. [Google Scholar] [CrossRef]
- Martín, L.; Rodríguez, M.; Matulewicz, M.; Fissore, E.; Gerschenson, L.; Leonardi, P. Seasonal variation in agar composition and properties from Gracilaria gracilis (Gracilariales, Rhodophyta) of the Patagonian coast of Argentina. Phycol. Res. 2013, 61, 163–171. [Google Scholar] [CrossRef]
- Xu, Z. The Physiological Responses of Two Economic Marine Macroalga Species to Nutrients Supplies in Seawater. Master’s Thesis, Shantou University, Shantou, China, 2007. (In Chinese with English abstract). [Google Scholar]
- Cha, Y. Study on the Nutrients Uptake and Photosynthesis Based on Bioremediation in Two Species of Economic Marine Macroalgae. Master’s Thesis, South China University of Technology, Guangzhou, China, 2013. (In Chinese with English abstract). [Google Scholar]
- Li, D.P.; Ma, Z.; Li, H.; Ding, G.; Xin, M.; Wu, H.; Guo, W. NH4-N over-compensatory uptake of Sargassum horneri under the stress of nutrients deficiency. Oceanol. ET Limnol. Sin. 2018, 49, 904–909, (In Chinese with English abstract). [Google Scholar]
- McGlathery, K.; Pedersen, M.; Borum, J. Changes in intracellular nitrogen pools and feedback controls on nitrogen uptake in Chaetomorpha linum (Chlorophyta). J. Phycol. 1996, 32, 393–401. [Google Scholar] [CrossRef]
- Sutherland, J.; Lindstrom, C.; Nelson, W.; Brodie, J.; Lynch, M.; Hwang, M.; Choi, H. A new look at an ancient order: Generic revision of the Bangiales (Rhodophyta). J. Phycol. 2011, 47, 1131–1151. [Google Scholar] [CrossRef]
- Zhang, T.; Li, J.; Ma, F.; Lu, Q.; Shen, Z.; Zhu, J. Study of photosynthetic characteristics of the Pyropia yezoensis thallus during the cultivation process. J. Appl. Phycol. 2014, 26, 859–865. [Google Scholar] [CrossRef]
- He, P.; Zhang, Z.; Zhang, X.; Ma, J. Seaweed Cultivation; Science Press: Beijing, China, 2018. (In Chinese) [Google Scholar]
- Neori, A.; Chopin, T.; Troell, M.; Buschmann, A.; Kraemer, G.; Halling, C.; Shpigel, M.; Yarish, C. Integrated aquaculture: Rational, evolution and state of the art emphasizing seaweed biofiltration in modern mariculture. Aquaculture 2004, 231, 361–391. [Google Scholar] [CrossRef]
- Carmona, R.; Kraemer, G.; Yarish, C. Exploring Northeast American and Asian species of Porphyra for use in an integrated finfish–alga aquaculture system. Aquaculture 2006, 252, 54–65. [Google Scholar] [CrossRef]
- Kang, Y.; Kim, S.; Lee, J.; Chung, I.; Park, S. Nitrogen biofiltration capacities and photosynthetic activity of Pyropia yezoensis Ueda (Bangiales, Rhodophyta): Groundwork to validate its potential in Integrated Multi-Trophic Aquaculture (IMTA) J. Appl. Phycol. 2014, 26, 947–955. [Google Scholar] [CrossRef]
- Tian, J.B.; Wang, Y.; Sun, X.; Zhang, H.; Huang, B. Design and engineering of mariculture effluents purification system. Fish. Mod. 2008, 35, 1–5, (In Chinese with English abstract). [Google Scholar]
- Sun, Q. Nutrients Absorption of Aquaculture Wastewater and Bioremediation of Sea Area by Macroalgae. Master’s Thesis, Fujian Normal University, Fuzhou, China, 2013. (In Chinese with English abstract). [Google Scholar]
- He, P.; Xu, S.; Zhang, H.; Wen, S.; Dai, Y.; Lin, S.; Yarish, C. Bioremediation efficiency in the removal of dissolved inorganic nutrients by the red seaweed, Porphyra yezoensis, cultivated in the open sea. Water Res. 2008, 42, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Li, J. Marine Algae of China; Science Press: Beijing, China, 2017. (In Chinese) [Google Scholar]
- Xu, J.; Chen, W.; Song, Z.; Jiang, H.; Zhu, J.; Lu, Q. Effects of different culture conditions on growth and physiological response of Porphyra dentata thallus. J. Fish. China 2013, 37, 1319–1327, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Chen, P.; Huang, Z.; Zhu, J.; Lu, Q.; Chen, W. Effect of environmental factor on conchospore releasing, attachment and germination in Pyropia dentata. South. China Fish. Sci. 2015, 11, 55–61, (In Chinese with English abstract). [Google Scholar]
- Cheng, Q.; Zheng, B. Fish. System Retrieval of China; Science Press: Beijing, China, 1987. (In Chinese) [Google Scholar]
- Lei, L.; Liu, X. A primary study on culture of turbot Scophthalmus maeoticus. Mod. Fish. Inform. 1995, 10, 1–3, (In Chinese with English abstract). [Google Scholar]
- Xu, K.; Chen, H.; Wang, W.; Xu, Y.; Ji, D.; Chen, C.; Xie, C. Responses of photosynthesis and CO2 concentrating mechanisms of marine crop Pyropia haitanensis thalli to large pH variations at different time scales. Algal Res. 2017, 28, 200–210. [Google Scholar] [CrossRef]
- Jiang, H.; Zou, D.; Lou, W.; Deng, Y.; Zeng, X. Effects of seawater acidification and alkalization on the farmed seaweed, Pyropia haitanensis (Bangiales, Rhodophyta), grown under different irradiance conditions. Algal Res. 2018, 31, 413–420. [Google Scholar] [CrossRef]
- Xiong, Y.; Yang, R.; Sun, X.; Yang, T.; Chen, H. Effect of the epiphytic bacterium Bacillus sp. WPySW2 on the metabolism of Pyropia haitanensis. J. Appl. Phycol. 2018, 30, 1225–1237. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Xing, L.; Xu, K.; Ji, D.; Xu, Y.; Chen, C.; Xie, C. Salt stress-induced H2O2 and Ca2+ mediate K+ /Na+ homeostasis in Pyropia haitanensis. J. Appl. Phycol. 2020, 32, 4199–4210. [Google Scholar] [CrossRef]
- Huang, L.; Peng, L.; Yan, X. Multi-omics responses of red algae Pyropia haitanensis to intertidal desiccation during low tides. Algal Res. 2021, 58, 102376. [Google Scholar] [CrossRef]
- Zhong, Z.; Wang, W.; Sun, X.; Liu, F.; Liang, Z.; Wang, F.; Chen, W. Developmental and physiological properties of Pyropia dentata (Bangiales, Rhodophyta) conchocelis in culture. J. Appl. Phycol. 2016, 28, 3435–3445. [Google Scholar] [CrossRef]
- Li, S.; Liu, D. Improvement of spectrophotometric determination of phosphorus in water by phosphomolybdenum blue. Environ. Prot. Chem. Ind. 2006, 26, 78–80, (In Chinese with English abstract). [Google Scholar]
- Sun, X.; Hong, L.; Ye, H. Experiment determining nitrate nitrogen in water samples by on-line cadmium column reduction-flow injection method. Water Resour. Prot. 2010, 26, 75–77, (In Chinese with English abstract). [Google Scholar]
- Cao, Y.; Wang, W.; Liang, Z.R.; Liu, F.; Sun, X.; Yao, H.; Li, X.; Wang, F. Genetic and nutrient analysis of new Pyropia yezoensis strain “Huangyou No. 1”. Guangxi. Sci. 2016, 23, 131–137, (In Chinese with English abstract). [Google Scholar]
- Davison, R.; Pearson, G. Stress tolerance in intertidal seaweeds. J. Phycol. 1996, 32, 197–211. [Google Scholar] [CrossRef]
- Guo, G.L.; Dong, S. Effects of desiccation on the growth and photosynthetic rate of four intertidal macroalgae from different vertical locations. Trans. Oceanol. Limnol. 2011, 4, 78–84, (In Chinese with English abstract). [Google Scholar]
- Thomas, T.; Turpin, D.H.; Harrison, P. Desiccation enhanced nitrogen uptake rates in intertidal seaweeds. Mar. Biol. 1987, 94, 293–298. [Google Scholar] [CrossRef]
- Ji, Y.; Tanaka, J. Effects of desiccation on the photosynthesis of seaweeds from the intertidal zone in Honshu, Japan. Phycol. Res. 2002, 50, 145–153. [Google Scholar] [CrossRef]
- Gao, S.; Wang, G. The enhancement of cyclic electron flow around photosystem I improves the recovery of severely desiccated Porphyra yezoensis (Bangiales, Rhodophyta). J. Exp. Bot. 2012, 63, 4349–4358. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Cai, S. The Cultivation and Processing of Pyropia yezoensis; Science Press: Beijing, China, 1996. (In Chinese) [Google Scholar]
- Yoshida, T. Marine Algae of Japan; Uchida Roukakuho Publishing: Tokyo, Japan, 1998. (In Japanese) [Google Scholar]
- Raven, J.; Hurd, C. Ecophysiology of photosynthesis in macroalgae. Photosynth. Res. 2012, 113, 105–125. [Google Scholar] [CrossRef]
- Hurd, C. Shaken and stirred: The fundamental role of water motion in resource acquisition and seaweed productivity. Perspect. Phycol. 2017, 4, 73–81. [Google Scholar] [CrossRef]
- Kregting, L.; Hepburn, C.D.; Savidge, G. Seasonal differences in the effects of oscillatory and uni-directional flow on the growth and nitrate-uptake rates of juvenile Laminaria digitata (Phaeophyceae) J. Phycol. 2015, 51, 1116–1126. [Google Scholar] [CrossRef] [Green Version]
- Inoue, Y.; Terada, R.; Belleza, D.; Nishihara, G. Effect of water velocity on the physiology of a collapsing Sargassum siliquastrum canopy under a controlled environment. Phycol. Res. 2020, 68, 313–322. [Google Scholar] [CrossRef]
- Yang, W. Hydrodynamic, illumination, nitrogen and phosphorus on the response mechanism of Chlorella. sp. Master’s Thesis, Xi’an University of Architecture and Technology, Xi'an, China, 2018. (In Chinese with English abstract). [Google Scholar]
- Naldi, M.; Wheeler, P. Changes in nitrogen pools in Ulva fenestrata (Chlorophyta) and Gracilaria pacifica (Rhodophyta) under nitrate and ammonium enrichment. J. Phycol. 1999, 35, 70–77. [Google Scholar] [CrossRef]
- Yarish, C.; Redmond, S.; Kim, J. Gracilaria Culture Handbook for New England; Wrack Lines; University of Connecticut Sea Grant: Groton, CT, USA, 2012. [Google Scholar]
- Wang, Y.; Feng, Y.; Liu, X.; Zhong, M.; Chen, W.; Wang, F.; Du, H. Response of Gracilaria lemaneiformis to nitrogen deprivation. Algal Res. 2018, 34, 82–96. [Google Scholar] [CrossRef]
- Liu, X.; Wen, J.; Zheng, C.; Jia, H.; Chen, W.; Du, H. The impact of nitrogen deficiency and subsequent recovery on the photosynthetic performance of the red macroalga Gracilariopsis lemaneiformis. J. Appl. Phycol. 2019, 31, 2699–2707. [Google Scholar] [CrossRef]
- Liu, J.; Dong, S.; Ma, S. Effects of temperature and salinity on growth and NH4-N uptake of Gracilaria tenuistipitata var. liui, Ulva pertusa, Gracilaria filicina and NH4-N uptake of Gracilaria tenuistipitata var. liui. Acta. Oceanol. Sin. 2001, 23, 109–116. [Google Scholar]
- Perini, V.; Bracken, M. Nitrogen availability limits phosphorus uptake in an intertidal macroalga. Oecologia 2014, 175, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Sun, J.; Chen, Z.; Wei, T. Effect of N/P ratio on the growth of a red tide diatom Skeletonema costatum. Trans. Oceanol. Limnol. 2002, 2, 39–44, (In Chinese with English abstract). [Google Scholar]
- Friedlander, M.; Krom, M.; Ben-Amotz, A. The effect of light and ammonium on growth, epiphytes and chemical constituents of Gracilaria conferta in outdoor cultures. Bot. Mar. 1991, 34, 161–166. [Google Scholar] [CrossRef]

| Factors | df | F | P |
|---|---|---|---|
| RGR | |||
| Desiccation (D) | 4 | 5.973 | <0.01 |
| Species (S) | 1 | 1.187 | 0.346 |
| Interaction (D × S) | 4 | 0.851 | 2.061 |
| NO3-N removal rate | |||
| Desiccation (D) | 4 | 16.177 | <0.001 |
| Species (S) | 1 | 1.465 | 0.239 |
| Interaction (D × S) | 4 | 2.935 | <0.05 |
| PO4-P removal rate | |||
| Desiccation (D) | 4 | 0.834 | 2.947 |
| Species (S) | 1 | 1.181 | 0.385 |
| Interaction (D × S) | 4 | 0.655 | 6.421 |
| Factors | df | F | P |
|---|---|---|---|
| RGR | |||
| Water velocity (W) | 2 | 29.933 | <0.001 |
| Species (S) | 1 | 16.025 | <0.001 |
| Interaction (W × S) | 2 | 27.251 | <0.001 |
| NO3-N removal rate | |||
| Water velocity (W) | 2 | 20.070 | <0.001 |
| Species (S) | 1 | 0.773 | 4.197 |
| Interaction (W × S) | 2 | 5.773 | <0.01 |
| PO4-P removal rate | |||
| Water velocity (W) | 2 | 18.191 | <0.001 |
| Species (S) | 1 | 9.966 | <0.001 |
| Interaction (W × S) | 2 | 11.555 | <0.001 |
| Factors | df | F | P |
|---|---|---|---|
| RGR | |||
| Nitrogen limitation (N) | 2 | 5.190 | <0.01 |
| Species (S) | 1 | 25.021 | <0.001 |
| Interaction (N × S) | 2 | 19.201 | <0.001 |
| NO3-N removal rate | |||
| Nitrogen limitation (N) | 2 | 23.876 | <0.001 |
| Species (S) | 1 | 0.482 | 9.110 |
| Interaction (N × S) | 2 | 15.766 | <0.001 |
| PO4-P removal rate | |||
| Nitrogen limitation (N) | 2 | 28.171 | <0.001 |
| Species (S) | 1 | 8.960 | <0.001 |
| Interaction (N × S) | 2 | 12.095 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Cui, G.; Liu, Y.; Wang, Q.; Gong, Q.; Gao, X. Effects of Desiccation, Water Velocity, and Nitrogen Limitation on the Growth and Nutrient Removal of Neoporphyra haitanensis and Neoporphyra dentata (Bangiales, Rhodophyta). Water 2021, 13, 2745. https://doi.org/10.3390/w13192745
Li J, Cui G, Liu Y, Wang Q, Gong Q, Gao X. Effects of Desiccation, Water Velocity, and Nitrogen Limitation on the Growth and Nutrient Removal of Neoporphyra haitanensis and Neoporphyra dentata (Bangiales, Rhodophyta). Water. 2021; 13(19):2745. https://doi.org/10.3390/w13192745
Chicago/Turabian StyleLi, Jingyu, Guohua Cui, Yan Liu, Qiaohan Wang, Qingli Gong, and Xu Gao. 2021. "Effects of Desiccation, Water Velocity, and Nitrogen Limitation on the Growth and Nutrient Removal of Neoporphyra haitanensis and Neoporphyra dentata (Bangiales, Rhodophyta)" Water 13, no. 19: 2745. https://doi.org/10.3390/w13192745
APA StyleLi, J., Cui, G., Liu, Y., Wang, Q., Gong, Q., & Gao, X. (2021). Effects of Desiccation, Water Velocity, and Nitrogen Limitation on the Growth and Nutrient Removal of Neoporphyra haitanensis and Neoporphyra dentata (Bangiales, Rhodophyta). Water, 13(19), 2745. https://doi.org/10.3390/w13192745






