Experimental and Numerical Study of Biochar Fixed Bed Column for the Adsorption of Arsenic from Aqueous Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Used for the Laboratory Tests
2.2. Experimental Apparatus and Test Procedure
2.3. Numerical Model
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mishra, R.K.; Dubey, S.C. Fresh water availability and it’s global challenge. Int. J. Eng. Sci. Invent. Res. Dev. 2015, 2, 351–407. [Google Scholar]
- Verma, R.; Dwivedi, P. Heavy metal water pollution-A case study. Recent Res. Sci. Technol. 2013, 5, 98–99. [Google Scholar]
- Egboka, B.C.E.; Nwankwor, G.I.; Orajaka, I.P.; Ejiofor, A.O. Principles and problems of environmental pollution of groundwater resources with case examples from developing countries. Environ. Health Perspect. 1989, 83, 39–68. [Google Scholar] [CrossRef] [PubMed]
- Sbarato, V.M.; Sánchez, H.J. Analysis of arsenic pollution in groundwater aquifers by X-ray fluorescence. Appl. Radiat. Isot. 2001, 54, 737–740. [Google Scholar] [CrossRef]
- Gunduz, O.; Simsek, C.; Hasozbek, A. Arsenic pollution in the groundwater of Simav Plain, Turkey: Its impact on water quality and human health. Water Air Soil Pollut. 2010, 205, 43–62. [Google Scholar] [CrossRef]
- Liang, C.P.; Wang, S.W.; Kao, Y.H.; Chen, J.S. Health risk assessment of groundwater arsenic pollution in southern Taiwan. Environ. Geochem. Health 2016, 38, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Habuda-Stanić, M.; Kuleš, M.; Kalajdžić, B.; Romić, Ž. Quality of groundwater in eastern Croatia. The problem of arsenic pollution. Desalination 2007, 210, 157–162. [Google Scholar] [CrossRef]
- Shah, B.A. Status of groundwater arsenic pollution of Mirzapur district in Holocene aquifers from parts of the Middle Ganga Plain, India. Environ. Earth Sci. 2015, 73, 1505–1514. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, H.; Tang, J.; Qin, X.; Yu, A.Y. Attenuation of arsenic in a karst subterranean stream and correlation with geochemical factors: A case study at Lihu, South China. J. Environ. Sci. (China) 2014, 26, 2222–2230. [Google Scholar] [CrossRef]
- Seidl, M.; Balázs, G.; Scheer, M. The Chemistry of Yellow Arsenic. Chem. Rev. 2019, 119, 8406–8434. [Google Scholar] [CrossRef]
- Zhu, N.; Qiao, J.; Ye, Y.; Yan, T. Synthesis of mesoporous bismuth-impregnated aluminum oxide for arsenic removal: Adsorption mechanism study and application to a lab-scale column. J. Environ. Manag. 2018, 211, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Bretzler, A.; Lalanne, F.; Nikiema, J.; Podgorski, J.; Pfenninger, N.; Berg, M.; Schirmer, M. Corrigendum to “Groundwater arsenic contamination in Burkina Faso, West Africa: Predicting and verifying regions at risk” Sci. Total Environ. 584–585 (2017) 958–970]. Sci. Total Environ. 2017, 598, 562. [Google Scholar] [CrossRef] [PubMed]
- Ratnaike, R.N. Acute and chronic arsenic toxicity. Postgrad. Med. J. 2003, 79, 391–396. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; incorporating the 1st addendum; World Health Organization: Geneva, Switzerland, 2017; ISBN 978-92-4-154995-0. Available online: https://www.who.int/publications/i/item/9789241549950 (accessed on 26 March 2021).
- Nicomel, N.R.; Leus, K.; Folens, K.; Van Der Voort, P.; Du Laing, G. Technologies for arsenic removal from water: Current status and future perspectives. Int. J. Environ. Res. Public Health 2015, 13, 1–24. [Google Scholar] [CrossRef]
- Figoli, A.; Fuoco, I.; Apollaro, C.; Chabane, M.; Mancuso, R.; Gabriele, B.; De Rosa, R.; Vespasiano, G.; Barca, D.; Criscuoli, A. Arsenic-contaminated groundwaters remediation by nanofiltration. Sep. Purif. Technol. 2020, 238, 116461. [Google Scholar] [CrossRef]
- Sen, M.; Manna, A.; Pal, P. Removal of arsenic from contaminated groundwater by membrane-integrated hybrid treatment system. J. Memb. Sci. 2010, 354, 108–113. [Google Scholar] [CrossRef]
- Pal, P.; Chakrabortty, S.; Linnanen, L. A nanofiltration-coagulation integrated system for separation and stabilization of arsenic from groundwater. Sci. Total Environ. 2014, 476–477, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Košutić, K.; Furač, L.; Sipos, L.; Kunst, B. Removal of arsenic and pesticides from drinking water by nanofiltration membranes. Sep. Purif. Technol. 2005, 42, 137–144. [Google Scholar] [CrossRef]
- Baciocchi, R.; Chiavola, A.; Gavasci, R. Ion exchange equilibria of arsenic in the presence of high sulphate and nitrate concentrations. Water Sci. Technol. Water Supply 2005, 5, 67–74. [Google Scholar] [CrossRef]
- Chiavola, A.; D’Amato, E.; Baciocchi, R. Ion exchange treatment of groundwater contaminated by arsenic in the presence of sulphate. Breakthrough experiments and modeling. Water. Air. Soil Pollut. 2012, 223, 2373–2386. [Google Scholar] [CrossRef]
- Macur, R.E.; Jackson, C.R.; Botero, L.M.; McDermott, T.R.; Inskeep, W.P. Bacterial Populations Associated with the Oxidation and Reduction of Arsenic in an Unsaturated Soil. Environ. Sci. Technol. 2004, 38, 104–111. [Google Scholar] [CrossRef]
- González, M.M.; Gallego, M.; Valcárcel, M. Determination of arsenic in wheat flour by electrothermal atomic absorption spectrometry using a continuous precipitation-dissolution flow system. Talanta 2001, 55, 135–142. [Google Scholar] [CrossRef]
- Ghurye, G.L.; Clifford, D.A.; Tripp, A.R. Combined arsenic and nitrate removal by ion exchange. J. Am. Water Work. Assoc. 1999, 91, 85–96. [Google Scholar] [CrossRef]
- Zhang, H.; Selim, H.M. Kinetics of arsenate adsorption—Desorption in soils. Environ. Sci. Technol. 2005, 39, 6101–6108. [Google Scholar] [CrossRef] [PubMed]
- Boczkaj, G.; Fernandes, A. Wastewater treatment by means of advanced oxidation processes at basic pH conditions: A review. Chem. Eng. J. 2017, 320, 608–633. [Google Scholar] [CrossRef]
- Boni, M.R.; Chiavola, A.; Di Marcantonio, C.; Sbaffoni, S.; Biagioli, S.; Cecchini, G.; Frugis, A. A study through batch tests on the analytical determination and the fate and removal of methamphetamine in the biological treatment of domestic wastewater. Environ. Sci. Pollut. Res. 2018, 25, 27756–27767. [Google Scholar] [CrossRef]
- Chiavola, A.; Tedesco, P.; Boni, M.R. Fate of selected drugs in the wastewater treatment plants (WWTPs) for domestic sewage. Environ. Sci. Pollut. Res. 2019, 26, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Karimi, S.; Tavakkoli Yaraki, M.; Karri, R.R. A comprehensive review of the adsorption mechanisms and factors influencing the adsorption process from the perspective of bioethanol dehydration. Renew. Sustain. Energy Rev. 2019, 107, 535–553. [Google Scholar] [CrossRef]
- Chiavola, A.; Tedesco, P.; Boni, M.R. Fate of Some Endocrine Disruptors in Batch Experiments Using Activated and Inactivated Sludge. Water Air Soil Pollut. 2016, 227, 424. [Google Scholar] [CrossRef]
- Thirunavukkarasu, V.; Anuradha, C.V.; Viswanathan, P. Protective effect of fenugreek (Trigonella foenum graecum) seeds in experimental ethanol toxicity. Phyther. Res. 2003, 17, 737–743. [Google Scholar] [CrossRef]
- Pattanayak, J.; Mondal, K.; Mathew, S.; Lalvani, S.B. A Parametric evaluation of the removal of As(V) and As(III) by carbon-based adsorbents. Carbon N.Y. 2000, 38, 589–596. [Google Scholar] [CrossRef]
- Guha, S.; Chaudhury, P. Locating critical points and constructing reaction paths in noble gas clusters: A simulated annealing based study. J. Mol. Struct. THEOCHEM 2010, 945, 12–16. [Google Scholar] [CrossRef]
- Tchieda, V.K.; D’Amato, E.; Chiavola, A.; Parisi, M.; Chianese, A.; Amamra, M.; Kanaev, A. Removal of Arsenic by Alumina: Effects of Material Size, Additives, and Water Contaminants. Clean Soil Air Water 2016, 44, 496–505. [Google Scholar] [CrossRef]
- Chiavola, A.; D’Amato, E.; Boni, M.R. Comparison of different iron oxide adsorbents for combined arsenic, vanadium and fluoride removal from drinking water. Int. J. Environ. Sci. Technol. 2019, 16, 6053–6064. [Google Scholar] [CrossRef]
- Chiavola, A.; D’Amato, E.; Stoller, M.; Chianese, A.; Boni, M.R. Application of iron based nanoparticles as adsorbents for Arsenic removal from water. Chem. Eng. Trans. 2016, 47, 325–330. [Google Scholar] [CrossRef]
- Sawana, R.; Somasundar, Y.; Iyer, V.S.; Baruwati, B. Ceria modified activated carbon: An efficient arsenic removal adsorbent for drinking water purification. Appl. Water Sci. 2017, 7, 1223–1230. [Google Scholar] [CrossRef]
- Kalaruban, M.; Loganathan, P.; Nguyen, T.V.; Nur, T.; Hasan Johir, M.A.; Nguyen, T.H.; Trinh, M.V.; Vigneswaran, S. Iron-impregnated granular activated carbon for arsenic removal: Application to practical column filters. J. Environ. Manag. 2019, 239, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Vithanage, M.; Herath, I.; Joseph, S.; Bundschuh, J.; Bolan, N.; Ok, Y.S.; Kirkham, M.B.; Rinklebe, J. Interaction of arsenic with biochar in soil and water: A critical review. Carbon N.Y. 2017, 113, 219–230. [Google Scholar] [CrossRef]
- Igalavithana, A.D.; Kwon, E.E.; Vithanage, M.; Rinklebe, J.; Moon, D.H.; Meers, E.; Tsang, D.C.W.; Ok, Y.S. Soil lead immobilization by biochars in short-term laboratory incubation studies. Environ. Int. 2019, 127, 190–198. [Google Scholar] [CrossRef]
- Zimmerman, A.R.; Gao, B.; Ahn, M.Y. Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol. Biochem. 2011, 43, 1169–1179. [Google Scholar] [CrossRef]
- Viotti, P.; Tatti, F.; Rossi, A.; Luciano, A.; Marzeddu, S.; Mancini, G.; Boni, M.R. An Eco-Balanced and Integrated Approach for a More-Sustainable MSW Management. Waste Biomass Valorization 2020, 11, 5139–5150. [Google Scholar] [CrossRef]
- Stoller, M.; Sacco, O.; Vilardi, G.; Pulido, J.M.O.; Di Palma, L. Technical–economic evaluation of chromium recovery from tannery wastewater streams by means of membrane processes. Desalin. Water Treat. 2018, 127, 57–63. [Google Scholar] [CrossRef]
- Agegnehu, G.; Srivastava, A.K.; Bird, M.I. The role of biochar and biochar-compost in improving soil quality and crop performance: A review. Appl. Soil Ecol. 2017, 119, 156–170. [Google Scholar] [CrossRef]
- Glazunova, D.M.; Kuryntseva, P.A.; Selivanovskaya, S.Y.; Galitskaya, P.Y. Assessing the Potential of Using Biochar as a Soil Conditioner. In IOP Conference Series: Earth and Environmental Science, Proceedings of the 3rd International Conference Environment and Sustainable Development of Territories: Ecological Challenges of the 21st Century, Kazan, Russia, 27–29 September 2017; Institute of Physics Publishing: Bristol, UK, 2018; Volume 107, p. 12059. [Google Scholar]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, L.; Zheng, B. Biochar to improve soil fertility. A review. Agron. Sustain. Dev. 2016, 36, 1–18. [Google Scholar] [CrossRef]
- Fidel, R.; Laird, D.; Parkin, T. Effect of Biochar on Soil Greenhouse Gas Emissions at the Laboratory and Field Scales. Soil Syst. 2019, 3, 8. [Google Scholar] [CrossRef]
- Criscuoli, I.; Ventura, M.; Sperotto, A.; Panzacchi, P.; Tonon, G. Effect of woodchips biochar on sensitivity to temperature of soil greenhouse gases emissions. Forests 2019, 10, 594. [Google Scholar] [CrossRef]
- Keske, C.; Godfrey, T.; Hoag, D.L.K.; Abedin, J. Economic feasibility of biochar and agriculture coproduction from Canadian black spruce forest. Food Energy Secur. 2020, 9, e188. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Insight into biochar properties and its cost analysis. Biomass Bioenergy 2016, 84, 76–86. [Google Scholar] [CrossRef]
- Yao, Z.; You, S.; Ge, T.; Wang, C.H. Biomass gasification for syngas and biochar co-production: Energy application and economic evaluation. Appl. Energy 2018, 209, 43–55. [Google Scholar] [CrossRef]
- Ng, W.C.; You, S.; Ling, R.; Gin, K.Y.H.; Dai, Y.; Wang, C.H. Co-gasification of woody biomass and chicken manure: Syngas production, biochar reutilization, and cost-benefit analysis. Energy 2017, 139, 732–742. [Google Scholar] [CrossRef]
- Viglašová, E.; Galamboš, M.; Danková, Z.; Krivosudský, L.; Lengauer, C.L.; Hood-Nowotny, R.; Soja, G.; Rompel, A.; Matík, M.; Briančin, J. Production, characterization and adsorption studies of bamboo-based biochar/montmorillonite composite for nitrate removal. Waste Manag. 2018, 79, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Frišták, V.; Micháleková-Richveisová, B.; Víglašová, E.; Ďuriška, L.; Galamboš, M.; Moreno-Jimenéz, E.; Pipíška, M.; Soja, G. Sorption separation of Eu and As from single-component systems by Fe-modified biochar: Kinetic and equilibrium study. J. Iran. Chem. Soc. 2017, 14, 521–530. [Google Scholar] [CrossRef]
- Daňo, M.; Viglašová, E.; Galamboš, M.; Štamberg, K.; Kujan, J. Surface Complexation Models of Pertechnetate on Biochar/Montmorillonite Composite—Batch and Dynamic Sorption Study. Materials 2020, 13, 3108. [Google Scholar] [CrossRef] [PubMed]
- Sizmur, T.; Quilliam, R.; Puga, A.P.; Moreno-Jiménez, E.; Beesley, L.; Gomez-Eyles, J.L. Application of Biochar for Soil Remediation. 98104 2015, 295–324. [Google Scholar] [CrossRef]
- Hu, X.; Ding, Z.; Zimmerman, A.R.; Wang, S.; Gao, B. Batch and column sorption of arsenic onto iron-impregnated biochar synthesized through hydrolysis. Water Res. 2015, 68, 206–216. [Google Scholar] [CrossRef]
- Qambrani, N.A.; Rahman, M.M.; Won, S.; Shim, S.; Ra, C. Biochar properties and eco-friendly applications for climate change mitigation, waste management, and wastewater treatment: A review. Renew. Sustain. Energy Rev. 2017, 79, 255–273. [Google Scholar] [CrossRef]
- Boni, M.R.; Chiavola, A.; Marzeddu, S. Remediation of Lead-Contaminated Water by Virgin Coniferous Wood Biochar Adsorbent: Batch and Column Application. Water Air Soil Pollut. 2020, 231, 171. [Google Scholar] [CrossRef]
- Tatti, F.; Papini, M.P.; Sappa, G.; Raboni, M.; Arjmand, F.; Viotti, P. Contaminant back-diffusion from low-permeability layers as affected by groundwater velocity: A laboratory investigation by box model and image analysis. Sci. Total Environ. 2018, 622–623, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Tatti, F.; Petrangeli Papini, M.; Torretta, V.; Mancini, G.; Boni, M.R.; Viotti, P. Experimental and numerical evaluation of Groundwater Circulation Wells as a remediation technology for persistent, low permeability contaminant source zones. J. Contam. Hydrol. 2019, 222, 89–100. [Google Scholar] [CrossRef]
- Grund, S.C.; Hanusch, K.; Wolf, H.U. Arsenic and Arsenic Compounds. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; Volume 100 C, pp. 41–93. [Google Scholar]
- Boni, M.R.; Leoni, S.; Sbaffoni, S. Co-landfilling of pretreated waste: Disposal and management strategies at lab-scale. J. Hazard. Mater. 2007, 147, 37–47. [Google Scholar] [CrossRef]
- Chiavola, A.; Marzeddu, S.; Boni, M.R. Remediation of Water Contaminated by Pb(II) Using Virgin Coniferous Wood Biochar as Adsorbent. In Frontiers in Water-Energy-Nexus—Nature-Based Solutions, Advanced Technologies and Best Practices for Environmental Sustainability. Advances in Science, Technology & Innovation (IEREK Interdisciplinary Series for Sustainable Development); Proceedings of the 2nd WaterEnergyNEXUS Conference; Salerno, Italy, 14–17 November 2018, Naddeo, V., Balakrishnan, M., Choo, K.-H., Eds.; Springer, Cham: Salerno, Italy, 2020; pp. 363–366. ISBN 978-3-030-13067-1. [Google Scholar] [CrossRef]
- Rose, P.; Hager, S.; Glas, K.; Rehmann, D.; Hofmann, T. Coating techniques for glass beads as filter media for removal of manganese from water. Water Sci. Technol. Water Supply 2017, 17, 95–106. [Google Scholar] [CrossRef][Green Version]
- Boni, M.R.; Chiavola, A.; Antonucci, A.; Di Mattia, E.; Marzeddu, S. A novel treatment for Cd-contaminated solution through adsorption on beech charcoal: The effect of bioactivation. Desalin. Water Treat. 2018, 127, 104–110. [Google Scholar] [CrossRef]
- Boni, M.R.; Chiavola, A.; Marzeddu, S. Application of Biochar to the Remediation of Pb-Contaminated Solutions. Sustainability 2018, 10, 4440. [Google Scholar] [CrossRef]
- Metodi analitici per le acque; Belli, M., Centioli, D., De Zorzi, P., Sansone, U., Capri, S., Pagnotta, R., Pettine, M., Eds.; APAT—IRSA/CNR: Rome, Italy, 2003; ISBN 88-448-0083-7. [Google Scholar]
- Clesceri, L.S.; Greenberg, A.E.; Eaton, A.D. (Eds.) Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association (APHA): Washington, DC, USA, 1998; ISBN 978-0875532356. [Google Scholar]
- Pickens, J.F.; Grisak, G.E. Scale-dependent dispersion in a stratified granular aquifer. Water Resour. Res. 1981, 17, 1191–1211. [Google Scholar] [CrossRef]
- Luciano, A.; Viotti, P.; Torretta, V.; Mancini, G. Numerical approach to modelling pulse-mode soil flushing on a Pb-contaminated soil. J. Soils Sediments 2013, 13, 43–55. [Google Scholar] [CrossRef]
- Vilardi, G.; Mpouras, T.; Dermatas, D.; Verdone, N.; Polydera, A.; Di Palma, L. Nanomaterials application for heavy metals recovery from polluted water: The combination of nano zero-valent iron and carbon nanotubes. Competitive adsorption non-linear modeling. Chemosphere 2018, 201, 716–729. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Karahan, H. Unconditional stable explicit finite difference technique for the advection-diffusion equation using spreadsheets. Adv. Eng. Softw. 2007, 38, 80–86. [Google Scholar] [CrossRef]
- Saul’yev, V.K. Integration of Equations of Parabolic Type by the Method of Nets; Pergamon Press Ltd.: Oxford, UK, 1964; ISBN 978-0-08-010195-8. [Google Scholar]
- Bhattacharya, P.; Welch, A.H.; Stollenwerk, K.G.; McLaughlin, M.J.; Bundschuh, J.; Panaullah, G. Arsenic in the environment: Biology and Chemistry. Sci. Total Environ. 2007, 379, 109–120. [Google Scholar] [CrossRef]
- Cullen, W.R.; Reimer, K.J. Arsenic speciation in the environment. Chem. Rev. 1989, 89, 713–764. [Google Scholar] [CrossRef]
- Tan, K.L.; Hameed, B.H. Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2017, 74, 25–48. [Google Scholar] [CrossRef]
- Vilardi, G.; Di Palma, L.; Verdone, N. Heavy metals adsorption by banana peels micro-powder: Equilibrium modeling by non-linear models. Chinese, J. Chem. Eng. 2018, 26, 455–464. [Google Scholar] [CrossRef]
- Jin, H.; Capareda, S.; Chang, Z.; Gao, J.; Xu, Y.; Zhang, J. Biochar pyrolytically produced from municipal solid wastes for aqueous As(V) removal: Adsorption property and its improvement with KOH activation. Bioresour. Technol. 2014, 169, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Begum, S.A.; Golam Hyder, A.H.M.; Vahdat, N. Adsorption isotherm and kinetic studies of As(V) removal from aqueous solution using cattle bone char. J. Water Supply Res. Technol.—AQUA 2016, 65, 244–252. [Google Scholar] [CrossRef]
- Niazi, N.K.; Bibi, I.; Shahid, M.; Ok, Y.S.; Burton, E.D.; Wang, H.; Shaheen, S.M.; Rinklebe, J.; Lüttge, A. Arsenic removal by perilla leaf biochar in aqueous solutions and groundwater: An integrated spectroscopic and microscopic examination. Environ. Pollut. 2018, 232, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Sattar, M.S.; Shakoor, M.B.; Ali, S.; Rizwan, M.; Niazi, N.K.; Jilani, A. Comparative efficiency of peanut shell and peanut shell biochar for removal of arsenic from water. Environ. Sci. Pollut. Res. 2019, 26, 18624–18635. [Google Scholar] [CrossRef] [PubMed]

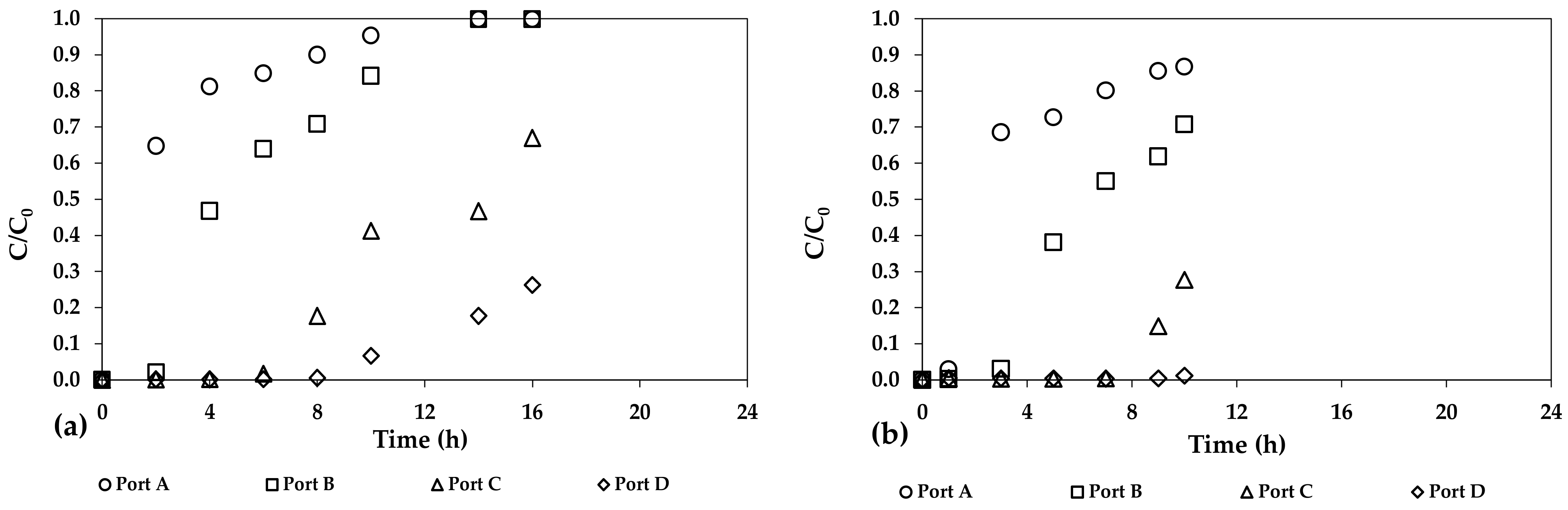

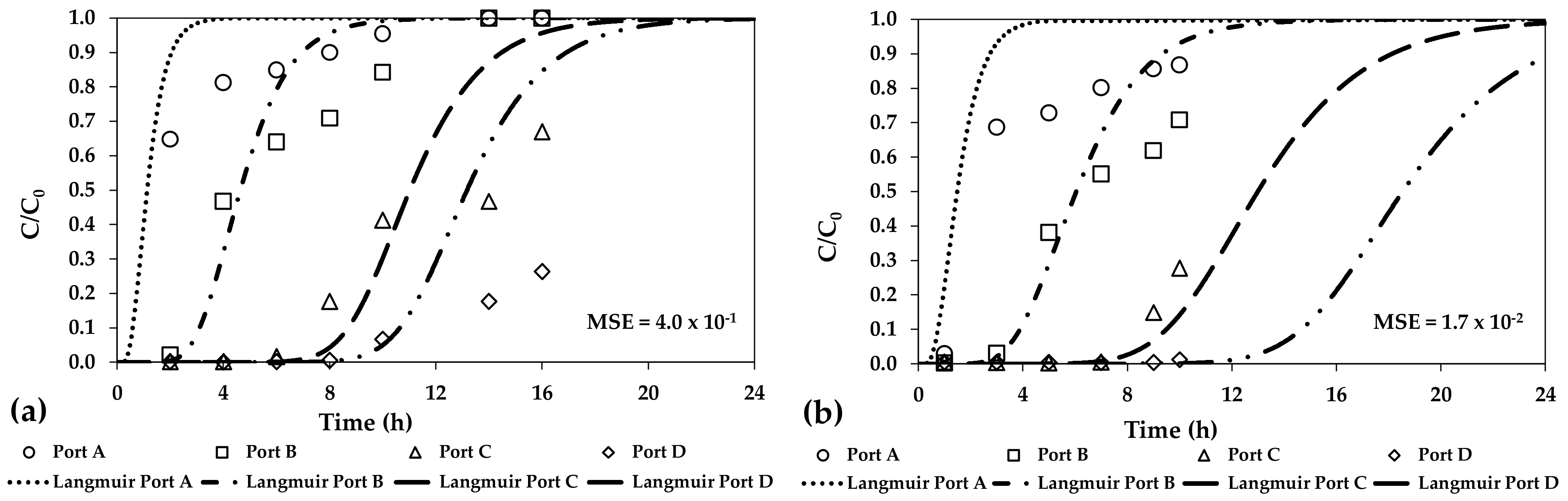
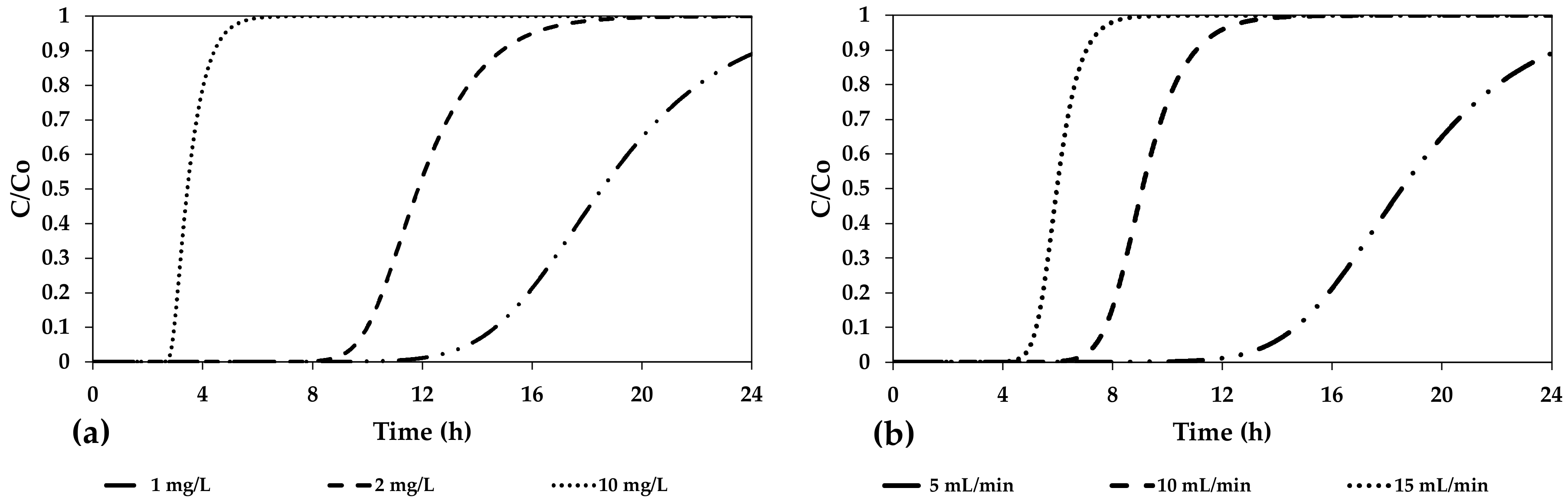
| Adsorbent Media | As Concentration (μg/L) | pH (-) | Average Size (mm) | Specific Surface (m2/g) | Adsorption Capacity (mg/g) | References |
|---|---|---|---|---|---|---|
| Alumina APS Alumina AMESO Alumina APS-TiO2 250 °C Alumina APS-TiO2 450 °C | 400–600 | 6.5–7.0 | 0.106 0.020 0.106 0.106 | 155 110 155 155 | 8.310–9.223 19.800 9.162–10.448 8.500 | [34] |
| Granular ferric hydroxides | 20 | 7 ± 0.1 | 0.930 0.600 1.400 | 120–200 300 300 | 0.431 0.250 0.286 | [35] |
| Magnetite nanoparticles | 10,000 | 6.0 | 9 × 10−6 | n.r. | 8.25 | [36] |
| PAC-CeO2 | 330 | 7.8 | n.r. | 1050 | ~12.000 | [37] |
| GAC GAC-Fe | 100 | 6.0 | 300–600 × 10−3 | 1124 876 | 0.180–0.130 0.300–0.440 | [38] |
| Material | Parameter | Unit | Value | Method |
|---|---|---|---|---|
| Biochar | Apparent bulk density | kg/L | 0.142 | UNI EN 13040:2008 |
| compacted in the laboratory | ||||
| pH | - | 12.4 ± 0.46 | UNI EN 13040:2008 + UNI EN | |
| 13037:2012 | ||||
| Electric conductibility | mS/m | 802 ± 13 | UNI EN 13040:2008 + UNI EN | |
| 13038:2012 | ||||
| Humidity | % m/m | 5.3 ± 0.53 | UNI EN 13040:2008 | |
| Ash content (550 °C) | % m/m | 31.26 ± 3.13 | UNI EN 14775:2010 | |
| Particle-size fraction < 5 mm | % m/m s.s. | 100 ± 10 | UNI EN 15428:2008 | |
| Particle-size fraction < 2 mm | % m/m s.s. | 97 ± 10 | UNI EN 15428:2008 | |
| Particle-size fraction < 0.5 mm | % m/m | 70 ± 7 | UNI EN 15428:2008 | |
| Quartz Sand | Dry density | kg/L | 2.65 | UNI EN 13242 |
| Medium grain size d50 | μm | 700 | UNI EN 13242 | |
| Medium grain size d10 | μm | 450 | UNI EN 13242 | |
| Medium grain size d60 | μm | 800 | UNI EN 13242 | |
| Hydraulic conductivity | m/s | 3.0 × 10−3 | - |
| Parameter | Unit | Value | |
|---|---|---|---|
| Test 1 | Test 2 | ||
| p | - | 0.25 | 0.30 |
| Dz | m | 1.4 × 10−2 | 1.4 × 10−2 |
| u | m min−1 | 4.0 × 10−3 | 3.3 × 10−3 |
| D | m2 min−1 | 5 × 10−5 | 5 × 10−5 |
| ρb | g cm−3 | 1.6 | 1.6 g |
| qmax | mg g−1 | 2.6 | 4.0 |
| KL | L mg−1 | 9 × 10−1 | 9 × 10−1 |
| Kd | L mg-1 | 1.6 | 1.8 |
| Adsorbent Media | As Conc. | Langmuir Isotherm | References | |
|---|---|---|---|---|
| (mg L−1) | qmax (mg/g) | b (L/mg) | ||
| Biochar produced from municipal solid wastes | 5–400 | 18.06–24.49 | 7.2 × 10−2–7.8 × 10−2 | [80] |
| Cattle bone char | 0.10–1.00 | 0.399 | 1.04 × 10−3 | [81] |
| Perilla leaf biochar | 0.05–7.00 | 3.85–7.21 | 1.08 × 10−3–2.14 × 10−3 | [82] |
| Peanut shell biochar | 5.00 | 7.94 | 2.17 × 10−3 | [83] |
| Virgin coniferous wood biochar | 1.0 | 1.8 | 9 × 10−2 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boni, M.R.; Marzeddu, S.; Tatti, F.; Raboni, M.; Mancini, G.; Luciano, A.; Viotti, P. Experimental and Numerical Study of Biochar Fixed Bed Column for the Adsorption of Arsenic from Aqueous Solutions. Water 2021, 13, 915. https://doi.org/10.3390/w13070915
Boni MR, Marzeddu S, Tatti F, Raboni M, Mancini G, Luciano A, Viotti P. Experimental and Numerical Study of Biochar Fixed Bed Column for the Adsorption of Arsenic from Aqueous Solutions. Water. 2021; 13(7):915. https://doi.org/10.3390/w13070915
Chicago/Turabian StyleBoni, Maria Rosaria, Simone Marzeddu, Fabio Tatti, Massimo Raboni, Giuseppe Mancini, Antonella Luciano, and Paolo Viotti. 2021. "Experimental and Numerical Study of Biochar Fixed Bed Column for the Adsorption of Arsenic from Aqueous Solutions" Water 13, no. 7: 915. https://doi.org/10.3390/w13070915
APA StyleBoni, M. R., Marzeddu, S., Tatti, F., Raboni, M., Mancini, G., Luciano, A., & Viotti, P. (2021). Experimental and Numerical Study of Biochar Fixed Bed Column for the Adsorption of Arsenic from Aqueous Solutions. Water, 13(7), 915. https://doi.org/10.3390/w13070915











