Disinfection Kinetics of Free Chlorine, Monochloramines and Chlorine Dioxide on Ammonia-Oxidizing Bacterium Inactivation in Drinking Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. N. europaea and Disinfectants
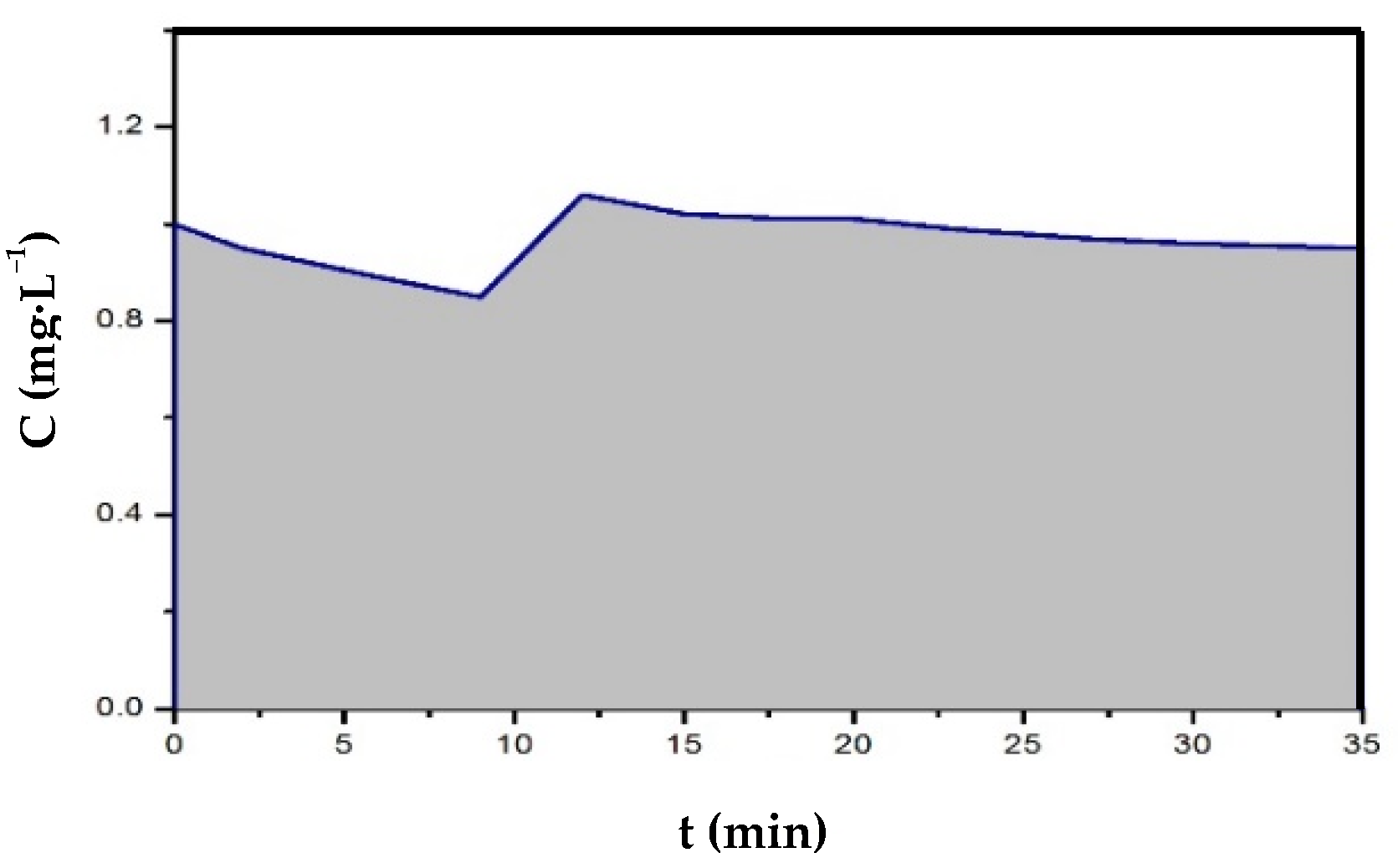
2.2. Experimental Protocol for Batch Disinfection Experiments
2.3. Flow Cytometry for Viable Bacteria Enumeration
3. Results and Discussion
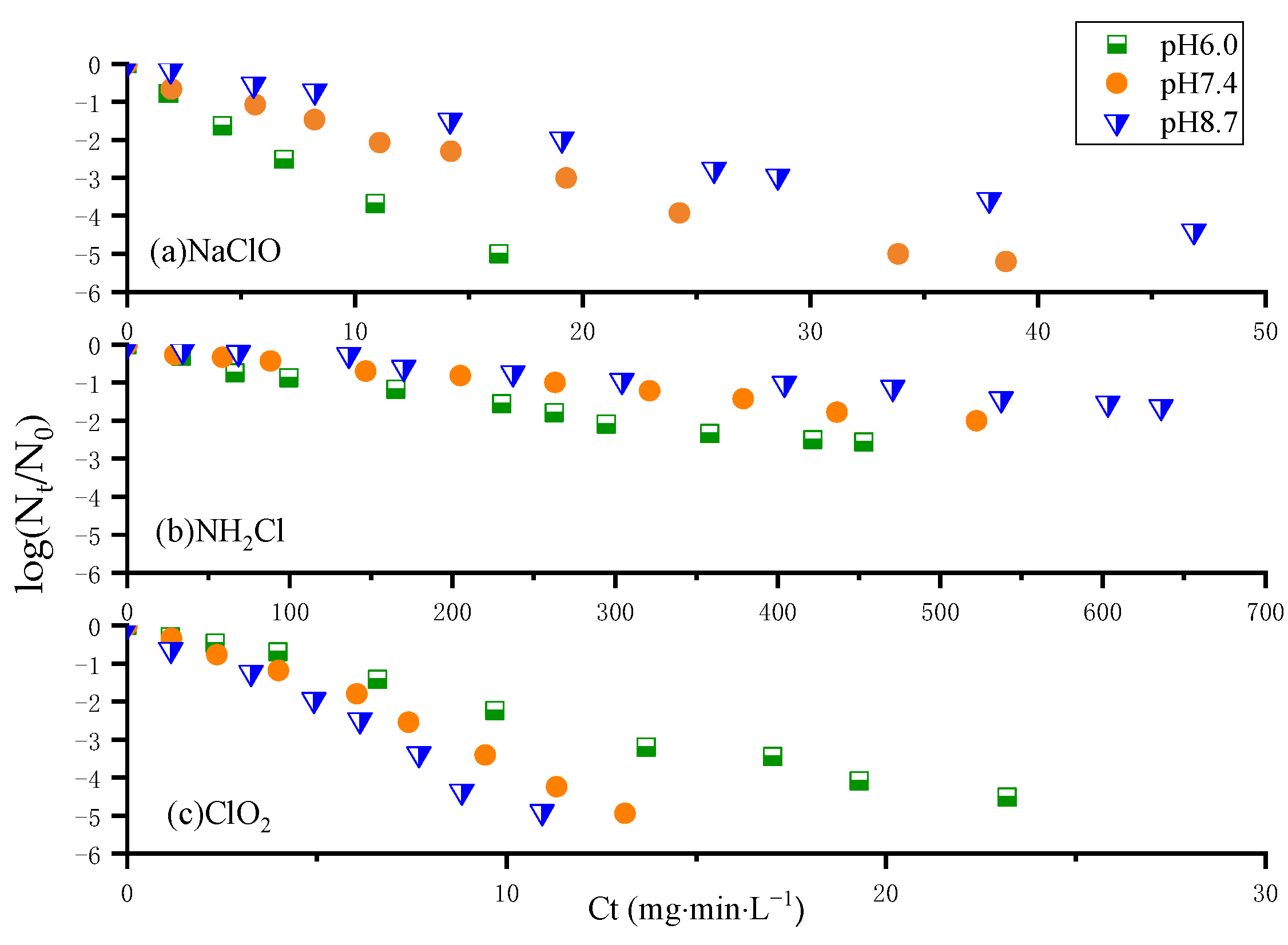
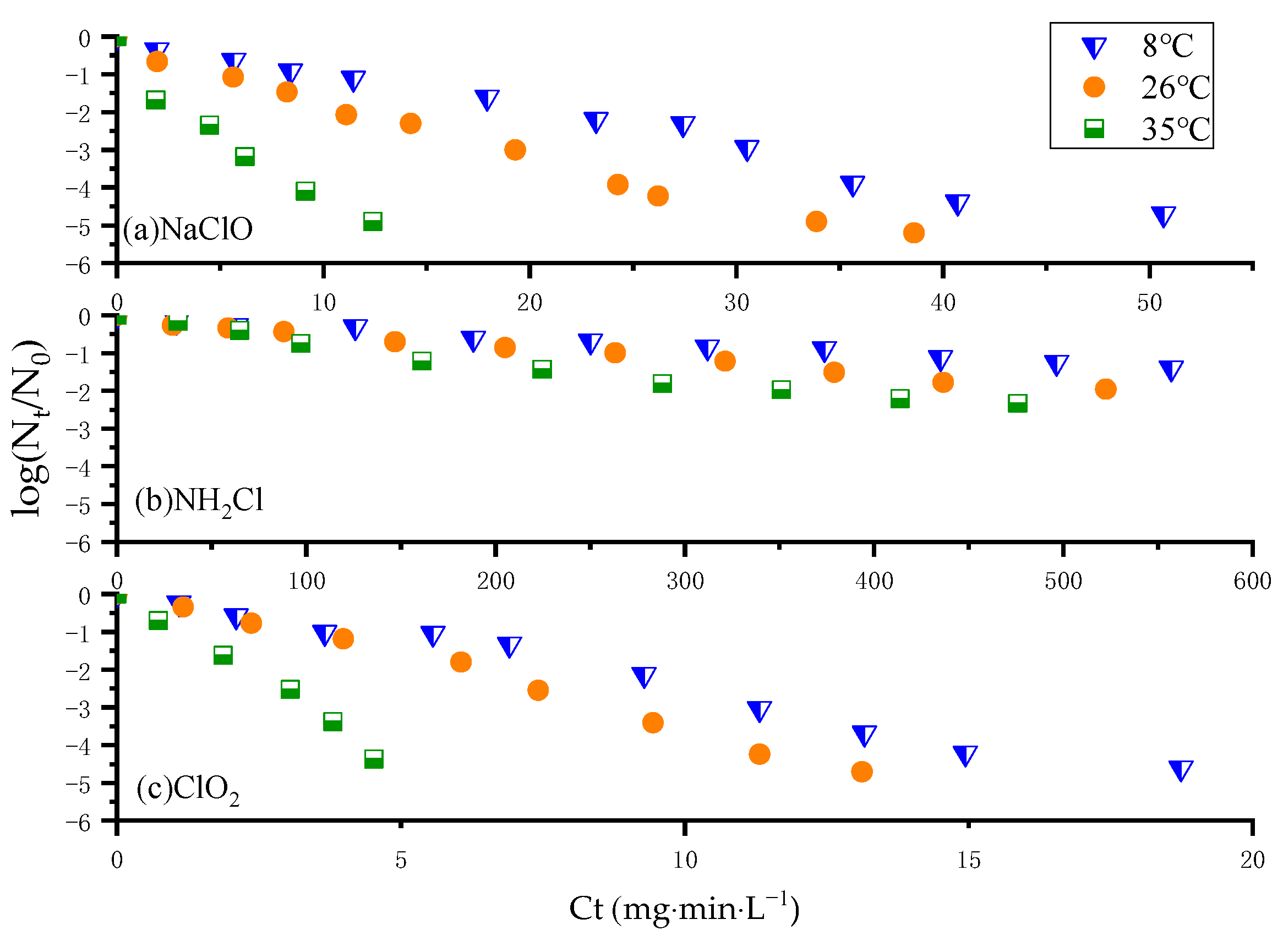
3.1. Kinetic Analysis of N. europaea Inactivation Experiments
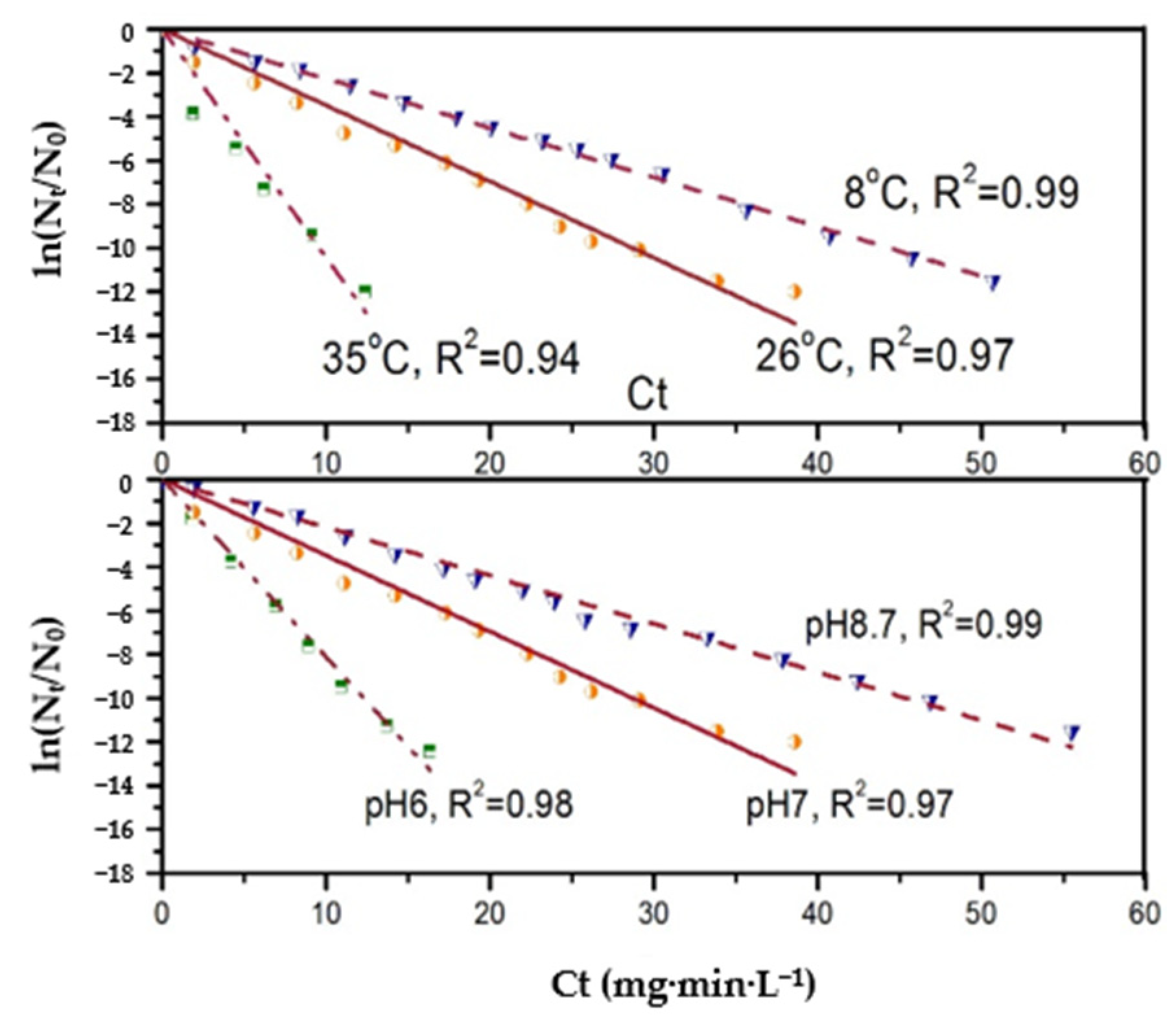
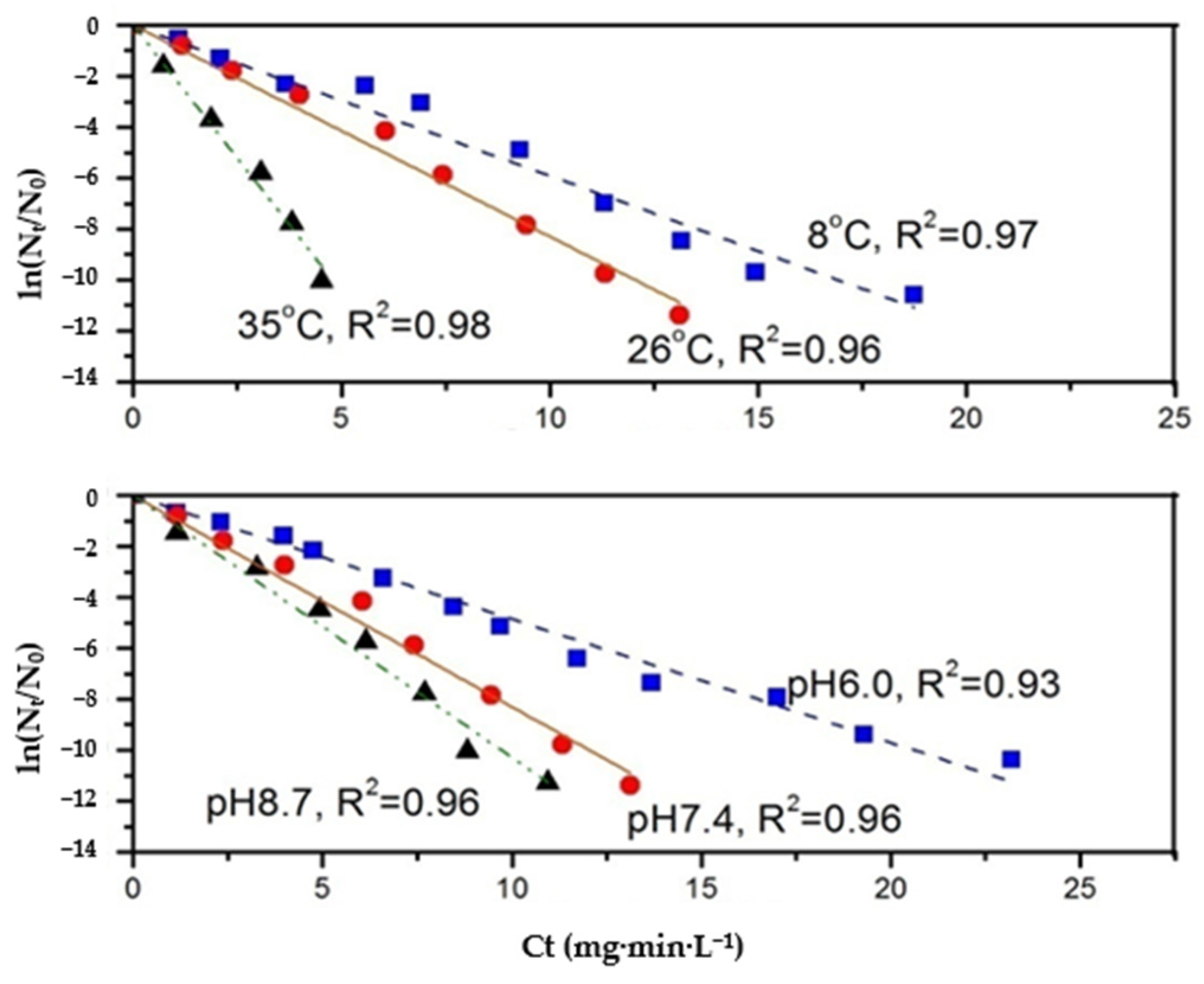
3.2. Effect of pH and Temperature on N. europaea Disinfection Kinetics
3.3. Development of the Disinfection Kinetic Model
- kinetics of free chlorine disinfection:ln(Nt/N0) = −7.14(0.59)pH(1.04)T(Ct)
- kinetics of monochloramine:ln(Nt/N0) = −0.07(0.7)pH(1.03)T(Ct)
- kinetics of chlorine dioxide:ln(Nt/N0) = −0.05(1.32)pH(1.03)T(Ct)
3.4. Model Validation
4. Conclusions
- (1)
- The inactivation effect of N. europaea by the three disinfectants increases with increasing temperature. Free chlorine and chlorine dioxide react more significantly to increased temperature than monochloramine. The inactivation effect of these three disinfectants on N. europaea showed different trends with increasing pH. Among them, the inactivation effect of free chlorine and monochloramine on N. europaea decreased, while that of chlorine dioxide increased.
- (2)
- The inactivation effects of the three disinfectants on N. europaea were ranked in descending order as chlorine dioxide, free chlorine and monochloramine, with monochloramine being much less effective than the others. There is a potential risk of nitrification in the DWDS when monochloramine is used for disinfection; monochloramine can stop being added regularly and strong disinfectants such as free chlorine and chlorine dioxide can be used to inactivate AOB.
- (3)
- The inactivation coefficient k-value of disinfectant on N. europaea varied with pH and temperature, and the effect of pH and temperature on the k-value was not linear.
- (4)
- The inactivation coefficient k-value was expressed as a function of pH and temperature, and it was substituted into the Chick–Watson model to fit the disinfection prediction model containing pH and temperature T. This model can better quantify the inactivation process of AOB in three disinfectants from the perspective of kinetics.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quintiliani, C.; Di Cristo, C.; Leopardi, A. Vulnerability Assessment to Trihalomethane Exposure in Water Distribution Systems. Water 2018, 10, 912. [Google Scholar] [CrossRef] [Green Version]
- Seidel, C.J.; McGuire, M.J.; Summers, R.S.; Via, S. Have utilities switched to chloramines? J. Am. Water Work. Assoc. 2005, 97, 87–97. [Google Scholar] [CrossRef]
- LeChevallier, M.W.; Welch, N.J.; Smith, D.B. Full-scale studies of factors related to coliform regrowth in drinking water. Appl. Environ. Microbiol. 1996, 62, 2201. [Google Scholar] [CrossRef] [Green Version]
- Qi, Y.; Shang, C.; Lo, I.M.C. Formation of haloacetic acids during monochloramination. Water Res. 2004, 38, 2375–2383. [Google Scholar] [CrossRef] [PubMed]
- Vikesland, P.J.; Ozekin, K.; Valentine, R.L. Monochloramine Decay in Model and Distribution System Waters. Water Res. 2001, 35, 1766–1776. [Google Scholar] [CrossRef]
- Zhang, Y.; Love, N.; Edwards, M. Nitrification in Drinking Water Systems. Crit. Rev. Environ. Sci. Technol. 2009, 39, 153–208. [Google Scholar] [CrossRef]
- Bradley, T.C.; Haas, C.N.; Sales, C.M. Nitrification in Premise Plumbing: A Review. Water 2020, 12, 830. [Google Scholar] [CrossRef] [Green Version]
- Wilczak, A.; Jacangelo, J.G.; Marcinko, J.P.; Odell, L.H.; Kirmeyer, G.J. Occurrence of nitrification in chloraminated distribution systems. J.—Am. Water Works Assoc. 1996, 88, 74–85. [Google Scholar] [CrossRef]
- Cunliffe, D.A. Bacterial nitrification in chloraminated water supplies. Appl. Environ. Microbiol. 1991, 57, 3399–3402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.L.; Sun, Y.L.; Li, G.L.; Zhang, L.Q.; Wang, D.T.; Li, G.B. Investigation of nitrite status in drinking water distribution system of a city in south China. J. Harbin Inst. Technol. 2006, 38, 1047–1050. [Google Scholar]
- Oldenburg, P.S.; Regan, J.M.; Harrington, G.W.; Noguera, D.R. Kinetics of Nitrosomonas europaea INACTIVATION by chloramine. J.—Am. Water Works Assoc. 2002, 94, 100–110. [Google Scholar] [CrossRef]
- Chauret, C.; Smith, C.; Baribeau, H. Inactivation of Nitrosomonas europaea and pathogenic Escherichia coli by chlorine and monochloramine. J. Water Health 2008, 6, 315–322. [Google Scholar] [CrossRef] [Green Version]
- Wahman, D.G.; Wulfeck-Kleier, K.A.; Pressman, J.G. Monochloramine Disinfection Kinetics of Nitrosomonas europaea by Propidium Monoazide Quantitative PCR and Live/Dead BacLight Methods. Appl. Environ. Microbiol. 2009, 75, 5555–5562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, H.E. A Note on the Variation of the Rate of Disinfection with Change in the Concentration of the Disinfectant. Epidemiol. Infect. 1908, 8, 536–542. [Google Scholar] [CrossRef] [Green Version]
- Peleg, M. Modeling the dynamic kinetics of microbial disinfection with dissipating chemical agents—A theoretical investigation. Appl. Microbiol. Biotechnol. 2021, 105, 539–549. [Google Scholar] [CrossRef]
- Wahman, D.G.; Schrantz, K.A.; Pressman, J.G. Determination of the Effects of Medium Composition on the Monochloramine Disinfection Kinetics of Nitrosomonas europaea by the Propidium Monoazide Quantitative PCR and Live/Dead BacLight Methods. Appl. Environ. Microbiol. 2010, 76, 8277–8280. [Google Scholar] [CrossRef] [Green Version]
- Ofori, I.; Maddila, S.; Lin, J.; Jonnalagadda, S.B. Chlorine dioxide inactivation of Pseudomonas aeruginosa and Staphylococcus aureus in water: The kinetics and mechanism. J. Water Process. Eng. 2018, 26, 46–54. [Google Scholar] [CrossRef]
- Yang, W.; Yang, D.; Zhu, S.-Y.; Chen, B.-Y.; Huo, M.-X.; Li, J.-W. The synergistic effect of Escherichia coli inactivation by sequential disinfection with low level chlorine dioxide followed by free chlorine. J. Water Health 2012, 10, 557–564. [Google Scholar] [CrossRef] [Green Version]
- McGuire, M.J.; Lieu, N.I.; Pearthree, M.S. Using chlorite ion to control nitrification. J.—Am. Water Works Assoc. 1999, 91, 52–61. [Google Scholar] [CrossRef]
- Ruffell, K.M.; Rennecker, J.L.; Mariñas, B.J. Inactivation of Cryptosporidium parvum oocysts with chlorine dioxide. Water Res. 2000, 34, 868–876. [Google Scholar] [CrossRef]
- Kampf, G. Efficacy of ethanol against viruses in hand disinfection. J. Hosp. Infect. 2018, 98, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.W.; Huo, X.C.; Lin, T.F. Exposure of Microcystis aeruginosa to hydrogen peroxide and titanium dioxide under visible light conditions: Modeling the impact of hydrogen peroxide and hydroxyl radical on cell rupture and microcystin degradation. Water Res. 2018, 141, 217–226. [Google Scholar] [CrossRef]
- Armstrong, A.M.; Sobsey, M.D.; Casanova, L.M. Disinfection of bacteriophage MS2 by copper in water. Appl. Microbiol. Biotechnol. 2017, 101, 6891–6897. [Google Scholar] [CrossRef] [PubMed]
- John, D.E.; Haas, C.N.; Nwachuku, N.; Gerba, C.P. Chlorine and ozone disinfection of Encephalitozoon intestinalis spores. Water Res. 2005, 39, 2369–2375. [Google Scholar] [CrossRef]
- Gyurek, L.L.; Finch, G.R. Modeling water treatment chemical disinfection kinetics. J. Environ. Eng.-ASCE 1998, 124, 783–793. [Google Scholar] [CrossRef]
- Haas, C.N.; Joffe, J. Disinfection under dynamic conditions: Modification of Hom’s model for decay. Environ. Sci. Technol. 1994, 28, 1367–1369. [Google Scholar] [CrossRef] [PubMed]


| Experimental Conditions | Free Chlorine | Monochloramine | Chlorine Dioxide | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| k (L·mg−1·min−1) | Ct3log (mg·min·L−1) | R2 | K (L·mg−1·min−1) | Ct3log (mg·min·L−1) | R2 | K (L·mg−1·min−1) | Ct3log (mg·min·L−1) | R2 | ||
| pH | 6 | 0.81 | 7 | 0.98 | 0.015 | 463.7 | 0.94 | 0.48 | 14.4 | 0.93 |
| 7 | 0.35 | 9.2 | 0.97 | 0.009 | 731.7 | 0.98 | 0.83 | 8.3 | 0.96 | |
| 8.7 | 0.22 | 32.9 | 0.99 | 0.006 | 1123.6 | 0.96 | 1.03 | 6.7 | 0.96 | |
| Temperature (°C) | 8 | 0.23 | 23.8 | 0.99 | 0.006 | 1132.1 | 0.97 | 0.59 | 11.7 | 0.97 |
| 26 | 0.35 | 9.2 | 0.97 | 0.009 | 731.7 | 0.98 | 0.83 | 8.3 | 0.96 | |
| 35 | 1.04 | 3.4 | 0.94 | 0.014 | 488.6 | 0.98 | 2.09 | 3.3 | 0.98 | |
| Disinfectant | r | RMSE |
|---|---|---|
| Free chlorine | 0.929 | 0.234 |
| monochloramine | 0.978 | 0.046 |
| Chlorine dioxide | 0.917 | 0.168 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Qiu, J.; Xu, X.; Zhou, L. Disinfection Kinetics of Free Chlorine, Monochloramines and Chlorine Dioxide on Ammonia-Oxidizing Bacterium Inactivation in Drinking Water. Water 2021, 13, 3026. https://doi.org/10.3390/w13213026
Zhang Y, Qiu J, Xu X, Zhou L. Disinfection Kinetics of Free Chlorine, Monochloramines and Chlorine Dioxide on Ammonia-Oxidizing Bacterium Inactivation in Drinking Water. Water. 2021; 13(21):3026. https://doi.org/10.3390/w13213026
Chicago/Turabian StyleZhang, Yongji, Jie Qiu, Xianfang Xu, and Lingling Zhou. 2021. "Disinfection Kinetics of Free Chlorine, Monochloramines and Chlorine Dioxide on Ammonia-Oxidizing Bacterium Inactivation in Drinking Water" Water 13, no. 21: 3026. https://doi.org/10.3390/w13213026






