The Role of Aquatic Ecosystems (River Tua, Portugal) as Reservoirs of Multidrug-Resistant Aeromonas spp.
Abstract
:1. Introduction
2. Material and Methods
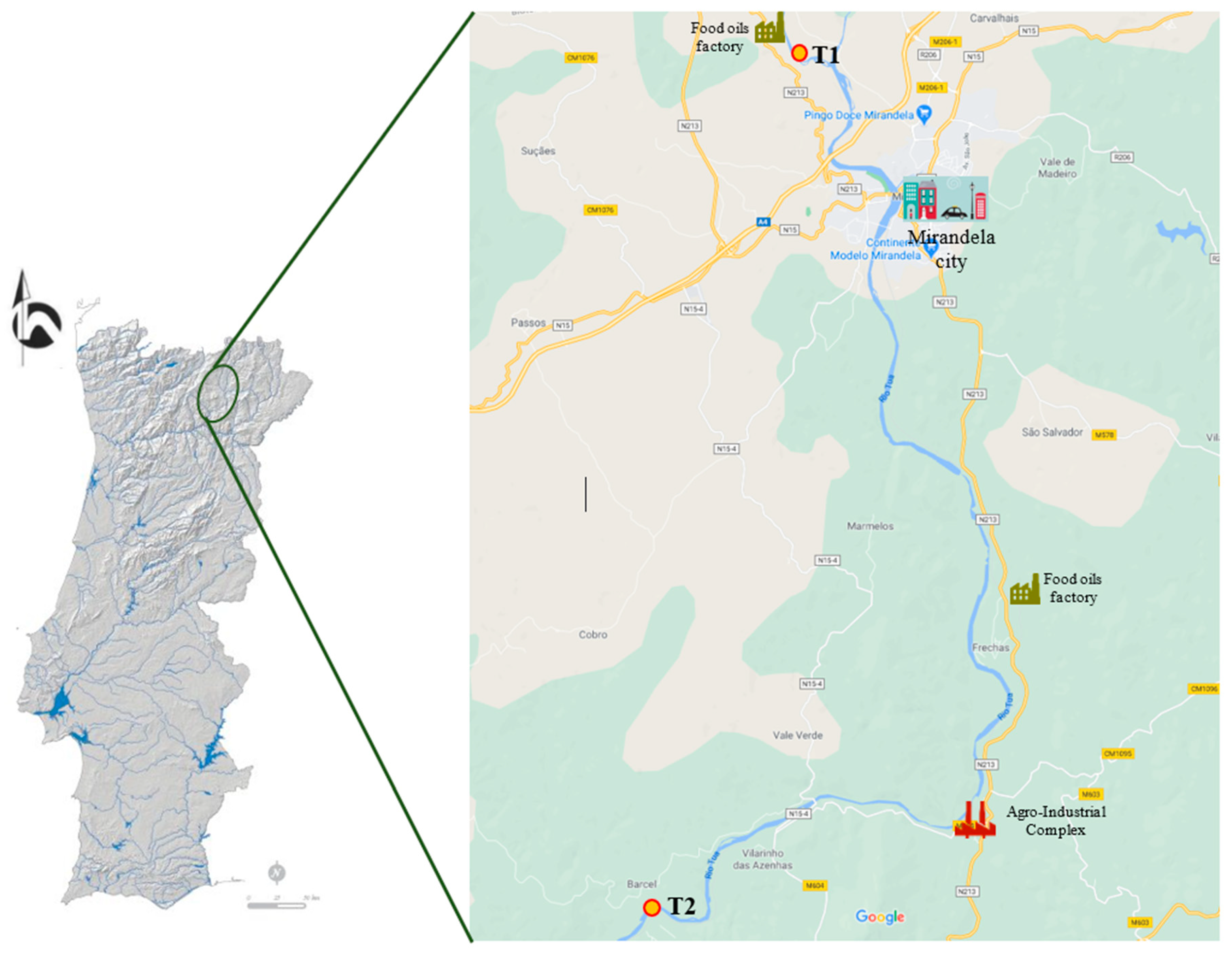
2.1. Study Area
2.2. Sample Processing and Isolation
2.3. Identification of Isolates
2.4. Antimicrobial Susceptibility Testing
2.5. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMC | Amoxicillin/clavulanic acid |
| AMR | Antimicrobial resistance |
| AML | Amoxicillin |
| APA | Environment Portuguese Agency |
| ARB | Antibiotic resistant bacteria |
| ARG | Antibiotic resistance genes |
| ATM | Aztreonam |
| BHI | Brain Hearth Infusion |
| BOD | Biological oxygen demand |
| C | Chloramphenicol |
| CIP | Ciprofloxacin |
| CLSI | Clinical Laboratory Standard Institute |
| CN | Gentamicin |
| COD | Chemical oxygen demand |
| CTX | Cefotaxime |
| FD | Foodborne Diseases |
| ESBL | Extended-Spectrum β-lactamases |
| FOS | Fosfomycin |
| GSP | Glutamate Starch Phenol |
| GSP–IMP | Glutamate Starch Phenol imipenem |
| IMP | Imipenem |
| IPMA | Portuguese Institute of the Sea and the Atmosphere |
| K | Kanamycin |
| KF | Cephalothin |
| MDR | Multidrug-resistant |
| NA | Nalidixic acid |
| PRL | Piperacillin |
| SXT | Cotrimoxazole |
| SXT | Trimethoprim/sulfamethoxazole |
| T1 | Site T1 (Chelas) |
| T2 | Site T2 (Barcel) |
| TE | Tetracycline |
| TIC | Ticarcillin |
| TIM | Ticarcillin/clavulanic acid |
| TOB | Tobramycin |
| TSA | Tryptone Soya Agar |
| TSS | Total suspended solids |
| TZP | Piperacillin/tazobactam |
| WWTP | Wastewater treatment plant |
References
- Páll, E.; Niculae, M.; Kiss, T.; Şandru, C.D.; Spînu, M. Human impact on the microbiological water quality of the rivers. J. Med. Microbiol. 2013, 62, 1635–1640. [Google Scholar] [CrossRef]
- Felis, E.; Kalka, J.; Sochacki, A.; Kowalska, K.; Bajkacz, S.; Harnisz, M.; Korzeniewska, E. Antimicrobial pharmaceuticals in the aquatic environment—Occurrence and environmental implications. Eur. J. Pharmacol. 2020, 866, 172813. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Antimicrobial Resistance: Global Report on Surveillance. 2018. Available online: https://www.who.int/medicines/areas/rational_use/who-amr-amc-report20181109.pdf (accessed on 3 February 2021).
- Baquero, F.; Martínez, J.; Cantón, R. Antibiotics and antibiotic resistance in water environments. Curr. Opin. Biotechnol. 2008, 19, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.D. Antibiotic resistance in the environment: A link to the clinic? Curr. Opin. Microbiol. 2010, 13, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Ogo, M.; Koike, T.; Takada, H.; Newman, B. Sulfonamide and tetracycline resistance genes in total- and culturable-bacterial assemblages in South African aquatic environments. Front. Microbiol. 2015, 6, 796. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, S.A.; Ramachandran, A.; Perron, G.G. Antibiotic Pollution in the Environment: From Microbial Ecology to Public Policy. Microorganisms 2019, 7, 180. [Google Scholar] [CrossRef] [Green Version]
- Vipin, C.; Saptami, K.; Fida, F.; Mujeeburahiman, M.; Rao, S.S.; Athmika Arun, A.B.; Rekha, P.D. Potential synergistic activity of quercetin with antibiotics against multidrug-resistant clinical strains of Pseudomonas aeruginosa. PLoS ONE 2020, 15, e0241304. [Google Scholar] [CrossRef]
- He, Y.; Yuan, Q.; Mathieu, J.; Stadler, L.; Senehi, N.; Sun, R.; Alvarez, P.J.J. Antibiotic resistance genes from livestock waste: Occurrence, dissemination, and treatment. NPJ Clean Water 2020, 3, 4. [Google Scholar] [CrossRef] [Green Version]
- Michael, I.; Rizzo, L.; McArdell, C.S.; Manaia, C.M.; Merlin, C.; Schwartz, T.; Dagot, C.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: A review. Water Res. 2013, 47, 957–995. [Google Scholar] [CrossRef] [Green Version]
- Ghaderpour, A.; Ho, W.S.; Chew, L.L.; Bong, C.W.; Chong, V.C.; Thong, K.L.; Chai, L.C. Diverse and abundant multi-drug resistant E. coli in Matang mangrove estuaries. Malaysia Front. Microbiol. 2015, 10. [Google Scholar] [CrossRef] [Green Version]
- Figueira, V.; Vaz-Moreira, I.; Silva, M.; Manaia, C.M. Diversity and antibiotic resistance of Aeromonas spp. in drinking and waste water treatment plants. Water Res. 2011, 45, 5599–5611. [Google Scholar] [CrossRef] [PubMed]
- Henriques, I.S.; Fonseca, F.; Alves, A.; Saavedra, M.J.; Correia, A. Occurrence and diversity of integrons and β-lactamase genes among ampicillin-resistant isolates from estuarine waters. Res. Microbiol. 2006, 157, 938–947. [Google Scholar] [CrossRef]
- Picão, R.C.; Poirel, L.; Demarta, A.; Silva, C.S.F.; Corvaglia, A.R.; Petrini, O.; Nordmann, P. Plasmid-mediated quinolone resistance in Aeromonas allosaccharophila recovered from a Swiss lake. J. Antimicrob. Chemother. 2008, 62, 948–950. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, A.S.; Bruun, M.S.; Dalsgaard, I.; Larsen, J.L. Incidence, distribution, and spread of tetracycline resistance determinants and integron-associated antibiotic resistance genes among motile aeromonads from a fish farming environment. Appl. Environ. Microbiol. 2001, 67, 5675–5682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saavedra, M.J.; Guedes-Novais, S.; Alves, A.; Rema, P.; Tacão, M.; Correia, A.; Martínez-Murcia, A. Resistance to beta-lactam antibiotics in Aeromonas hydrophila isolated from rainbow trout (Oncorhynchus mykiss). Int. Microbiol. 2004, 7, 207–211. [Google Scholar]
- Sørum, H. Antimicrobial Drug Resistance in Fish Pathogens. In Antimicrobial Resistance in Bacteria of Animal Origin; Aarestrup, F.M., Ed.; American Society for Microbiology Press: Washington, DC, USA, 2006; pp. 213–238. [Google Scholar]
- Carvalho, M.J.; Martínez-Murcia, A.; Esteves, A.C.; Correia, A.; Saavedra, M.J. Phylogenetic diversity, antibiotic resistance and virulence traits of Aeromonas spp. from untreated waters for human consumption. Int. J. Food Microbiol. 2012, 159, 230–239. [Google Scholar] [CrossRef]
- Fernández-Bravo, A.; Figueras, M.J. An Update on the genus Aeromonas: Taxonomy, epidemiology, and pathogenicity. Microorganisms 2020, 8, 129. [Google Scholar] [CrossRef] [Green Version]
- Figueras, M.J.; Alperi, A.; Saavedra, M.J.; Ko, W.C.; Gonzalo, N.; Navarro, M.; Martínez-Murcia, A.J. Clinical relevance of the recently described species Aeromonas aquariorum. J. Clin. Microbiol. 2009, 47, 3742–3746. [Google Scholar] [CrossRef] [Green Version]
- Beaz-Hidalgo, R.; Martínez-Murcia, A.; Figueras, M.J. Reclassification of Aeromonas hydrophila subsp. dhakensis Huys and Aeromonas aquariorum Martínez-Murcia et al. 2008 as Aeromonas dhakensis sp. nov. comb nov. and emendation of the species Aeromonas hydrophila. Syst. Appl. Microbiol. 2013, 36, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.L.; Lamy, B.; Ko, W.C. Aeromonas dhakensis, an increasingly recognized human pathogen. Front. Microbiol. 2016, 7, 793. [Google Scholar] [CrossRef]
- Janda, J.M.; Abbott, S.L. The genus Aeromonas: Taxonomy, pathogenicity, and infection. Clin. Microbiol. Rev. 2010, 23, 35–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Court of Auditors. Addressing Antimicrobial Resistance: Progress in the Animal Sector, But this Health Threat Remains A Challenge for the EU. Special Report Nº 21.41. 2019. Available online: http://europeanmemoranda.cabinetoffice.gov.uk/memorandum/court-of-auditors-special-report-no-2019-21-addressing-antimicrobial-resistance-progress-in-the-animal (accessed on 24 February 2021).
- Jobbins, S.E.; Alexander, K.A. Whence they came—Antibiotic-resistant Escherichia coli in African wildlife. J. Wildl. Dis. 2015, 51, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Furness, L.E.; Campbell, A.; Zhang, L.; Gaze, W.H.; Robbie, A.; McDonald, R.A. Wild small mammals as sentinels for the environmental transmission of antimicrobial resistance. Environ. Res. 2017, 154, 28–34. [Google Scholar] [CrossRef]
- Usui, M.; Tagaki, C.; Fukuda, A.; Okubo, T.; Boonla, C.; Suzuki, S. Use of Aeromonas spp. as general indicators of antimicrobial susceptibility among bacteria in aquatic environments in Thailand. Front. Microbiol. 2016, 7, 710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varela, A.R.; Nunes, O.C.; Manaia, C.M. Quinolone resistant Aeromonas spp. as carriers and potential tracers of acquired antibiotic resistance in hospital and municipal wastewater. Sci. Total Environ. 2016, 15, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Baron, S.; Granier, S.A.; Larvor, E.; Jouy, E.; Cineux, M.; Wilhelm, A.; Gassilloud, B.; Le Bouquin, S.; Kempf, I.; Chauvin, C. Aeromonas diversity and antimicrobial susceptibility in freshwater-an attempt to set generic epidemiological cut-off values. Front. Microbiol. 2017, 8, 503. [Google Scholar] [CrossRef] [Green Version]
- Abbott, S.L.; Cheung, W.K.W.; Janda, J.M. The genus Aeromonas: Biochemical characteristics, atypical reactions, and phenotypic identification schemes. J. Clin. Microbiol. 2003, 41, 2348–2357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2020. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria, 3rd ed.; CLSI Guideline M45; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2016. [Google Scholar]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2011, 268–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, W.C.; Yu, K.W.; Liu, C.Y.; Huang, C.T.; Leu, H.S.; Chuang, Y.C. Increasing antibiotic resistance in clinical isolates of Aeromonas strains in Taiwan. Antimicrob. Agents Chemother. 1996, 40, 1260–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saengsitthisak, B.; Chaisri, W.; Punyapornwithaya, V.; Mektrirat, R.; Klayraung, S.; Bernard, J.K.; Pikulkaew, S. Occurrence and antimicrobial susceptibility profiles of multidrug-resistant Aeromonads isolated from freshwater ornamental fish in chiang Mai Province. Pathogens 2020, 9, 973. [Google Scholar] [CrossRef] [PubMed]
- Dias, C.; Borges, A.; Saavedra, M.J.; Simões, M. Biofilm formation and multidrug-resistant Aeromonas spp. from wild animals. J. Glob. Antimicrob. Resist. 2018, 12, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Lupo, A.; Coyne, S.; Berendonk, T.U. Origin and evolution of antibiotic resistance: The common mechanisms of emergence and spread in water bodies. Front. Microbiol. 2012, 3, 18. [Google Scholar] [CrossRef] [Green Version]
- Holmes, P.; Nicolls, L.M.; Sartory, D.P. The ecology of mesophilic Aeromonas in the aquatic environment. In The Genus Aeromonas; John Wiley & Sons Ltd.: West Sussex, UK, 1996; pp. 127–150. [Google Scholar]
- McLellan, S.L.; Huse, S.M.; Mueller-Spitz, S.R.; Andreishcheva, E.N.; Sogin, M.L. Diversity and population structure of sewage-derived microorganisms in wastewater treatment plant influent. Environ. Microbiol. 2010, 12, 378–392. [Google Scholar] [CrossRef] [Green Version]
- Dias, C.; Mota, V.; Martinez-Murcia, A.; Saavedra, M.J. Antimicrobial resistance patterns of Aeromonas spp. isolated from ornamental fish. J. Aquac. Res. Dev. 2012, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Dias, C.; Serra, C.R.; Simões, L.C.; Simões, M.; Martinez-Murcia, A.; Saavedra, M.J. Extended-spectrum β-lactamase and carbapenemase-producing Aeromonas species in wild animals from Portugal. Vet. Rec. 2014, 24, 532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goňi-Urriza, U.; Capdepuy, M.C.; Arpin, C.; Raymond, N.; Caumette, P.; Quentin, C. Impact of an urban effluent on antibiotic resistance of riverine Enterobacteriaceae and Aeromonas spp. Appl. Environ. Microbiol. 2000, 66, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Rawat, D.; Nair, D. Extended-spectrum β-lactamases in Gram Negative Bacteria. J. Glob. Infect. Dis. 2010, 2, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shang, X.; Hu, F.; Lao, X.; Gao, X.; Zheng, H.; Yao, W. β-Lactamase Inhibitors: An Update. Mini-Rev. Med. Chem. 2013, 13, 1846–1861. [Google Scholar] [CrossRef]
- Codjoe, F.S.; Donkor, E.S. Carbapenem resistance: A review. Med. Sci. 2017, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Critically Important Antimicrobials for Human Medicine, 6th ed.; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- World Organization for Animal Health. OIE List of Antimicrobial Agents of Veterinary Importance; World Organization for Animal Health: Paris, France, 2018. [Google Scholar]
- Piotrowska, M.; Przygodzinska, D.; Matyjewicz, K.; Popowska, M. Occurrence and variety of β-Lactamase genes among Aeromonas spp. isolated from urban wastewater treatment plant. Front. Microbiol. 2017, 8, 863. [Google Scholar] [CrossRef] [Green Version]
- Picão, R.C.; Cardoso, J.P.; Campana, E.H.; Nicoletti, A.G.; Petrolin, F.V.; Assis, D.M.; Juliano, L.; Gales, A.C. The route of antimicrobial resistance from the hospital effluent to the environment: Focus on the occurrence of KPC-producing Aeromonas spp. and Enterobacteriaceae in sewage. Diagn. Microbiol. Infect. Dis. 2013, 76, 80–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knapp, C.W.; Lima, L.; Olivares-Rieumont, S.; Bowen, E.; Werner, D.; Graham, D.W. Seasonal variations in antibiotic resistance gene transport in the almendares river, havana, cuba. Front. Microbiol. 2012, 3, 396. [Google Scholar] [CrossRef] [Green Version]
- Decker, E.H.; Kerkhoff, A.J.; Moses, M.E. Global patterns of city size Distributions and their fundamental drivers. PLoS ONE 2007, 2, e934. [Google Scholar] [CrossRef] [PubMed]
- United Nations (UN). Revision of World Urbanization Prospects. 2009. Available online: www.unpopulation.org (accessed on 21 January 2021).
- Abraham, W.-R. Megacities as sources for pathogenic bacteria in rivers and their fate downstream. Int. J. Microbiol. 2011, 2011, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Salvat, M.J.F.; Ashbolt, N. Aeromonas in Global Water Pathogen Project; University of Alberta: Edmonton, AB, Canada, 2019. [Google Scholar]
- He, L.; Du, P.; Chen, Y.; Lu, H.; Cheng, X.; Chang, B.; Wang, Z. Advances in microbial fuel cells for wastewater treatment. Renew. Sustain. Energy Rev. 2017, 71, 388–403. [Google Scholar] [CrossRef]
- Hassani, L.; Imziln, B.; Boussaid, A.; Gauthier, M.J. Seasonal incidence of and antibiotic resistance among Aeromonas species isolated from domestic wastewater before and after treatment in stabilization ponds. Microb. Ecol. 1992, 23, 227–237. [Google Scholar] [CrossRef]
- Latif-Eugenín, F.; Beaz-Hidalgo, R.; Figueras, M.J. Evaluation of different conditions and culture media for the recovery of Aeromonas spp. from water and shellfish samples. J. Appl. Microbiol. 2016, 121, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Latif-Eugenin, F.; Hidalgo, R.B.; Silvera, C.; Fernandez-Cassi, X.; Figueras, M.J. Chlorinated and ultraviolet radiation-treated reclaimed irrigation water is the source of Aeromonas found in vegetables used for human consumption. Environ. Res. 2017, 154, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Daskalov, H. The importance of Aeromonas hydrophila in food safety. Food Control. 2006, 17, 474–483. [Google Scholar] [CrossRef]
- Lijon, M.B.; Khatun, M.M.; Islam, A.; Khatun, M.M.; Islam, M.A. Detection of multidrug resistance Aeromonas hydrophila in farm raised fresh water prawns. J. Adv. Vet. Anim. Res. 2015, 2, 469–474. [Google Scholar] [CrossRef]
- Martínez-Murcia, A.; Beaz-Hidalgo, R.; Navarro, A.; Carvalho, M.J.; Aravena-Román, M.; Correia, A.; Figueras, M.J.; Saavedra, M.J. Aeromonas lusitana sp. nov., isolated from untreated water and vegetables. Curr. Microbiol. 2016, 72, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Patil, H.J.; Benet-Perelberg, A.; Naor, A.; Smirnov, M.; Ofek, T.; Nasser, A.; Minz, D.; Cytryn, E. Evidence of increased antibiotic resistance in phylogenetically-diverse Aeromonas isolates from semi-intensive fish ponds treated with antibiotics. Front. Microbiol. 2016, 7, 1875. [Google Scholar] [CrossRef] [Green Version]
- Borella, L.; Salogni, C.; Vitale, N.; Scali, F.; Moretti, V.M.; Pasquali, P.; Alborali, G.L. Motile aeromonads from farmed and wild freshwater fish in northern Italy: An evaluation of antimicrobial activity and multidrug resistance during 2013 and 2016. Acta Vet. Scand. 2020, 23, 6. [Google Scholar] [CrossRef]
- Bhunia, A.K. Opportunistic and Emerging Foodborne Pathogens: Aeromonas hydrophila, Plesiomonas shigelloides, Cronobacter sakazakii, and Brucella abortus. In Foodborne Microbial Pathogens; Food Science Text Series; Springer: New York, NY, USA, 2018. [Google Scholar]
- Hirsch, R.; Ternes, T.; Haberer, K.; Kratz, K.L. Occurrence of antibiotics in the aquatic environment. Sci. Total Environ. 1999, 12, 109–118. [Google Scholar] [CrossRef]
- Huang, J.J.; Hu, H.Y.; Lu, S.Q.; Li, Y.; Tang, F.; Lu, Y.; Wei, B. Monitoring and evaluation of antibiotic-resistant bacteria at a municipal wastewater treatment plant in China. Environ. Int. 2012, 42, 31–36. [Google Scholar] [CrossRef]
- Vaz-Moreira, I.; Nunes, O.C.; Manaia, C.M. Bacterial diversity and antibiotic resistance in water habitats: Searching the links with the human microbiome. FEMS Microbiol. Rev. 2014, 38, 761–778. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, S.D.; Murby, J.; Bates, J. Ecosystem response to antibiotics entering the aquatic environment. Mar. Pollut. Bull. 2005, 51, 218–223. [Google Scholar] [CrossRef]
- Bengtsson-Palme, J.; Kristiansson, E.; Larsson, D.G.J. Environmental factors influencing the development and spread of antibiotic resistance. FEMS Microbiol. Rev. 2018, 42, 68–80. [Google Scholar] [CrossRef]
- Lima, T.; Domingues, S.; Da Silva, G.J. Manure as a Potential Hotspot for Antibiotic Resistance Dissemination by Horizontal Gene Transfer Events. Vet. Sci. 2020, 7, 110. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, S.; Fernandes, C.; Monteiro, S.; Cabecinha, E.; Teixeira, A.; Varandas, S.; Saavedra, M.J. The Role of Aquatic Ecosystems (River Tua, Portugal) as Reservoirs of Multidrug-Resistant Aeromonas spp. Water 2021, 13, 698. https://doi.org/10.3390/w13050698
Gomes S, Fernandes C, Monteiro S, Cabecinha E, Teixeira A, Varandas S, Saavedra MJ. The Role of Aquatic Ecosystems (River Tua, Portugal) as Reservoirs of Multidrug-Resistant Aeromonas spp. Water. 2021; 13(5):698. https://doi.org/10.3390/w13050698
Chicago/Turabian StyleGomes, Sónia, Conceição Fernandes, Sandra Monteiro, Edna Cabecinha, Amílcar Teixeira, Simone Varandas, and Maria José Saavedra. 2021. "The Role of Aquatic Ecosystems (River Tua, Portugal) as Reservoirs of Multidrug-Resistant Aeromonas spp." Water 13, no. 5: 698. https://doi.org/10.3390/w13050698
APA StyleGomes, S., Fernandes, C., Monteiro, S., Cabecinha, E., Teixeira, A., Varandas, S., & Saavedra, M. J. (2021). The Role of Aquatic Ecosystems (River Tua, Portugal) as Reservoirs of Multidrug-Resistant Aeromonas spp. Water, 13(5), 698. https://doi.org/10.3390/w13050698









