Validation of Water Quality Monitoring Algorithms for Sentinel-2 and Sentinel-3 in Mediterranean Inland Waters with In Situ Reflectance Data
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Field Data Collection and Laboratory Measurements
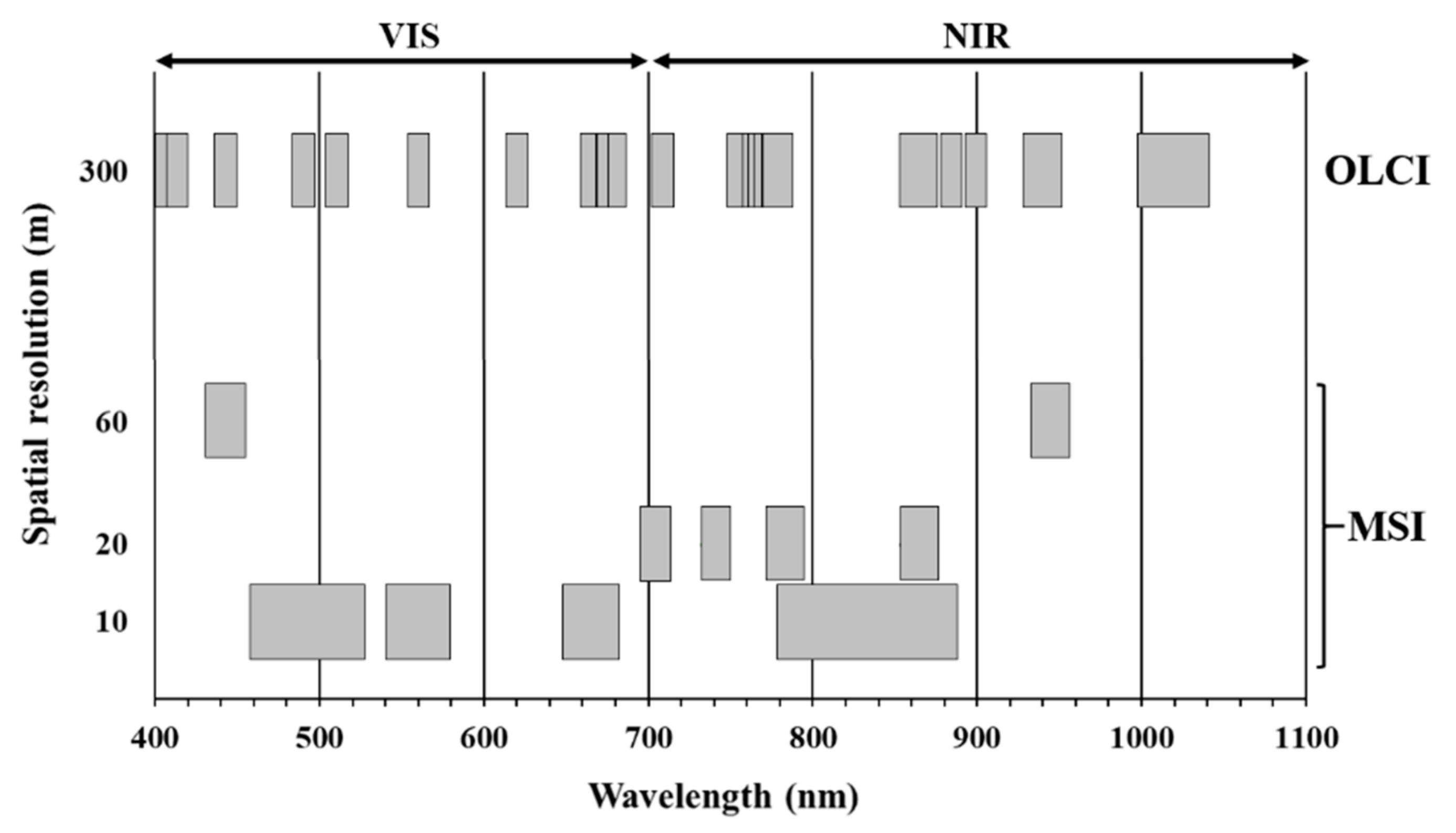
2.3. Algorithms Retrieval
3. Results
3.1. Measured Data
3.2. Algorithms Retrieval
3.2.1. SDD
3.2.2. CDOM
3.2.3. TSS
3.2.4. Chl_a
3.2.5. PC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Name | Position | Depth | Volume | Elevation | Res. Time | Climate | Visits a | Samples | # of Spectroradiometry Samples | # of Variables Samples | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lat. | Lon. | m (max.) | ×106 m3 | m.a.s.l. | years | ASD-FR | HandHeld 2 | HR4000 | SDD | CDOM | TSS | Chl_a | PC | |||||||||||||||||||||
| d.dd | d.dd | m | µg/L QSE | mg/L | mg/m3 | mg/m3 | ||||||||||||||||||||||||||||
| Aguilar | 42.80 | −4.32 | 48 | 247 | 942 | 0.78 | Cfb | 2 | 4 | 4 | 4 | 4 | 0 | 4 | 4 | |||||||||||||||||||
| Alarcón | 39.60 | −2.17 | 71 | 1118 | 806 | 2.15 | Csa | 2 | 4 | 4 | 4 | 4 | 0 | 4 | 4 | |||||||||||||||||||
| Albufera | 39.34 | −0.35 | 2 | 360 | 1 | 10.0 | Csa | 7 | 36 | 29 | 7 | 36 | 18 | 6 | 28 | 19 | ||||||||||||||||||
| Alcántara | 39.75 | −6.75 | 135 | 3200 | 218 | 0.43 | Csa | 2 | 8 | 8 | 8 | 2 | 4 | 7 | 2 | |||||||||||||||||||
| Alcorlo | 41.02 | −3.02 | 62 | 180 | 920 | 2.22 | Csa | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | |||||||||||||||||||
| Almendra | 41.22 | −6.28 | 202 | 2413 | 730 | 1.57 | Csb | 3 | 13 | 13 | 13 | 3 | 6 | 9 | 3 | |||||||||||||||||||
| Bellús | 38.93 | −0.47 | 34 | 69 | 144 | 0.24 | Csa | 3 | 7 | 6 | 1 | 7 | 6 | 7 | 7 | 5 | ||||||||||||||||||
| Benaixeve | 39.73 | −1.09 | 90 | 221 | 450 | 0.63 | Csb | 4 | 13 | 13 | 13 | 13 | 13 | 13 | 5 | |||||||||||||||||||
| Beniarrés | 38.80 | −0.35 | 53 | 27 | 318 | 0.35 | Csa | 3 | 4 | 1 | 3 | 4 | 4 | 3 | 4 | 2 | ||||||||||||||||||
| Bornos | 36.80 | −5.73 | 52 | 215 | 104 | 0.72 | Csa | 1 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | |||||||||||||||||||
| Brovales | 38.35 | −6.68 | 25 | 7 | 303 | n/a | Csa | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | |||||||||||||||||||
| Buendía | 40.40 | −2.77 | 79 | 1458 | 712 | 2.73 | Csa | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | |||||||||||||||||||
| Burguillo | 40.42 | −4.60 | 91 | 198 | 729 | 0.47 | Csa | 2 | 4 | 4 | 4 | 4 | 0 | 4 | 4 | |||||||||||||||||||
| Canelles | 42.03 | 0.65 | 150 | 201 | 506 | 1.22 | Cfb | 1 | 4 | 4 | 4 | 4 | 0 | 4 | 0 | |||||||||||||||||||
| Cernadilla | 42.02 | −6.47 | 69 | 233 | 889 | 0.47 | Csb | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | |||||||||||||||||||
| Cijara | 39.37 | −5.00 | 80 | 1470 | 428 | 1.68 | Csa | 1 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | |||||||||||||||||||
| Contreras | 39.62 | −1.53 | 129 | 852 | 669 | 1.48 | Csa | 5 | 17 | 2 | 8 | 7 | 15 | 12 | 13 | 15 | 3 | |||||||||||||||||
| Cortes | 39.23 | −0.92 | 112 | 118 | 326 | 0.08 | Csa | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | |||||||||||||||||||
| Cuerda del Pozo | 41.85 | −2.75 | 40 | 200 | 1,078 | 1.96 | Cfb | 2 | 7 | 7 | 7 | 0 | 6 | 6 | 0 | |||||||||||||||||||
| Ebro | 42.97 | −4.07 | 34 | 540 | 838 | 1.55 | Cfb | 2 | 3 | 3 | 3 | 3 | 0 | 2 | 2 | |||||||||||||||||||
| El Atazar | 40.90 | −3.53 | 141 | 426 | 873 | 1.19 | Csa | 3 | 4 | 4 | 4 | 3 | 0 | 4 | 4 | |||||||||||||||||||
| Entrepeñas | 40.50 | −2.72 | 85 | 874 | 718 | 1.04 | Csa | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | |||||||||||||||||||
| Giribaile | 38.08 | −3.48 | 84 | 475 | 346 | 1.28 | Csa | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | |||||||||||||||||||
| Guadalcacín | 36.65 | −5.75 | 44 | 800 | 102 | 2.40 | Csa | 1 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | |||||||||||||||||||
| Guadalén | 38.17 | −3.47 | 55 | 173 | 350 | 1.25 | Csa | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | |||||||||||||||||||
| Guadalteba | 36.95 | −4.83 | 84 | 173 | 362 | 1.44 | Csa | 1 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | |||||||||||||||||||
| Iznájar | 37.25 | −4.30 | 120 | 980 | 421 | 1.63 | Csa | 3 | 14 | 14 | 14 | 4 | 6 | 9 | 3 | |||||||||||||||||||
| Jándula | 38.25 | −3.92 | 88 | 322 | 360 | 1.29 | Csa | 1 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | |||||||||||||||||||
| La Serena | 38.88 | −5.17 | 89 | 2828 | 355 | 3.59 | Csa | 2 | 4 | 4 | 4 | 4 | 0 | 4 | 4 | |||||||||||||||||||
| Maria Cristina | 40.02 | −0.16 | 38 | 18 | 100 | 5.96 | Csa | 2 | 5 | 5 | 5 | 4 | 4 | 4 | 0 | |||||||||||||||||||
| Navalcán | 40.03 | −5.10 | 26 | 34 | 370 | 0.46 | Bsk | 1 | 3 | 3 | 3 | 3 | 0 | 3 | 3 | |||||||||||||||||||
| Negratín | 37.55 | −2.93 | 75 | 496 | 638 | 1.87 | Csb | 1 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | |||||||||||||||||||
| Pinilla | 40.93 | −3.55 | 33 | 38 | 1089 | 0.27 | Csb | 3 | 4 | 4 | 4 | 4 | 0 | 4 | 3 | |||||||||||||||||||
| Regajo | 39.89 | −0.52 | 23 | 6 | 407 | 0.14 | Csa | 2 | 7 | 4 | 3 | 7 | 7 | 7 | 7 | 4 | ||||||||||||||||||
| Rialb | 41.97 | 1.23 | 99 | 402 | 430 | 0.36 | Cfa | 1 | 4 | 4 | 4 | 4 | 0 | 4 | 0 | |||||||||||||||||||
| Riaño | 42.97 | −5.02 | 101 | 654 | 1100 | 0.95 | Csb | 1 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | |||||||||||||||||||
| Ricobayo | 41.63 | −5.90 | 100 | 1048 | 684 | 0.27 | Csa | 2 | 4 | 4 | 4 | 4 | 0 | 4 | 4 | |||||||||||||||||||
| Rosarito | 40.10 | −5.30 | 38 | 84 | 307 | 0.25 | Csa | 13 | 54 | 54 | 31 | 43 | 2 | 38 | 41 | |||||||||||||||||||
| San Juan | 40.38 | −4.33 | 78 | 148 | 580 | 0.26 | Csa | 1 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | |||||||||||||||||||
| Sanabria | 42.12 | −6.70 | 88 | 188 | 998 | 0.26 | Csb | 1 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | |||||||||||||||||||
| Santa Teresa | 40.65 | −5.58 | 59 | 496 | 887 | 0.80 | Csb | 1 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | |||||||||||||||||||
| Santillana | 40.72 | −3.83 | 40 | 91 | 894 | 0.83 | Csa | 2 | 3 | 3 | 3 | 3 | 0 | 3 | 3 | |||||||||||||||||||
| Sitjar | 40.01 | −0.23 | 58 | 49 | 160 | 0.37 | Csa | 3 | 6 | 6 | 6 | 6 | 6 | 6 | 2 | |||||||||||||||||||
| Terradets | 42.05 | 0.88 | 47 | 33 | 372 | 0.04 | Cfa | 1 | 2 | 2 | 2 | 1 | 0 | 1 | 0 | |||||||||||||||||||
| Tous | 39.13 | −0.65 | 110 | 378 | 135 | 0.28 | Csa | 3 | 9 | 6 | 3 | 9 | 6 | 9 | 9 | 3 | ||||||||||||||||||
| Tremp | 42.22 | 0.97 | 86 | 184 | 501 | 0.18 | Cfa | 1 | 4 | 4 | 4 | 4 | 0 | 4 | 0 | |||||||||||||||||||
| Ullívarri | 42.93 | −2.58 | 37 | 139 | 547 | 0.30 | Cfb | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | |||||||||||||||||||
| Valdecañas | 39.80 | −5.43 | 98 | 1446 | 315 | 0.36 | Csa | 3 | 5 | 5 | 5 | 5 | 0 | 4 | 4 | |||||||||||||||||||
| Valmayor | 40.53 | −4.03 | 60 | 124 | 831 | 3.54 | Csa | 2 | 4 | 4 | 4 | 4 | 0 | 4 | 4 | |||||||||||||||||||
| Valparaíso | 41.97 | −6.30 | 64 | 145 | 833 | 0.23 | Csb | 2 | 3 | 3 | 3 | 3 | 0 | 2 | 2 | |||||||||||||||||||
| Valuengo | 38.30 | −6.67 | 32.7 | 10.0 | 297 | 0.08 | Csa | 1 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | |||||||||||||||||||
| Vega de Jabalón | 38.75 | −3.78 | 25 | 33.5 | 639 | 0.62 | Bsk | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | |||||||||||||||||||
| Total | 109 | 296 | 224 | 58 | 14 | 271 | 222 | 92 | 254 | 170 | ||||||||||||||||||||||||
Appendix B. Test of Chl_a and TSS Algorithms Showed in Table 10 Using Image Satellite
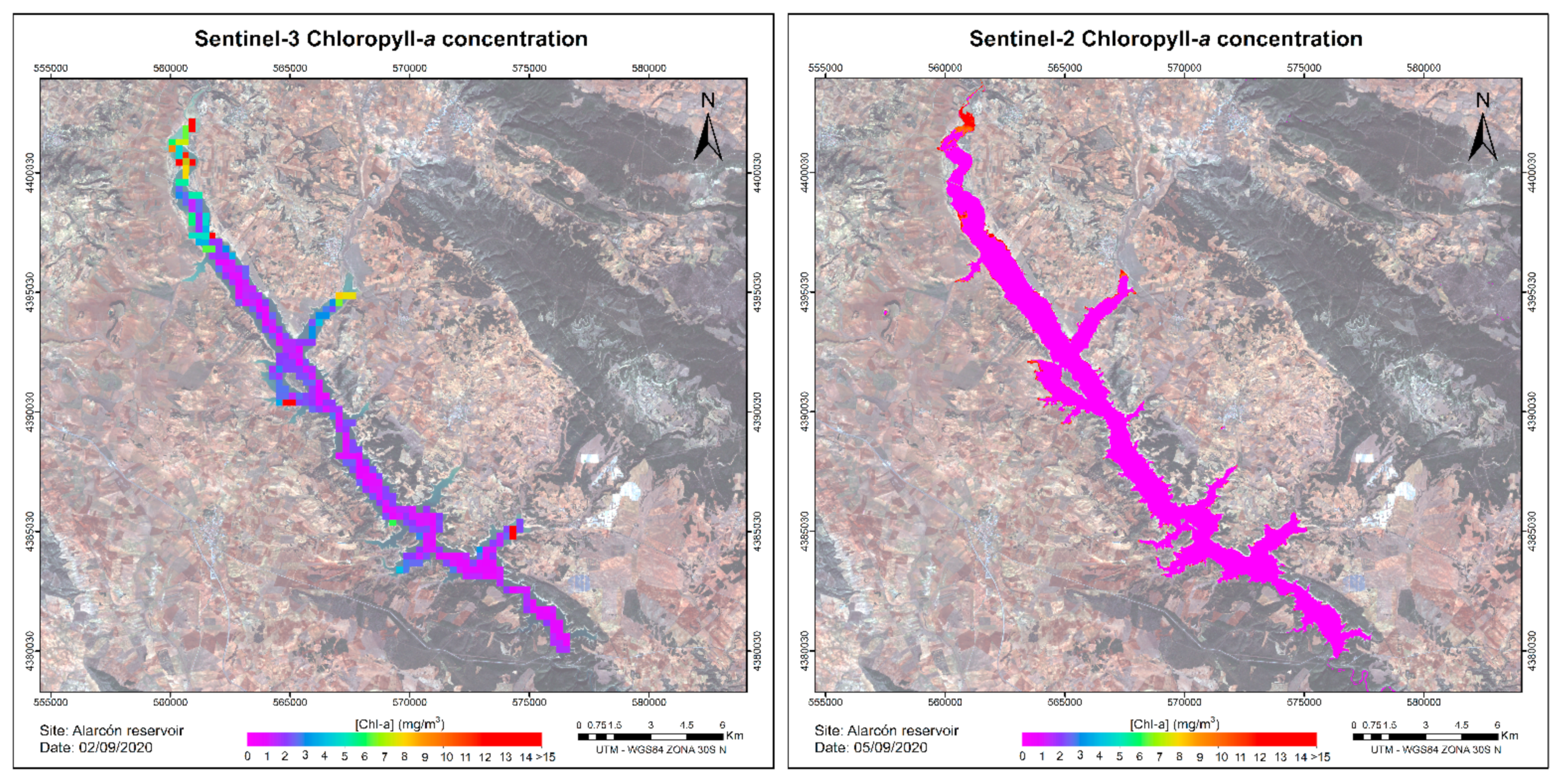

References
- Stanley, E.H.; Powers, S.M.; Lottig, N.R.; Buffam, I.; Crawford, J.T. Contemporary changes in dissolved organic carbon (DOC) in human-dominated rivers: Is there a role for DOC management? Freshw. Biol. 2012, 57, 26–42. [Google Scholar] [CrossRef]
- Lehner, B.; Döll, P.; Alcamo, J.; Henrichs, T.; Kaspar, F. Estimating the impact of global change on flood and drought risks in Europe: A continental, integrated analysis. Clim. Chang. 2006, 75, 273–299. [Google Scholar] [CrossRef]
- Marcé, R.; Armengol, J.; Dolz, J. Els efectes als embassaments i la seva rellevancia en la quantitat i la qualitat de l’aigua per la garantia del recurs. In Aigua i Canvi Climatic; Agencia Catalana de l’Aigua; Generalitat de Catalunya: Barcelona, Spain, 2009; pp. 221–238. Available online: https://aca-web.gencat.cat/aca/documents/ca/publicacions/impactes_sobre_ecosistemes/capitol17_lowress.pdf (accessed on 27 February 2021).
- Hestir, E.L.; Brando, V.E.; Bresciani, M.; Giardino, C.; Matta, E.; Villa, P.; Dekker, A.G. Measuring freshwater aquatic ecosystems: The need for a hyperspectral global mapping satellite mission. Remote Sens. Environ. 2015, 167, 181–195. [Google Scholar] [CrossRef] [Green Version]
- Navalgund, R.R.; Jayaraman, V.; Roy, P.S. Remote sensing applications: An overview. Curr. Sci. 2007, 93, 1747–1766. [Google Scholar]
- Östlund, C.; Flink, P.; Strömbeck, N.; Pierson, D.; Lindell, T. Mapping of the water quality of Lake Erken, Sweden, from imaging spectrometry and Landsat Thematic Mapper. Sci. Total. Environ. 2001, 268, 139–154. [Google Scholar] [CrossRef]
- Drusch, M.; Del Bello, U.; Carlier, S.; Colin, O.; Fernandez, V.; Gascon, F.; Hoersch, B.; Isola, C.; Laberinti, P.; Martimort, P.; et al. Sentinel-2: ESA’s optical high-resolution mission for GMES operational services. Remote Sens. Environ. 2012, 120, 25–36. [Google Scholar] [CrossRef]
- Fletcher, K. (Ed.) Sentinel-3: ESA’s Global Land and Ocean Mission for GMES Operational Services; ESA Communications: Noordwijk, The Netherlands, 2012; Available online: https://sentinel.esa.int/documents/247904/351187/S3_SP-1322_3.pdf (accessed on 27 February 2021).
- Nõges, P.; Van De Bund, W.; Cardoso, A.C.; Solimini, A.G.; Heiskanen, A.S. Assessment of the ecological status of European surface waters: A work in progress. Hydrobiologia 2009, 633, 197–211. [Google Scholar] [CrossRef]
- Dall’Olmo, G.; Gitelson, A.; Rundquist, D. Towards a unified approach for remote estimation of chlorophyll-a in both terrestrial vegetation and turbid productive waters. Geophys. Res. Let. 2003, 30, 1938. [Google Scholar] [CrossRef] [Green Version]
- Cairo, C.; Barbosa, C.; Lobo, F.; Novo, E.; Carlos, F.; Maciel, D.; Flores Júnior, R.; Silva, E.; Curtarelli, V. Hybrid Chlorophyll-a Algorithm for Assessing Trophic States of a Tropical Brazilian Reservoir Based on MSI/Sentinel-2 Data. Remote Sens. 2020, 12, 40. [Google Scholar] [CrossRef] [Green Version]
- Moses, W.J.; Saprygin, V.; Gerasyuk, V.; Povazhnyy, V.; Berdnikov, S.; Gitelson, A.A. OLCI-based NIR-red models for estimating chlorophyll-a concentration in productive coastal waters—A preliminary evaluation. Environ. Res. Commun. 2019, 1, 011002. [Google Scholar] [CrossRef]
- Pereira-Sandoval, M.; Urrego, P.; Ruiz-Verdú, A.; Tenjo, C.; Delegido, J.; Soria-Perpinyà, X.; Vicente, E.; Soria, J.; Moreno, J. Calibration and validation of algorithms for the estimation of chlorophyll-a concentration and Secchi depth in inland waters with Sentinel-2. Limnetica 2019, 38, 471–487. [Google Scholar] [CrossRef]
- Xu, M.; Liu, H.; Beck, R.; Lekki, J.; Yang, B.; Shu, S.; Liu, Y.; Benko, T.; Anderson, R.; Tokars, R.; et al. Regionally and Locally Adaptive Models for Retrieving Chlorophyll-a Concentration in Inland Waters From Remotely Sensed Multispectral and Hyperspectral Imagery. IEEE Trans. Geosci. Remote Sens. 2019, 57, 4758–4774. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, W.; Tian, Y.Q.; Yu, Q.; Zheng, Y.; Huang, L. Remote estimation of colored dissolved organic matter and chlorophyll-a in Lake Huron using Sentinel-2 measurements. J. Appl. Remote Sens. 2017, 11, 036007. [Google Scholar] [CrossRef]
- Lins, R.C.; Martinez, J.M.; Motta Marques, D.D.; Cirilo, J.A.; Fragoso, C.R. Assessment of chlorophyll-a remote sensing algorithms in a productive tropical estuarine-lagoon system. Remote Sens. 2017, 9, 516. [Google Scholar] [CrossRef] [Green Version]
- Lindholm, T.; Eriksson, J.E.; Meriluoto, J.A. Toxic cyanobacteria and water quality problems—Examples from a eutrophic lake on Åland, South West Finland. Water Res. 1989, 23, 481–486. [Google Scholar] [CrossRef]
- Falconer, I.R. An overview of problems caused by toxic blue–green algae (cyanobacteria) in drinking and recreational water. Environ. Toxicol. 1999, 14, 5–12. [Google Scholar] [CrossRef]
- Hilborn, E.D.; Beasley, V.R. One health and cyanobacteria in freshwater systems: Animal illnesses and deaths are sentinel events for human health risks. Toxins 2015, 7, 1374–1395. [Google Scholar] [CrossRef] [Green Version]
- Kwon, Y.S.; Pyo, J.; Kwon, Y.H.; Duan, H.; Cho, K.H.; Park, Y. Drone-based hyperspectral remote sensing of cyanobacteria using vertical cumulative pigment concentration in a deep reservoir. Remote Sens. Environ. 2020, 236, 111517. [Google Scholar] [CrossRef]
- Sòria-Perpinyà, X.; Vicente, E.; Urrego, P.; Pereira-Sandoval, M.; Ruíz-Verdú, A.; Delegido, J.; Soria, J.M.; Moreno, J. Remote sensing of cyanobacterial blooms in a hypertrophic lagoon (Albufera of València, Eastern Iberian Peninsula) using multitemporal Sentinel-2 images. Sci. Total Environ. 2020, 698, 134305. [Google Scholar] [CrossRef] [PubMed]
- Beck, R.; Xu, M.; Zhan, S.; Liu, H.; Johansen, R.A.; Tong, S.; Yang, B.; Shu, S.; Wu, Q.; Wang, S.; et al. Comparison of satellite reflectance algorithms for estimating phycocyanin values and cyanobacterial total biovolume in a temperate reservoir using coincident hyperspectral aircraft imagery and dense coincident surface observations. Remote Sens. 2017, 9, 538. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Simis, S.G.; Li, L.; Wang, Q.; Li, Y.; Song, K.; Lyu, H.; Zheng, Z.; Shi, K. A four-band semi-analytical model for estimating phycocyanin in inland waters from simulated MERIS and OLCI data. IEEE Trans. Geosci. Remote Sens. 2017, 56, 1374–1385. [Google Scholar] [CrossRef]
- Simis, S.G.; Ruiz-Verdú, A.; Domínguez-Gómez, J.A.; Peña-Martinez, R.; Peters, S.W.; Gons, H.J. Influence of phytoplankton pigment composition on remote sensing of cyanobacterial biomass. Remote Sens. Environ. 2007, 106, 414–427. [Google Scholar] [CrossRef]
- Giardino, C.; Bresciani, M.; Braga, F.; Cazzaniga, I.; Keukelaere, L.; Knaeps, E.; Brando, V.E. Bio-optical Modeling of Total Suspended Solids. In Bio-optical Modeling and Remote Sensing of Inland Waters; Mishra, D.R., Ogashawara, I., Gitelson, A.A., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 129–156. [Google Scholar] [CrossRef]
- Kirk, J.T.O. Light and photosynthesis in aquatic ecosystems, 3rd ed.; Cambridge University Press: Cambridge, UK, 2011; p. 649. [Google Scholar]
- Matthews, M.W. A current review of empirical procedures of remote sensing in inland and near-coastal transitional waters. Int. J. Remote Sens. 2011, 32, 6855–6899. [Google Scholar] [CrossRef]
- Soomets, T.; Uudeberg, K.; Jakovels, D.; Brauns, A.; Zagars, M.; Kutser, T. Validation and Comparison of Water Quality Products in Baltic Lakes Using Sentinel-2 MSI and Sentinel-3 OLCI Data. Sensors 2020, 20, 742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sòria-Perpinyà, X.; Urrego, E.P.; Pereira-Sandoval, M.; Ruiz-Verdú, A.; Soria, J.M.; Delegido, J.; Vicente, E.; Moreno, J. Monitoring water transparency of a hypertrophic lake (the Albufera of València) using multitemporal Sentinel-2 satellite images. Limnetica 2020, 39, 373–386. [Google Scholar] [CrossRef]
- Delegido, J.; Urrego, P.; Vicente, E.; Sòria-Perpinyà, X.; Soria, J.M.; Pereira-Sandoval, M.; Ruiz-Verdú, A.; Peña, R.; Moreno, J. Turbidez y profundidad de disco de Secchi con Sentinel-2 en embalses con diferente estado trófico en la Comunidad Valenciana. Revista de Teledetección 2019, 54, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Ruddick, K.G.; De Cauwer, V.; Park, Y.J.; Moore, G. Seaborne measurements of near infrared water-leaving reflectance: The similarity spectrum for turbid waters. Limnol. Oceanogr. 2006, 51, 1167–1179. [Google Scholar] [CrossRef] [Green Version]
- Kutser, T.; Koponen, S.; Kallio, K.; Fincke, T.; Paavel, B. Bio-optical Modeling of Colored Dissolved Organic Matter. In Bio-optical Modeling and Remote Sensing of Inland Waters; Mishra, D.R., Ogashawara, I., Gitelson, A.A., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Magnus, P.; Jaakkola, J.J.; Skrondal, A.; Alexander, J.; Becher, G.; Krogh, T.; Dybing, E. Water chlorination and birth defects. Epidemiology 1999, 10, 513–517. [Google Scholar] [CrossRef] [PubMed]
- McDonald, T.A.; Komulainen, H. Carcinogenicity of the chlorination disinfection by-product MX. J. Environ. Sci. Health C 2005, 23, 163–214. [Google Scholar] [CrossRef]
- Kutser, T.; Pierson, D.C.; Kallio, K.Y.; Reinart, A.; Sobek, S. Mapping lake CDOM by satellite remote sensing. Remote Sens. Environ. 2005, 94, 535–540. [Google Scholar] [CrossRef]
- Kutser, T. The possibility of using the Landsat image archive for monitoring long time trends in coloured dissolved organic matter concentration in lake waters. Remote Sens. Environ. 2012, 123, 334–338. [Google Scholar] [CrossRef]
- Al-Kharusi, E.S.; Tenenbaum, D.E.; Abdi, A.M.; Kutser, T.; Karlsson, J.; Bergström, A.K.; Berggren, M. Large-Scale Retrieval of Coloured Dissolved Organic Matter in Northern Lakes Using Sentinel-2 Data. Remote Sens. 2020, 12, 157. [Google Scholar] [CrossRef] [Green Version]
- Toming, K.; Kutser, T.; Laas, A.; Sepp, M.; Paavel, B.; Nõges, T. First experiences in mapping lake water quality parameters with Sentinel-2 MSI imagery. Remote Sens. 2016, 8, 640. [Google Scholar] [CrossRef] [Green Version]
- Brezonik, P.L.; Olmanson, L.G.; Finlay, J.C.; Bauer, M.E. Factors affecting the measurement of CDOM by remote sensing of optically complex inland waters. Remote Sens. Environ. 2015, 157, 199–215. [Google Scholar] [CrossRef]
- Ruescas, A.B.; Hieronymi, M.; Mateo-Garcia, G.; Koponen, S.; Kallio, K.; Camps-Valls, G. Machine learning regression approaches for colored dissolved organic matter (CDOM) retrieval with S2–MSI and S3–OLCI simulated data. Remote Sens. 2018, 10, 786. [Google Scholar] [CrossRef] [Green Version]
- Wetzel, R.G.; Likens, G.E. Composition and biomass of phytoplankton. In Limnological Analyses; Springer: New York, NY, USA, 2000; pp. 147–174. [Google Scholar]
- Gordon, H.R.; McCluney, W.R. Estimation of the depth of sunlight penetration in the sea for remote sensing. Appl. Opt. 1975, 14, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Mobley, C.D. Estimation of the remote-sensing reflectance from above-surface measurements. Appl. Opt. 1999, 38, 7442–7455. [Google Scholar] [CrossRef]
- European Space Agency, Sentinel-2 Spectral Response Functions (S2-SRF) v3.0. Available online: https://earth.esa.int/web/sentinel/user-guides/sentinel-2-msi/document-library/-/asset_publisher/Wk0TKajiISaR/content/sentinel-2a-spectral-responses (accessed on 16 April 2020).
- European Space Agency, Sentinel-3 OLCI-A Spectral Response Function (S3-SRF) v2.0. Available online: https://earth.esa.int/web/sentinel/user-guides/sentinel-3-olci/document-library/-/asset_publisher/hkf7sg9Ny1d5/content/sentinel-3-olci-a-spectral-response-functions (accessed on 16 April 2020).
- Shoaf, W.T.; Lium, B.W. Improved extraction of chlorophyll a and b from algae using dimethyl sulphoxide. Limnol. Oceanogr. 1976, 21, 926–928. [Google Scholar] [CrossRef]
- Jeffrey, S.T.; Humphrey, G.F. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem. Physiol. Pflanz. 1975, 167, 191–194. [Google Scholar] [CrossRef]
- Sòria-Perpinyà, X.; Sancho-Tello, V.; Rodriguez, M.J.; Durán, C.; Soria, J.M.; Vicente, E. Influence of chlorophyll a quantification methods in ecological quality indices. Inland Waters 2019, 9, 104–112. [Google Scholar] [CrossRef]
- Korshin, G.V.; Li, C.W.; Benjamin, M.M. Monitoring the properties of natural organic matter through UV spectroscopy: A consistent theory. Water Res. 1997, 31, 1787–1795. [Google Scholar] [CrossRef]
- Caballero, I.; Steinmetz, F.; Navarro, G. Evaluation of the first year of operational Sentinel-2A data for retrieval of suspended solids in medium-to high-turbidity waters. Remote Sens. 2018, 10, 982. [Google Scholar] [CrossRef] [Green Version]
- Ha, N.T.T.; Thao, N.T.P.; Koike, K.; Nhuan, M.T. Selecting the best band ratio to estimate chlorophyll-a concentration in a tropical freshwater lake using sentinel 2A images from a case study of Lake Ba Be (Northern Vietnam). ISPRS Int. J. Geo-Inf. 2017, 6, 290. [Google Scholar] [CrossRef]
- Yuan, L.; Yulong, G.; Chunmei, C.; Yibo, Z.; Yaoduo, H.; Zhong, X.; Shun, B. Remote estimation of total suspended matter concentration in the Hangzhou Bay based on OLCI and its water colour product applicability analysis. Haiyang Xuebao 2019, 41, 156–169. [Google Scholar]
- Li, J.; Tian, L.; Song, Q.; Huang, J.; Li, W.; Wei, A. A near-infrared band-based algorithm for suspended sediment estimation for turbid waters using the experimental Tiangong 2 moderate resolution wide-wavelength imager. IEEE J. Sel Top. Appl Earth Obs. Remote Sens. 2019, 12, 774–787. [Google Scholar] [CrossRef]
- Hou, X.; Feng, L.; Duan, H.; Chen, X.; Sun, D.; Shi, K. Fifteen-year monitoring of the turbidity dynamics in large lakes and reservoirs in the middle and lower basin of the Yangtze River, China. Remote Sens. Environ. 2017, 190, 107–121. [Google Scholar] [CrossRef]
- O’Reilly, J.E.; Werdell, P.J. Chlorophyll algorithms for ocean color sensors-OC4, OC5 & OC6. Remote Sens. Environ. 2019, 229, 32–47. [Google Scholar] [CrossRef]
- Koponen, S.; Pulliainen, J.; Kallio, K.; Hallikainen, M. Lake water quality classification with airborne hyperspectral spectrometer and simulated MERIS data. Remote Sens. Environ. 2002, 79, 51–59. [Google Scholar] [CrossRef]
- Gurlin, D.; Gitelson, A.A.; Moses, W.J. Remote estimation of chl-a concentration in turbid productive waters-Return to a simple two-band NIR-red model? Remote Sens. Environ. 2011, 115, 3479–3490. [Google Scholar] [CrossRef]
- Alikas, K.; Kratzer, S.; Reinart, A.; Kauer, T.; Paavel, B. Robust remote sensing algorithms to derive diffuse attenuation coefficient for lakes and coastal waters. Limnol. Oceanogr. Methods. 2015, 13, 402–415. [Google Scholar] [CrossRef]
- Koponen, S.; Attila, J.; Pulliainen, J.; Kallio, K.; Pyhälahti, T.; Lindfors, A.; Rasmus, K.; Hallikainen, M. A case study of airborne and satellite remote sensing of a spring bloom event in the Gulf of Finland. Cont. Shelf Res. 2007, 27, 228–244. [Google Scholar] [CrossRef]
- Zhu, W.; Yu, Q.; Tian, Y.Q.; Becker, B.L.; Zheng, T.; Carrick, H.J. An assessment of remote sensing algorithms for coloured dissolved organic matter in complex freshwater environments. Remote Sens. Environ. 2014, 140, 766–778. [Google Scholar] [CrossRef]
- Doxaran, D.; Cherukuru, R.C.N.; Lavender, S.J. Use of reflectance band ratios to estimate suspended and dissolved matter concentrations in estuarine waters. Int J. Remote Sens. 2005, 26, 1763–1769. [Google Scholar] [CrossRef]
- Kallio, K.; Kutser, T.; Hannonen, T.; Koponen, S.; Pulliainen, J.; Vepsäläinen, J.; Pyhälahti, T. Retrieval of water quality from airborne imaging spectrometry of various lake types in different seasons. Sci. Total Environ. 2001, 268, 59–77. [Google Scholar] [CrossRef]
- Gitelson, A.; Dall’Olmo, G.; Moses, W.; Rundquist, D.; Barrow, T.; Fisher, T.; Gurlin, D.; Holz, J. A simple semi-analytical model for remote estimation of chlorophyll-a in turbid waters: Validation. Remote Sens. Environ. 2008, 112, 3582–3593. [Google Scholar] [CrossRef]
- Jeppesen, E.; Kronvang, B.; Olesen, J.E.; Audet, J.; Søndergaard, M.; Hoffmann, C.C.; Andersen, H.E.; Lauridsen, T.L.; Liboriussen, L.; Larsen, S.E.; et al. Climate change effects on nitrogen loading from cultivated catchments in Europe: Implications for nitrogen retention, ecological state of lakes and adaption. Hydrobiologia 2011, 663, 1–21. [Google Scholar] [CrossRef]









| Dataset | CEDEX | ESAQS | |
|---|---|---|---|
| Instrument | ASD-FR | ASD FieldSpec® HandHeld 2 | Ocean Optics HR4000-UV-NIR |
| Manufacturer | Analytical Spectral Devices, Inc.; Boulder, CO, USA | Ocean Optics; Largo, FL, USA | |
| Acceptance angle | 8° | 8° | 8° |
| Spectral interval | 1.4 nm | 1 nm | 0.2 nm |
| Spectral range | 350–1000 nm | 325–1075 nm | 200–1100 nm |
| Reference | Sensor | Atmospheric Correction | Variables | Bands Relation | N | R2 | RMSE | Data Range | |
|---|---|---|---|---|---|---|---|---|---|
| [11] | S2-MSI | 6S | Chl_a | 8 | 0.78 | 5.34 | 2.89–22.83 mg/m3 | ||
| 6 | 0.93 | 12.09 | 19.51–87.63 mg/m3 | ||||||
| R740/R560 | 7 | 0.98 | 58.90 | 75.89–938.97 mg/m3 | |||||
| [12] | S3-OLCI | Bright Pixel Atmospheric Correction | Chl_a | R709/R665 | 15 | 0.95 | 6.53 | 1.81–96.41 mg/m3 | |
| 0.95 | 7 | ||||||||
| [13] | S2-MSI | Simulated water leaving radiance (Hydrolight) | Chl_a | 392 | 0.99 | 23 (MAE) | 10–169 mg/m3 | ||
| log10 [max. (R443; R490)/R560] | 392 | 0.97 | 0.89 (MAE) | <10 mg/m3 | |||||
| Sen2cor | SD | R490/R705 | 60 | 0.68 | 0.88 (MAE) | 0.25–10 m | |||
| [14] | S2-MSI | Empirical line method | Chl_a | R709/R665 | 56 | 0.76 | 4.39 | 7.84–60.95 mg/m3 | |
| [15] | S2-MSI | Sen2Cor | CDOM | R560/R705 | 41 | 0.88 | 0.73 | 0.11–8.46 m−1 a (440) | |
| Chl_a | R705/R665 | 0.49 | 9.97 | 1.62–51.68 mg/m3 | |||||
| [16] | S2-MSI | In situ reflectance | Chl_a | R705/R665 | 72 | 0.78 | 10.44 | 0.97–117.24 mg/m3 | |
| S3-OLCI | R709/R665 | 0.76 | 10.77 | ||||||
| [20] | Dron | PC | R709/R620 | 92 | 0.95 | – | 0.43–13.07 mg/m3 | ||
| [21] | S2-MSI | Sen2cor | PC | R740/R665 | 21 | 0.84 | 141 | 10–1287 mg/m3 | |
| [22] | S2-MSI | In situ reflectance | PC | R740–R665 | 29 | 0.70 | 4.82 | 0–23 | Relative Fluorescence Units |
| S3-OLCI | R707/R679 | 9 | 0.86 | 1.45 | |||||
| [23] | S3-OLCI | In situ reflectance | PC | 216 | 0.69 | 27.69 | 0.33–317.74 mg/m3 | ||
| [24] | MERIS | In situ reflectance | PC | R620; R665; R709; R779 | 373 | 0.74 | – | 0.4–1000 mg/m3 | |
| [27]* | S2-MSI S3-OLCI | C2RCC C2X TOA | Chl_a | R665/R709 | 49 | 0.7 | 8.9 | 18.9 (115.7) ** mg/m3 | |
| TSS | R700 | 0.7 | 3.5 | 8.9 (52.1) ** mg/L | |||||
| CDOM | R665/R490 | 0.6 | 0.8 | 5.5 (11.7) ** m−1 a (400) | |||||
| SD | R490/R709; R560/R709 | 0.8 | 0.4 | 0.9 (6.27)** m | |||||
| [28] | S2-MSI | Sen2cor | SD | R560/R704 | 79 | 0.67 | 0.06 | 0.19–0.62 m | |
| [29] | S2-MSI | Polymer | SD | R490/R560 | 82 | 0.8 | 1.4 | 0.5–10.5 m | |
| [37] | S2-MSI | Sen2Cor | CDOM | R560/R665 | 41 | 0.65 | 1.71 | 0.14–12.24 m−1 a(420) | |
| [38] | S2-MSI | TOA | Chl_a | 23 | 0.83 | – | 3.6–72.9 mg/m3 | ||
| CDOM | R560/R665 | 0.72 | – | 1.77–15.8 mg/L a(380) | |||||
| [39] | S2-MSI | In situ reflectance | CDOM | Ln(R490/R740) | 32 | 0.86 | 0.44 | 0.71–4.3 m–1 a(440) | |
| S3-OLCI | Ln(R510/R753) | 0.86 | 0.44 | ||||||
| [40] | S2-MSI | Simulated water leaving radiance (Hydrolight) | CDOM | R705/R490 | – | 0.97 | 0.17 | 1–86 m−1 a(400) | |
| S3-OLCI | 0.96 | 0.19 | |||||||
| [50] | S2-MSI | ACOLITE POLYMER (sun-glint) | TSS | R664 | 48 | 0.63 | 25.06% | <150 mg/L | |
| R865 | 0.95 | 10.28% | >150 mg/L | ||||||
| [51] | S2-MSI | Empirical line method | Chl_a | R560/R665 | 30 | 0.68 | 0.14 (SEE) | 1.58–6.00 mg/m3 | |
| [52] | S3-OLCI | UV-AC | TSS | R779/R510 | 50 | 0.91 | 19.29 | 33.88–695.24 mg/L | |
| [53] | Tiangong 2 MWI | UV-AC | TSS | NISSI = R820 − R’820 R’820 = R750 + (R980 − R750) × (820 − 750)/(980 − 750) | 92 20 | 0.85 0.76 | 22.7 | 1.78–330.43 mg/L 23.12–208.89 mg/L | |
| [54] | MODIS | Land-based atmospheric correction method | TSS | R645/R555 | 92 | 0.88 | 34.20% | 1–300 mg/L | |
| [55] | OLCI | In situ reflectance | Chl_a | log10 [max. (R443; R490; R510)/R560] | 2720 | 0.86 | – | 0.012 to 77.9 mg/m3 | |
| n | Min. | Max. | Mean | Median | SD | |
|---|---|---|---|---|---|---|
| SDD (m) | 271 | 0.10 | 11.50 | 2.47 | 1.76 | 2.26 |
| CDOM (µg/L QSE) | 222 | 0.03 | 17.14 | 1.90 | 1.60 | 1.66 |
| TSS (mg/L) | 92 | 0.67 | 78.82 | 9.03 | 3.01 | 15.92 |
| Chl_a (mg/m3) | 254 | 0.53 | 704.97 | 53.26 | 10.28 | 108.87 |
| PC (mg/m3) | 170 | 0.10 | 1040.00 | 106.37 | 17.27 | 194.60 |
| p < 0.001 | CDOM | TSS | Chl_a | PC |
|---|---|---|---|---|
| SDD | −0.588 (204) | −0.831 (91) | −0.845 (251) | −0.815 (157) |
| CDOM | 0.551 (55) | 0.680 (202) | 0.376 (159) | |
| TSS | 0.649 (92) | 0.734 (21) | ||
| Chl_a | 0.848 (160) |
| Sensor | Bands Relation | Calibration | Validation | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | R2 | n | R2 | RMSE | RRMSE | Bias | ||||
| SDD | S2 | R492/R705 | 134 | 0.65 | 131 | 0.71 | 1.30 | 55.19 | 0.35 | |
| S3 | R490/R709 | 0.64 | 0.69 | 1.36 | 57.65 | 0.36 | ||||
| S2 | R560/R705 | 135 | 0.63 | 131 | 0.77 | 1.03 | 43.64 | 0.31 | ||
| S3 | R560/R709 | 0.62 | 0.77 | 1.05 | 44.46 | 0.32 | ||||
| S2 | R492/R560 | 135 | 0.60 | 131 | 0.65 | 1.21 | 51.22 | 0.10 | ||
| S3 | R490/R560 | 0.60 | 0.65 | 1.19 | 50.95 | 0.13 | ||||
| Sensor | Bands Relation | Calibration | Validation | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | R2 | n | R2 | RMSE | RRMSE | bias | ||||
| CDOM | S2 | Ln(R492/R740) | 108 | 0.46 (l.) | 109 | 0.48 | 0.93 | 50.46 | 0.04 | |
| S3 | Ln(R510/R753) | 0.45 (l.) | 0.47 | 0.93 | 50.85 | 0.05 | ||||
| S2 | R560/R665 | 107 | 0.51 (p.) | 107 | 0.51 | 0.95 | 52.34 | 0.29 | ||
| S3 | R560/R665 | 0.50 (p.) | 0.48 | 0.98 | 53.96 | 0.29 | ||||
| S2 | R560/R705 | 108 | 0.51 (e.) | 110 | 0.55 | 0.91 | 49.88 | 0.30 | ||
| S3 | R560/R709 | 0.50 (e.) | 0.55 | 0.92 | 50.15 | 0.30 | ||||
| S2 | R665/R492 | 108 | 0.49 (l.) | 110 | 0.53 | 0.88 | 47.94 | 0.03 | ||
| S3 | R665/R490 | 0.47 (l.) | 0.51 | 0.90 | 48.90 | 0.03 | ||||
| S2 | R705/R492 | 108 | 0.48 (p.) | 108 | 0.48 | 1.03 | 56.24 | 0.26 | ||
| S3 | R709/R490 | 0.47 (p.) | 0.45 | 1.09 | 59.14 | 0.25 | ||||
| Sensor | Bands Relation | Calibration | Validation | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | R2 | n | R2 | RMSE | RRMSE | Bias | ||||
| TSS | S2 | R865 | 45 | 0.71 (e.) | 44 | 0.91 | 6.13 | 65.98 | 2.09 | |
| S3 | R865 | 0.71 (e.) | 0.91 | 6.03 | 64.87 | 2.01 | ||||
| S2 | R783/R492 | 45 | 0.90 (l.) | 41 | 0.93 | 4.57 | 49.69 | 0.27 | ||
| S3 | R779/R510 | 0.90 (l.) | 0.94 | 4.35 | 47.22 | 0.38 | ||||
| S2 | NISSI (R842) | 45 | 0.90 (l.) | 44 | 0.84 | 6.74 | 72.71 | 0.15 | ||
| S3 | NISSI (R779) | 42 | 0.92 (l.) | 0.81 | 7.74 | 83.27 | 0.63 | |||
| TSS < 10 mg/L | S2 | R665 | 38 | 0.54 (l.) | 35 | 0.55 | 1.42 | 45.27 | 0.08 | |
| S3 | R665 | 0.52 (l.) | 0.55 | 1.71 | 54.42 | 0.86 | ||||
| TSS < 20 mg/L | S2 | R700 | 40 | 0.84 (l.) | 37 | 0.85 | 1.79 | 42.89 | 0.39 | |
| S3 | R700 | 0.84 (l.) | 0.85 | 1.78 | 42.78 | 0.40 | ||||
| S2 | R665/R560 | 40 | 0.50 (p.) | 38 | 0.48 | 3.23 | 77.45 | 0.76 | ||
| S3 | R665/R560 | 0.48 (p.) | 0.47 | 4.25 | 84.70 | 0.75 | ||||
| TSS > 10 mg/L | S2 | R665 | 8 | 0.51 (l.) | 8 | 0.60 | 16.65 | 42.65 | 1.84 | |
| S3 | R665 | 0.50 (l.) | 0.59 | 16.71 | 43.12 | 2.00 | ||||
| TSS > 20 mg/L | S2 | R783/R492 | 6 | 0.77 (l.) | 5 | 0.80 | 11.44 | 22.77 | 4.36 | |
| S3 | R779/R510 | 0.78 (l.) | 0.81 | 11.20 | 22.29 | 4.78 | ||||
| S2 | NISSI (R842) | 6 | 0.77 (l.) | 5 | 0.40 | 20.44 | 41.96 | 1.15 | ||
| S3 | NISSI (R779) | 0.98 (l.) | 0.21 | 21.28 | 43.68 | 2.60 | ||||
| Sensor | Bands Relation | Calibration | Validation | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | R2 | n | R2 | RMSE | RRMSE | Bias | ||||
| Chl_a < 5 mg/m3 | S2 | log10 [max. (R443; R492)/R560] | 52 * | 0.68 (l.) | 53 | 0.55 | 0.94 | 43.18 | 0.09 | |
| S3 | log10 [max. (R443; R490)/R560] | 0.69 (l.) | 0.57 | 0.92 | 42.43 | 0.08 | ||||
| S3 | log10 [max. (R443; R490; R510)/R560] | 0.69 (l.) | 0.57 | 0.93 | 42.82 | 0.13 | ||||
| Chl_a > 5 mg/m3 | S2 | 73 | 0.92 (pl.) | 71 | 0.85 | 41.76 | 51.76 | 4.77 | ||
| S3 | 0.91 (pl.) | 0.84 | 79.35 | 98.36 | 29.87 | |||||
| S2 | 73 * | 0.82 (pl.) | 70 | 0.73 | 56.04 | 73.47 | 1.15 | |||
| S3 | 73 | 0.83 (pl.) | 0.82 | 54.97 | 72.07 | 14.44 | ||||
| S2 | R740/R560 | 73 | 0.91 (pl.) | 71 | 0.90 | 31.67 | 39.26 | 10.24 | ||
| S3 | R754/R560 | 0.91 (pl.) | 0.89 | 43.67 | 54.17 | 10.72 | ||||
| S2 | R705/R665 | 72 | 0.93 (p.) | 71 | 0.91 | 35.16 | 41.07 | 2.99 | ||
| S3 | R709/R665 | 0.93 (p.) | 0.91 | 37.10 | 43.95 | 3.40 | ||||
| S2 | R665/R705 | 73 * | 0.90 (e.) | 73 | 0.87 | 81.13 | 97.44 | 20.05 | ||
| S3 | R665/R709 | 0.89 (e.) | 72 | 0.87 | 60.13 | 71.50 | 15.82 | |||
| S2 | 73 * | 0.70 (p.) | 73 | 0.28 | 125.01 | 134.55 | 36.16 | |||
| S3 | 0.72 (pl.) | 71 | 0.55 | 81.48 | 95.54 | 15.89 | ||||
| S2 | log10 [max. (R443; R492)/R560] | 73 * | 0.47 (pl.) | 73 | 0.21 | 128.01 | 137.87 | 36.99 | ||
| S3 | log10 [max. (R443; R490)/R560] | 0.50 (pl.) | 0.23 | 124.86 | 134.38 | 33.83 | ||||
| S2 | R705/R560 | 72 * | 0.90 (pl.) | 71 | 0.89 | 39.74 | 48.00 | 2.22 | ||
| S3 | R709/R560 | 0.90 (pl.) | 72 | 0.90 | 37.04 | 43.88 | 0.49 | |||
| Sensor | Bands Relation | Calibration | Validation | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | R2 | n | R2 | RMSE | RRMSE | Bias | |||
| S2 | R705/R665 | 70 | 0.78 (p.) | 64 | 0.79 | 43.67 | 54.92 | 14.64 | |
| S3 | R709/R679 | 68 | 0.80 (p.) | 65 | 0.93 | 42.59 | 54.41 | 4.62 | |
| S3 | R709/R620 | 67 | 0.77 (p.) | 63 | 0.82 | 34.57 | 46.75 | 7.03 | |
| S2 | R740/R665 | 69 | 0.67 (p.) | 64 | 0.35 | 58.36 | 79.93 | 27.09 | |
| S3 | R754/R665 | 0.66 (p.) | 65 | 0.62 | 60.62 | 77.23 | 25.62 | ||
| S2 | 68 | 0.94 (l.) | 55 | 0.92 | 92.60 | 62.52 | 31.75 | ||
| S3 | 70 | 0.95 (l.) | 66 | 0.94 | 60.16 | 48.33 | 16.51 | ||
| S2 | Simis et al. [24] | 70 | 0.91 (l.) | 69 | 0.91 | 85.10 | 71.35 | 36.33 | |
| S3 | Simis et al. [24] | 70 | 0.96 (l.) | 69 | 0.96 | 39.98 | 33.52 | 2.65 | |
| Parameter | SENTINEL 2 | SENTINEL 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Range | n | Equation | R2 | RMSE | Equation | R2 | RMSE | |||
| SDD (m) | 0.1–9.55 | 266 | 0.5326 × (R560/R705) + 0.3818 | 0.69 | 1.14 | 0.4406 × (R560/R709) + 0.4729 | 0.68 | 1.16 | ||
| CDOM (µg/L QSE) | 0.03–5.30 | 217 | 2.4072 × (R665/R492) + 0.0709 | 0.52 | 0.88 | 2.235 × (R665/R490) + 0.1838 | 0.50 | 0.90 | ||
| TSS (mg/L) | 0.67–19.76 | 76 | 803.99 × R700 + 1.0947 | 0.85 | 1.55 | 813.45 × R700 + 1.2717 | 0.85 | 1.55 | ||
| 20.00–78.82 | 11 | 14.464 × (R783/R492) + 16.336 | 0.77 | 10.35 | 17.543 × (R779/R510) + 15.67 | 0.79 | 10.07 | |||
| Chl_a (mg/m3) | 0.53–4.92 | 106 | exp.10(−2.4792 × (log10[max.(R443;R492)/R560])–0.0389) | 0.62 | 0.91 | exp.10(−2.2251 × (log10[max.(R443;R490)/R560] –0.0306) | 0.64 | 0.90 | ||
| 5.16–674.70 | 144 | 19.866 × (R705/R665)2.3051 | 0.90 | 35.68 | 21.057 × (R709/R665)1.9516 | 0.90 | 37.29 | |||
| PC (mg/m3) | 0.13–1040 | 138 | 21.554 × (R705/R665)3.4791 | 0.79 | 44.48 | 0.96 | 41.47 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sòria-Perpinyà, X.; Vicente, E.; Urrego, P.; Pereira-Sandoval, M.; Tenjo, C.; Ruíz-Verdú, A.; Delegido, J.; Soria, J.M.; Peña, R.; Moreno, J. Validation of Water Quality Monitoring Algorithms for Sentinel-2 and Sentinel-3 in Mediterranean Inland Waters with In Situ Reflectance Data. Water 2021, 13, 686. https://doi.org/10.3390/w13050686
Sòria-Perpinyà X, Vicente E, Urrego P, Pereira-Sandoval M, Tenjo C, Ruíz-Verdú A, Delegido J, Soria JM, Peña R, Moreno J. Validation of Water Quality Monitoring Algorithms for Sentinel-2 and Sentinel-3 in Mediterranean Inland Waters with In Situ Reflectance Data. Water. 2021; 13(5):686. https://doi.org/10.3390/w13050686
Chicago/Turabian StyleSòria-Perpinyà, Xavier, Eduardo Vicente, Patricia Urrego, Marcela Pereira-Sandoval, Carolina Tenjo, Antonio Ruíz-Verdú, Jesús Delegido, Juan Miguel Soria, Ramón Peña, and José Moreno. 2021. "Validation of Water Quality Monitoring Algorithms for Sentinel-2 and Sentinel-3 in Mediterranean Inland Waters with In Situ Reflectance Data" Water 13, no. 5: 686. https://doi.org/10.3390/w13050686
APA StyleSòria-Perpinyà, X., Vicente, E., Urrego, P., Pereira-Sandoval, M., Tenjo, C., Ruíz-Verdú, A., Delegido, J., Soria, J. M., Peña, R., & Moreno, J. (2021). Validation of Water Quality Monitoring Algorithms for Sentinel-2 and Sentinel-3 in Mediterranean Inland Waters with In Situ Reflectance Data. Water, 13(5), 686. https://doi.org/10.3390/w13050686








