Ecology and Genetics of Cyperus fuscus in Central Europe—A Model for Ephemeral Wetland Plant Research and Conservation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling of Plant Material and Vegetation Data in the Field, Cultivation of Seeds from Soil
2.2. Vegetation Data Processing and Analyses
2.2.1. Data Sources
2.2.2. Compilation of the Vegetation Dataset
2.2.3. Vegetation Classification and Ordinations
2.3. Species Composition of the Soil Seed Bank
2.4. Chromosome Counts
2.5. Flow Cytometry
2.6. Relationship between Plant Community (Vegetation) and Genetic Diversity
2.7. Species Distribution in the Czech Republic
2.7.1. Temporal Changes of Species Distribution
2.7.2. Climatic Conditions
2.7.3. Climatic Niche Modelling
3. Results
3.1. Community Affiliation of Cyperus fuscus and Vegetation Species Composition
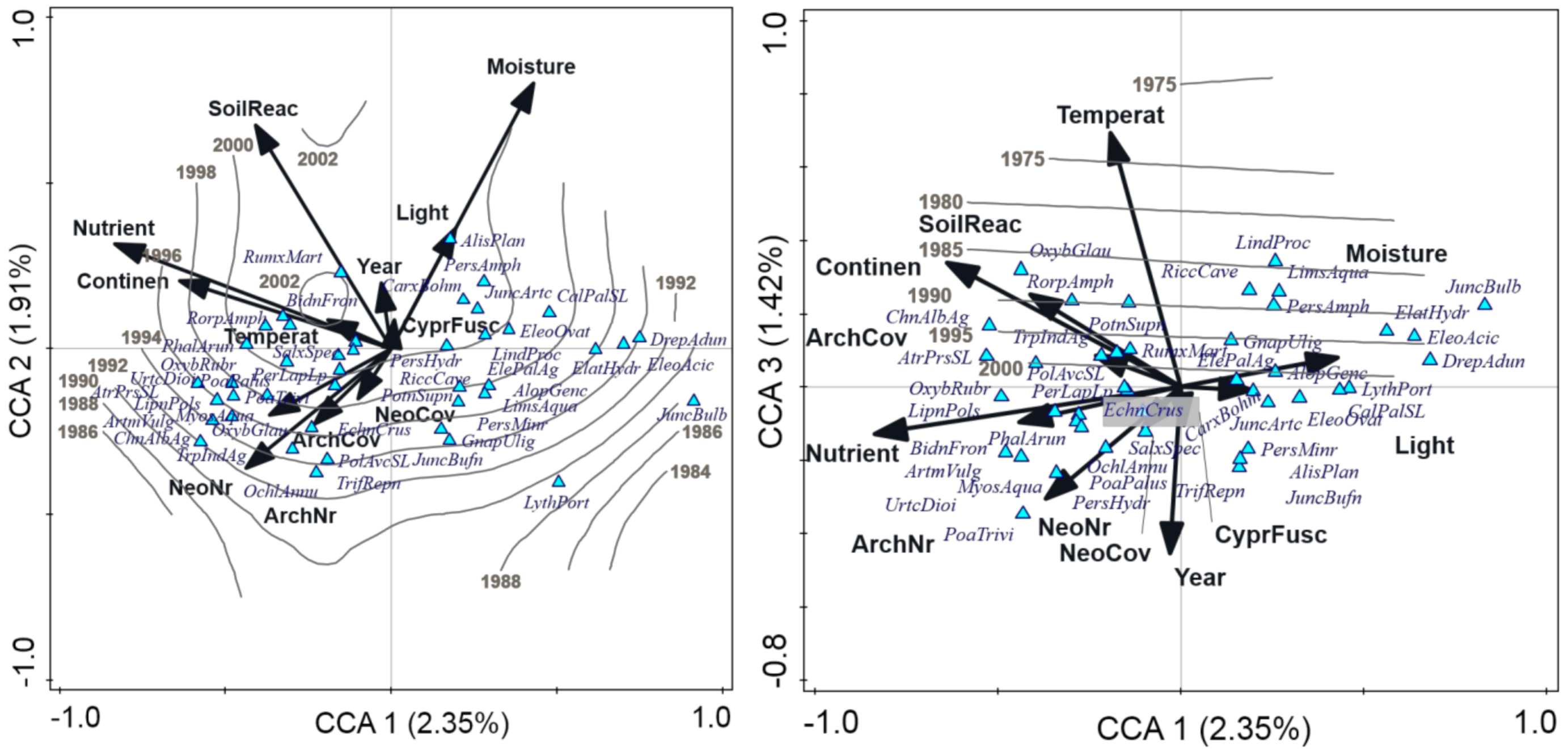
3.2. Factors Influencing Species Composition of the Vegetation with Cyperus fuscus
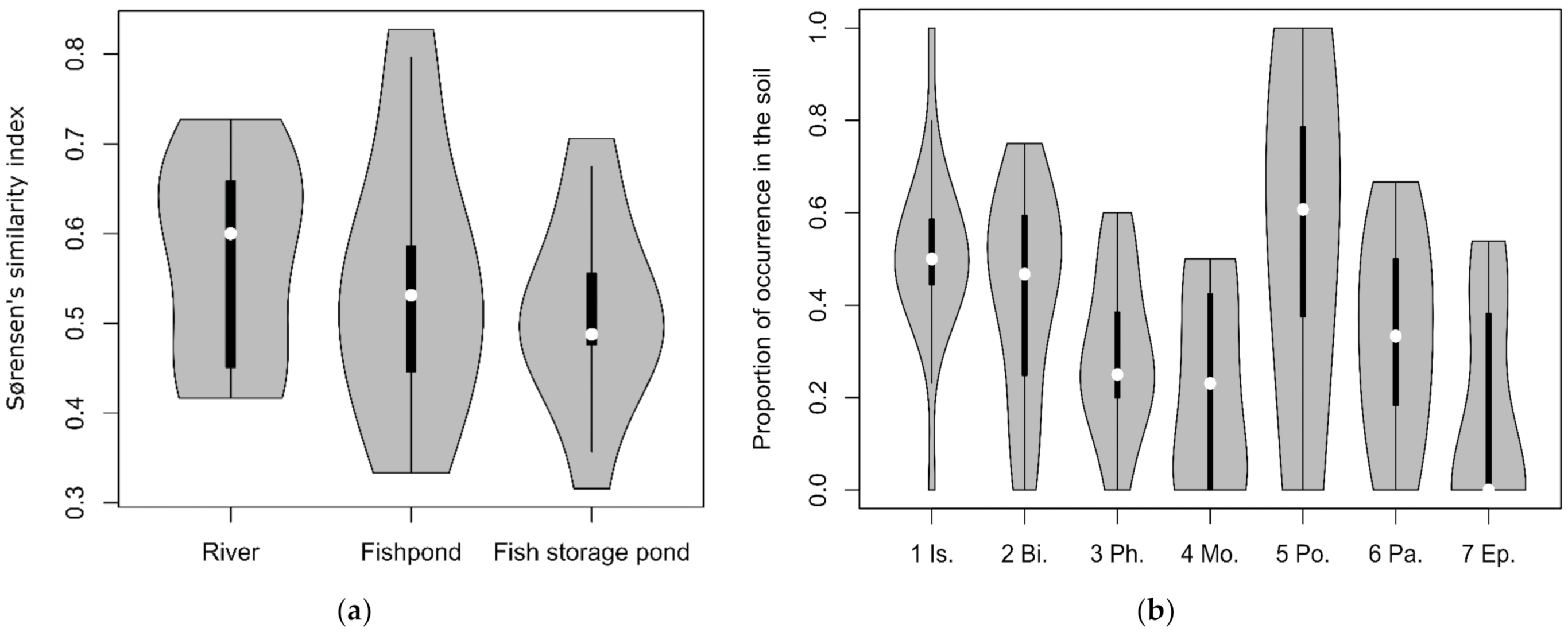
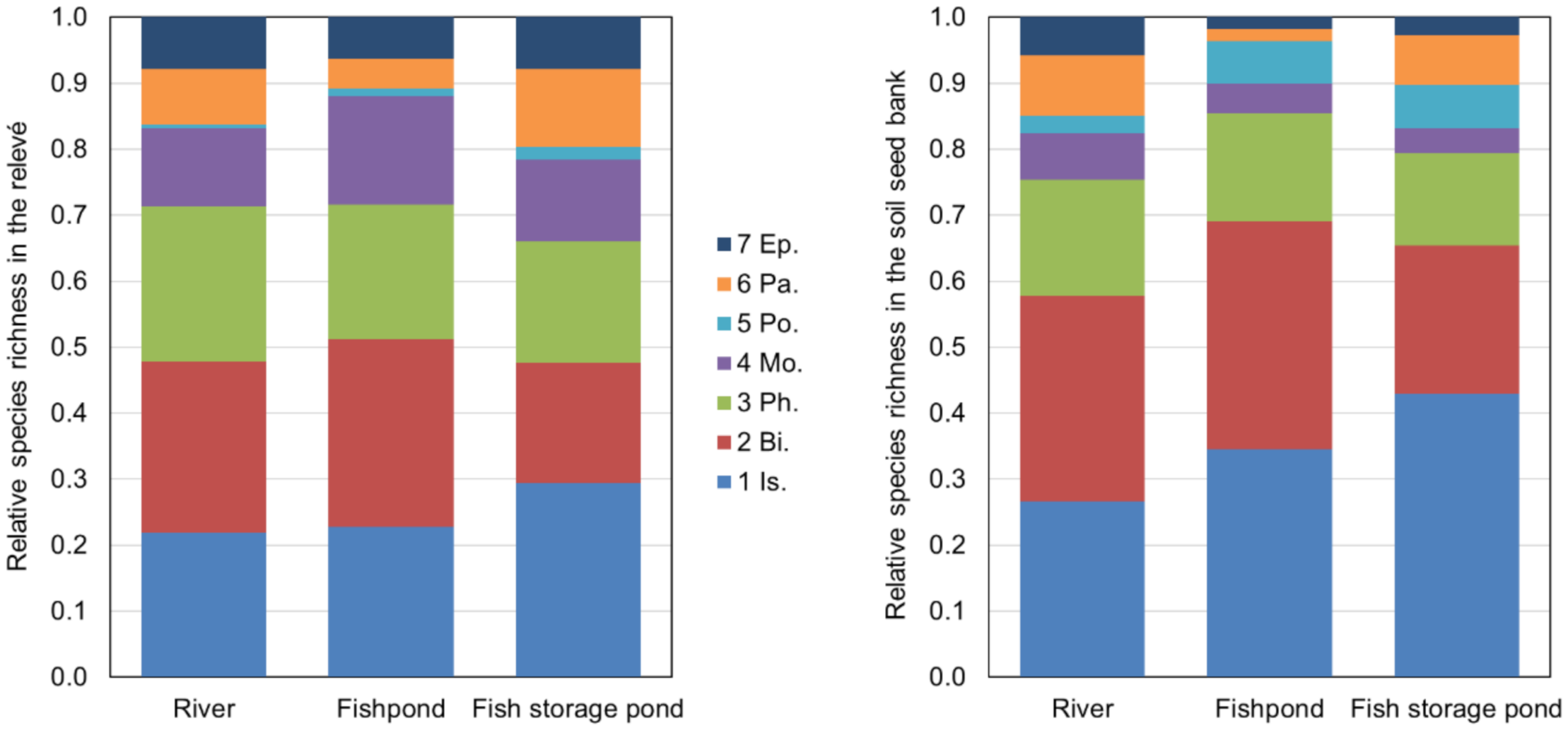
3.3. Similarity between Soil Seed Bank and Above-Ground Vegetation (Relevés)
3.4. Chromosome Number, Genome Size, and Ploidy Level
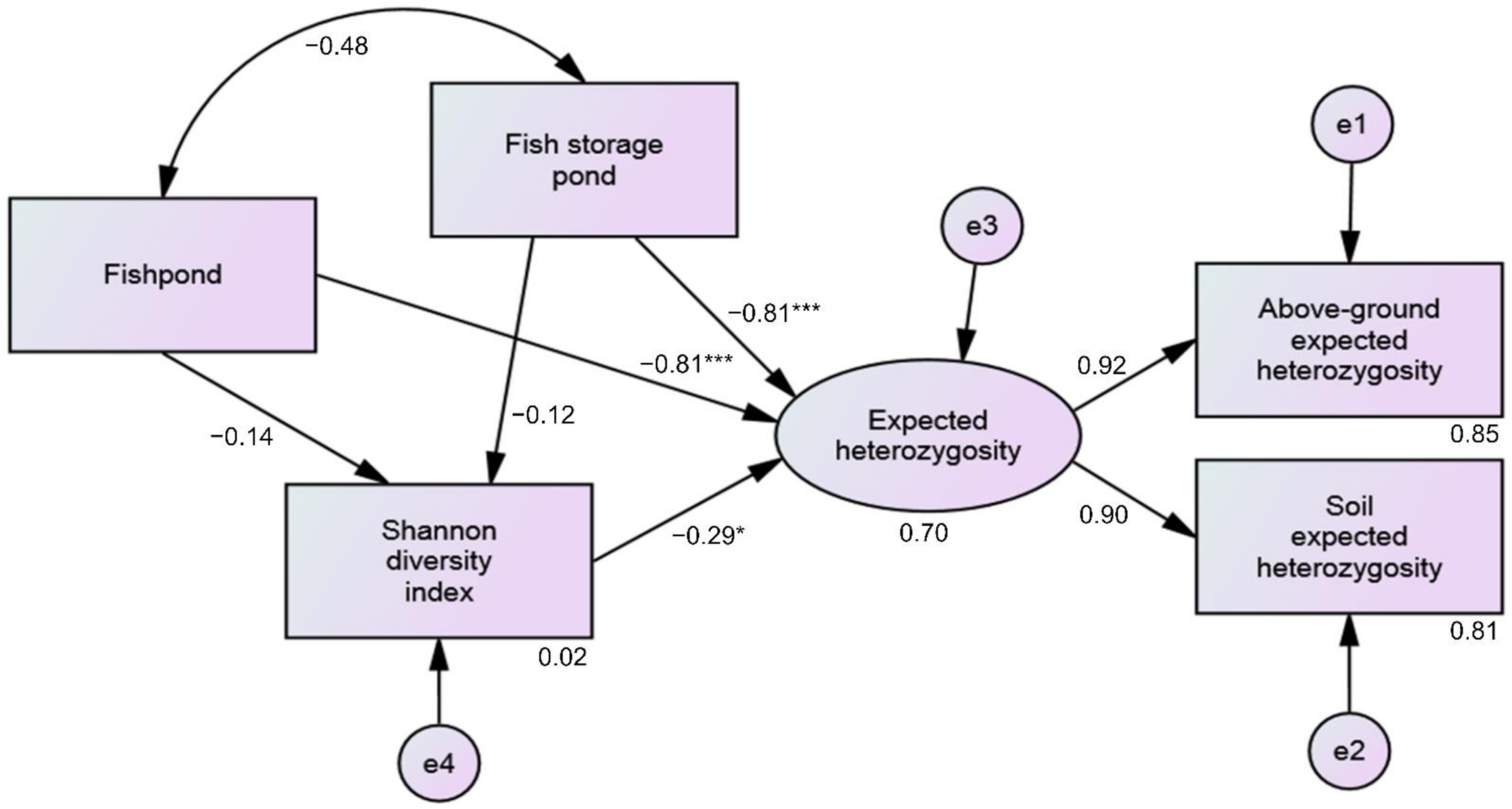
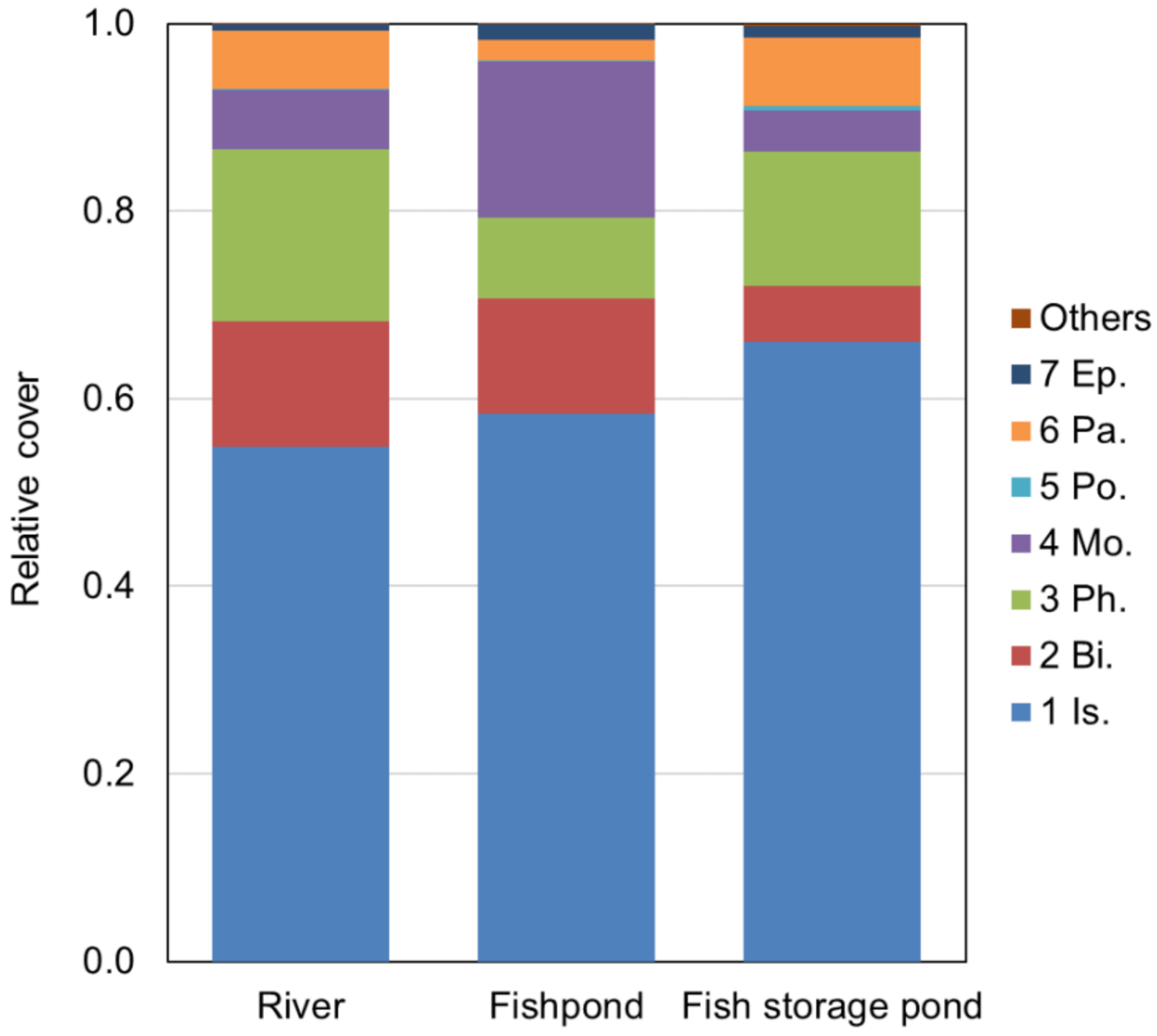
3.5. Effect of Plant Community (Vegetation) Diversity on Genetic Diversity in River and Anthropogenic Habitats
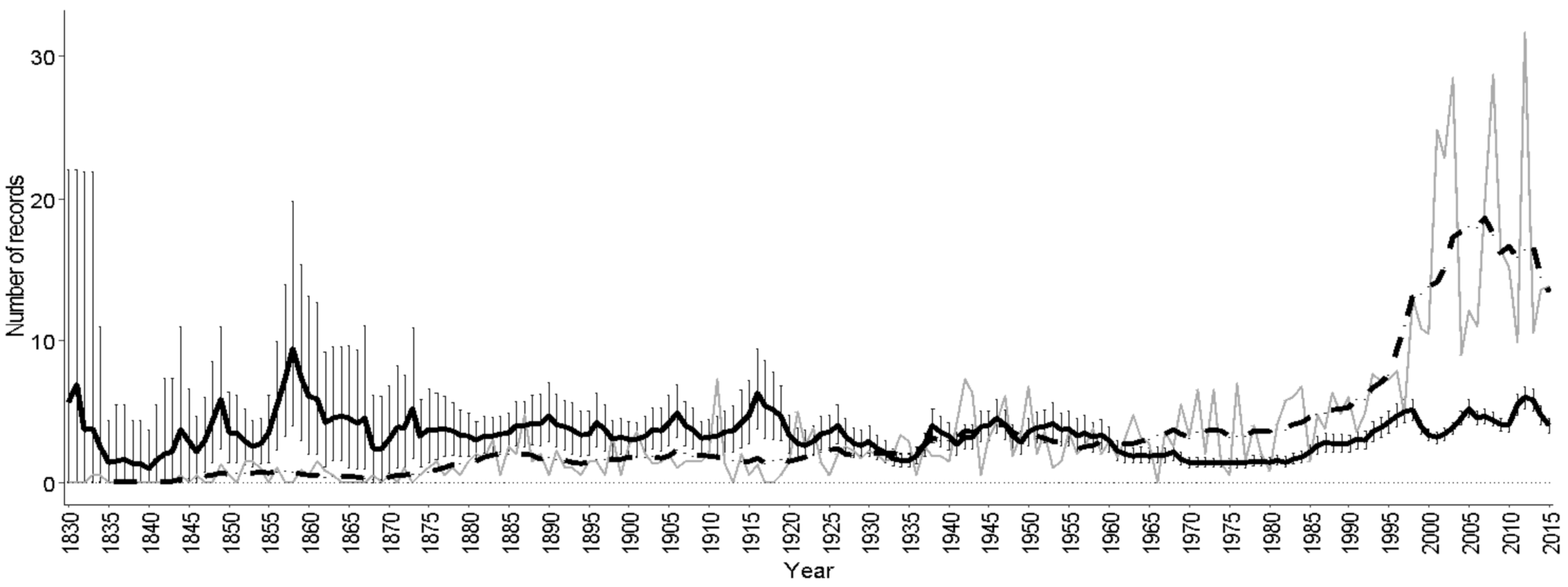
3.6. Distribution Trends
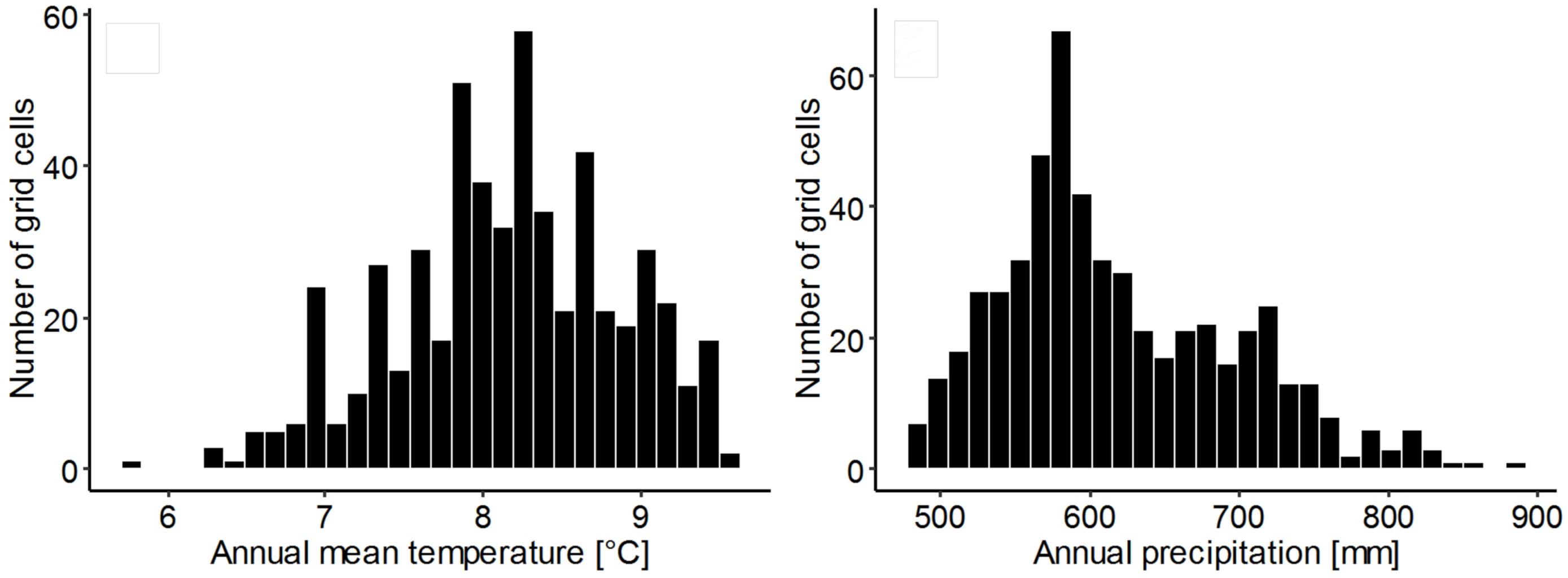
3.7. Climatic Conditions
3.8. Climatic Niche Modelling
4. Discussion
4.1. Vegetation and Cyperus fuscus Frequency Change over Time
4.2. Genetics and Soil Seed Bank
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Main Sources of Vegetation Data and General Rules Applied for their Selection
References
- Khan, F.A.; Ansari, A.A. Eutrophication: An ecological vision. Bot. Rev. 2005, 71, 449–482. [Google Scholar] [CrossRef]
- Mooij, W.M.; Hülsmann, S.; De Senerpont Domis, L.N.; Nolet, B.A.; Bodelier, P.L.E.; Boers, P.C.M.; Pires, L.M.D.; Gons, H.J.; Ibelings, B.W.; Noordhuis, R.; et al. The impact of climate change on lakes in the Netherlands: A review. Aquat. Ecol. 2005, 39, 381–400. [Google Scholar] [CrossRef]
- Usio, N.; Imada, M.; Nakagawa, M.; Akasaka, M.; Takamura, N. Effects of pond draining on biodiversity and water quality of farm ponds. Conserv. Biol. 2013, 27, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- Petsch, D.K. Causes and consequences of biotic homogenization in freshwater ecosystems. Int. Rev. Hydrobiol. 2016, 101, 113–122. [Google Scholar] [CrossRef]
- Zohary, T.; Ostrovsky, I. Ecological impacts of excessive water level fluctuations in stratified freshwater lakes. Inland Waters 2011, 1, 47–59. [Google Scholar] [CrossRef]
- Dhir, B. Status of aquatic macrophytes in changing climate: A perspective. J. Environ. Sci. Technol. 2015, 8, 139–148. [Google Scholar] [CrossRef]
- Short, F.T.; Kosten, S.; Morgan, P.A.; Malone, S.; Moore, G.E. Impacts of climate change on submerged and emergent wetland plants. Aquat. Bot. 2016, 135, 3–17. [Google Scholar] [CrossRef]
- Mucina, L.; Bültmann, H.; Dierßen, K.; Theurillat, J.P.; Raus, T.; Čarni, A.; Šumberová, K.; Willner, W.; Dengler, J.; Gavilán García, R.; et al. Vegetation of Europe: Hierarchical floristic classification system of vascular plant, bryophyte, lichen, and algal communities. Appl. Veg. Sci. 2016, 19 (Suppl. 1), 3–264. [Google Scholar] [CrossRef]
- Deil, U. A review on habitats, plant traits and vegetation of ephemeral wetlands—A global perspective. Phytocoenologia 2005, 35, 533–706. [Google Scholar] [CrossRef]
- Šumberová, K.; Ducháček, M.; Lososová, Z. Life-history traits controlling the survival of Tillaea aquatica: A threatened wetland plant species in intensively managed fishpond landscapes of the Czech Republic. Hydrobiologia 2012, 689, 91–110. [Google Scholar] [CrossRef]
- Richert, E.; Achtziger, R.; Dajdok, Z.; Günther, A.; Heilmeier, H.; Hübner, A.; John, H.; Šumberová, K. Rare wetland grass Coleanthus subtilis in Central and Western Europe—Current distribution, habitat types, and threats. Acta Soc. Bot. Polon. 2016, 85, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Gazaix, A.; Klesczewski, M.; Bouchet, M.A.; Cartereau, M.; Molina, J.; Michaud, H.; Muller, S.D.; Pirsoul, L.; Gauthier, P.; Grillas, P.; et al. A history of discoveries and disappearances of the rare annual plant Lythrum thesioides M.Bieb.: New insights into its ecology and biology. Bot. Lett. 2020, 167, 201–211. [Google Scholar] [CrossRef]
- Lansdown, R.V. Cyperus Fuscus. The IUCN Red List of Threatened Species 2013: eT164079A13545918. 2013. Available online: https://dx.doi.org/10.2305/IUCN.UK.2013-1.RLTS.T164079A13545918.en (accessed on 21 February 2021).
- Stoltze, M.; Pihl, S. Rødliste 1997 over Planter og Dyr i Danmark; Red List 1997 of Plants and Animals in Denmark; Miljø- og Energiministeriet, Danmarks Miljøundersøgelser & Skov- og Naturstyrelsen: Copenhagen, Denmark, 1998. [Google Scholar]
- Niklfeld, H.; Schratt-Ehrendorfer, L. (Eds.) Rote Liste gefährdeter Farn- und Blütenpflanzen (Pteridophyta und Spermatophyta) Österreichs. 2. Fassung. In Rote Listen Gefährdeter Pflanzen Österreichs. Grüne Reihe Bundesministerium Umwelt, Jugend und Familie 10; Austria Medien Service: Graz, Austria, 1999; pp. 33–130. [Google Scholar]
- Cheffings, C.M.; Farrell, L. The Vascular Plant Red Data List for Great Britain. Species Status 2005, 7, 1–116. [Google Scholar]
- Bornand, C.; Gygax, A.; Juillerat, P.; Jutzi, M.; Möhl, A.; Rometsch, S.; Sager, L.; Santiago, H.; Eggenberg, S. Rote Liste Gefässpflanzen. Gefährdete Arten der Schweiz; Bundesamt für Umwelt; Bern & Info Flora: Geneva, Switzerland, 2016. [Google Scholar]
- Metzing, D.; Garve, E.; Matzke-Hajek, G.; Adler, J.; Bleeker, W.; Breunig, T.; Caspari, S.; Dunkel, F.G.; Fritsch, R.; Gottschlich, G.; et al. Rote Liste und Gesamtartenliste der Farn- und Blütenpflanzen (Trachaeophyta) Deutschlands. In Rote Liste Gefährdeter Tiere, Pflanzen und Pilze Deutschlands. Band 7: Pflanzen; Metzing, D., Hofbauer, N., Ludwig, G., Matzke-Hajek, G., Eds.; Bundesamt für Naturschutz: Bonn-Bad Godesberg, Germany, 2018; Volume 70, pp. 13–358. [Google Scholar]
- Grulich, V.; Chobot, K. (Eds.) Červený seznam cévnatých rostlin ČR; The Red List of Vascular Plants of the Czech Republic; Agentura ochrany přírody a krajiny ČR: Praha, Czech Republic, 2017; Volume 35, pp. 75–132. [Google Scholar]
- Van Landuyt, W.; Hoste, I.; Vanhecke, L. Rode Lijst van de vaatplanten van Vlaanderen en het Brussels Hoofdstedelijk Gewest [Red List of vascular plants of Flanders and the Brussels capital region]. In Atlas van de Flora van Vlaanderen en het Brussels Gewest [Atlas of the Flora of Flanders and the Brussels Region]; Van Landuyt, W., Hoste, I., Vanhecke, L., Van den Bremt, P., Vercruysse, W., De Beer, D., Eds.; Instituut voor Natuur- en Bosonderzoek, Nationale Plantentuin van België & Flo.Wer.: Brussel, Belgium, 2006; pp. 69–81. [Google Scholar]
- Cyperus fuscus L. Estonian Red List of Threatened Species. eBiodiversity. Available online: http://vana.elurikkus.ut.ee/kirjeldus.php?id=4208&inst=&org=&lang=eng (accessed on 17 February 2021).
- Colling, G. Red List of the Vascular Plants of Luxembourg. Ferrantia 2005, 42, 1–77. [Google Scholar]
- Kaplan, Z.; Danihelka, J.; Štěpánková, J.; Ekrt, L.; Chrtek, J., Jr.; Zázvorka, J.; Grulich, V.; Řepka, R.; Prančl, J.; Ducháček, M.; et al. Distributions of vascular plants in the Czech Republic. Part 2. Preslia 2016, 88, 229–322. [Google Scholar]
- Von Lampe, M. Wuchsform, Wuchsrhythmus und Verbreitung der Arten der Zwergbinsengesellschaften. Diss. Bot. 1996, 266, 1–353. [Google Scholar]
- Ansong, M.; Pickering, C. Are weeds hitchhiking a ride on your car? A systematic review of seed dispersal on cars. PLoS ONE 2013, 8, e80275. [Google Scholar] [CrossRef] [Green Version]
- Šumberová, K.; Ducháček, M. Analysis of plant soil seed banks and seed dispersal vectors: Its potential and limits for forensic investigations. Forensic Sci. Int. 2017, 270, 121–128. [Google Scholar] [CrossRef]
- Böckelmann, J.; Tremetsberger, K.; Šumberová, K.; Grausgruber, H.; Bernhardt, K.G. Fitness and growth of the ephemeral mudflat species Cyperus fuscus in river and anthropogenic habitats in response to fluctuating water-levels. Flora 2017, 234, 135–149. [Google Scholar] [CrossRef]
- Böckelmann, J.; Tremetsberger, K.; Šumberová, K.; Kohl, G.; Grausgruber, H.; Bernhardt, K.G. Genetic variation in an ephemeral mudflat species: The role of the soil seed bank and dispersal in river and secondary anthropogenic habitats. Ecol. Evol. 2020, 10, 3620–3635. [Google Scholar] [CrossRef] [Green Version]
- Abernethy, V.J.; Willby, N.J. Changes along a disturbance gradient in the density and composition of propagule banks in floodplain aquatic habitats. Plant Ecol. 1999, 140, 177–190. [Google Scholar] [CrossRef]
- Aponte, C.; Kazakis, G.; Ghosn, D.; Papanastasis, V.P. Characteristics of the soil seed bank in Mediterranean temporary ponds and its role in ecosystem dynamics. Wetlands Ecol. Manage. 2010, 18, 243–253. [Google Scholar] [CrossRef] [Green Version]
- Koch, M.; Huthmann, M.; Bernhardt, K.G. Cardamine amara L. (Brassicaceae) in dynamic habitats: Genetic composition and diversity of seed bank and established populations. Basic Appl. Ecol. 2003, 4, 339–348. [Google Scholar] [CrossRef]
- Krahulcová, A. Chromosome numbers in selected monocotyledons (Czech Republic, Hungary, and Slovakia). Preslia 2003, 75, 97–113. [Google Scholar]
- Májovský, J. Index of chromosome numbers of Slovakian flora (Part 6). Acta Fac. Rerum Nat. Univ. Comen. Bot. 1978, 26, 1–42. [Google Scholar]
- Montgomery, L.; Khalaf, M.; Bailey, J.P.; Gornall, R.J. Contributions to a cytological catalogue of the British and Irish flora, 5. Watsonia 1997, 21, 365–368. [Google Scholar]
- Löve, Á. Chromosome number reports LXXXIX. Taxon 1985, 34, 727–730. [Google Scholar] [CrossRef]
- Löve, Á.; Löve, D. Cyto-taxonomical studies on boreal plants. III. Some new chromosome numbers of Scandinavian plants. Arkiv Bot. 1944, 31 A, 1–22. [Google Scholar]
- Levin, D.A. The Role of Chromosomal Change in Plant Evolution; Oxford University Press: Oxford, NY, USA, 2002. [Google Scholar]
- Čertner, M.; Kúr, P.; Kolář, F.; Suda, J. Climatic conditions and human activities shape diploid-tetraploid coexistence at different spatial scales in the common weed Tripleurospermum inodorum (Asteraceae). J. Biogeogr. 2019, 46, 1355–1366. [Google Scholar] [CrossRef]
- Hroudová, Z.; Zákravský, P. Ecology of two cytotypes of Butomus umbellatus. III. Distribution and habitat differentiation in the Czech and Slovak Republics. Folia Geobot. Phytotax. 1993, 28, 425–435. [Google Scholar] [CrossRef]
- Vellend, M. Species diversity and genetic diversity: Parallel processes and correlated patterns. Am. Nat. 2005, 166, 199–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vellend, M.; Geber, M.A. Connections between species diversity and genetic diversity. Ecol. Lett. 2005, 8, 767–781. [Google Scholar] [CrossRef]
- Van der Maarel, E. Transformation of cover-abundance values in phytosociology and its effect on community similarity. Vegetatio 1979, 38, 97–114. [Google Scholar]
- Dengler, J.; Jansen, F.; Glöckler, F.; Peet, R.K.; De Cáceres, M.; Chytrý, M.; Ewald, J.; Oldeland, J.; Finckh, M.; Lopez-Gonzalez, G.; et al. The Global Index of Vegetation-Plot Databases (GIVD): A new resource for vegetation science. J. Veg. Sci. 2011, 22, 582–597. [Google Scholar] [CrossRef]
- Willner, W.; Berg, C.; Heiselmayer, P. Austrian Vegetation Database. Biodivers. Ecol. 2012, 4, 333. [Google Scholar] [CrossRef] [Green Version]
- Chytrý, M.; Rafajová, M. Czech National Phytosociological Database: Basic statistics of the available vegetation-plot data. Preslia 2003, 75, 1–15. [Google Scholar]
- Kącki, Z.; Śliwiński, M. The Polish Vegetation Database: Structure, resources and development. Acta Soc. Bot. Polon. 2012, 81, 75–79. [Google Scholar] [CrossRef] [Green Version]
- Valachovič, M. Centrálna Databáza Fytocenologických Zápisov (CDF) na Slovensku [Central Database of Phytosociological Relevés (CDF) in Slovakia]. In Proceedings of the Zborník Referátov zo 7. Zjazdu SBS pri SAV, Hrabušice, Slovakia, 21–25 June 1999; Leskovjanská, A., Ed.; Správa NP Slovenský raj, Spišská Nová Ves & SBS: Bratislava, Slovakia, 1999; pp. 75–77. (In Slovak). [Google Scholar]
- Kalníková, V.; Kudrnovsky, H. Gravel Bar Vegetation Database. Phytocoenologia 2017, 47, 109–110. [Google Scholar] [CrossRef]
- Hennekens, S.M.; Schaminée, J.H.J. TURBOVEG, a comprehensive data base management system for vegetation data. J. Veg. Sci. 2001, 12, 589–591. [Google Scholar] [CrossRef]
- Chytrý, M.; Otýpková, Z. Plot sizes used for phytosociological sampling of European vegetation. J. Veg. Sci. 2003, 14, 563–570. [Google Scholar] [CrossRef]
- Tichý, L. JUICE, software for vegetation classification. J. Veg. Sci. 2002, 13, 451–453. [Google Scholar] [CrossRef]
- Euro+Med PlantBase. The Information Resource for Euro-Mediterranean Plant Diversity. Available online: https://www.emplantbase.org/home.html (accessed on 7 March 2021).
- Kącki, Z.; Łysko, A.; Dajdok, Z.; Kobierski, P.; Krawczyk, R.; Nowak, A.; Rosadziński, S.; Popiela, A. Formalized classification of ephemeral wetland vegetation (Isoëto-Nanojuncetea class) in Poland (Central Europe). PeerJ 2013. under review. [Google Scholar]
- Chytrý, M.; Tichý, L. National vegetation classification of the Czech Republic: A summary of the approach. Phytocoenologia 2018, 48, 121–131. [Google Scholar] [CrossRef]
- Ellenberg, H.; Weber, H.E.; Düll, R.; Wirth, W.; Werner, W.; Paulißen, D. Zeigerwerte von Pflanzen in Mitteleuropa. 2nd ed. Scr. Geobot. 1992, 18, 1–258. [Google Scholar]
- Wood, S.N. Thin-plate regression splines. J. R. Stat. Soc. Ser. B Stat. Methodol. 2003, 65, 95–114. [Google Scholar] [CrossRef]
- Wood, S.N. Stable and efficient multiple smoothing parameter estimation for generalized additive models. J. Am. Stat. Assoc. 2004, 99, 673–686. [Google Scholar] [CrossRef] [Green Version]
- Wood, S.N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. Ser. B Stat. Methodol. 2011, 73, 3–36. [Google Scholar] [CrossRef] [Green Version]
- Wood, S.N.; Pya, N.; Saefken, B. Smoothing parameter and model selection for general smooth models (with discussion). J. Am. Stat. Assoc. 2016, 111, 1548–1575. [Google Scholar] [CrossRef]
- Wood, S.N. Generalized Additive Models: An Introduction with R, 2nd ed.; CRC Press, Taylor & Francis Group: New York, NY, USA, 2017. [Google Scholar]
- Pyšek, P.; Danihelka, J.; Sádlo, J.; Chrtek, J., Jr.; Chytrý, M.; Jarošík, V.; Kaplan, Z.; Krahulec, F.; Moravcová, L.; Pergl, J.; et al. Catalogue of alien plants of the Czech Republic (2nd edition): Checklist update, taxonomic diversity and invasion patterns. Preslia 2012, 84, 155–255. [Google Scholar]
- Tokarska-Guzik, B.; Dajdok, Z.; Zając, M.; Zając, A.; Urbisz, A.; Danielewicz, W.; Hołdyński, C. Rośliny Obcego Pochodzenia w Polsce ze Szczególnym Uwzględnieniem Gatunków Inwazyjnych; Generalna Dyrekcja Ochrony Srodowiska: Warszawa, Poland, 2012.
- Sørensen, T. A method of establishing groups of equal amplitude in plant sociology based on similarity of species content and its application to analyses of the vegetation on Danish commons. Biol. Skr. Dan. Vid. Selsk. 1948, 5, 1–34. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 10 December 2020).
- Schwarzacher, T.; Heslop-Harrison, P. Practical In Situ Hybridisation, 2nd ed.; BIOS: Oxford, UK, 2000. [Google Scholar]
- Schönswetter, P.; Suda, J.; Popp, M.; Weiss-Schneeweiss, H.; Brochmann, C. Circumpolar phylogeography of Juncus biglumis (Juncaceae) inferred from AFLP fingerprints, cpDNA sequences, nuclear DNA content and chromosome numbers. Mol. Phylogenet. Evol. 2007, 42, 92–103. [Google Scholar] [CrossRef]
- Temsch, E.M.; Greilhuber, J.; Krisai, R. Genome size in liverworts. Preslia 2010, 82, 63–80. [Google Scholar]
- Otto, F.J.; Oldiges, H.; Göhde, W.; Jain, V.K. Flow cytometric measurement of nuclear DNA content variations as a potential in vivo mutagenicity test. Cytometry 1981, 2, 189–191. [Google Scholar] [CrossRef]
- Galbraith, D.W.; Harkins, K.R.; Maddox, J.M.; Ayres, N.M.; Sharma, D.P.; Firoozabady, E. Rapid flow cytometric analysis of the cell cycle in intact plant tissues. Science 1983, 220, 1049–1051. [Google Scholar] [CrossRef]
- Thiers, B.M. Index Herbariorum; The New York Botanical Garden: Bronx, NY, USA; Available online: http://sweetgum.nybg.org/science/ih/ (accessed on 21 February 2021).
- Wild, J.; Kaplan, Z.; Danihelka, J.; Petřík, P.; Chytrý, M.; Novotný, P.; Rohn, M.; Šulc, V.; Brůna, J.; Chobot, K.; et al. Plant distribution data for the Czech Republic integrated in the Pladias database. Preslia 2019, 91, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Petts, G.E. River regulation in Europe: An historical perspective. Regul. Rivers: Res. Manag. 1987, 1, 363–369. [Google Scholar]
- Danihelka, J.; Niklfeld, H.; Šípošová, H. Viola elatior, V. pumila and V. stagnina in Austria, Czechia and Slovakia: A story of decline. Preslia 2009, 81, 151–171. [Google Scholar]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Hijmans, R. R Package Raster: Geographic Data Analysis and Modeling. Version 3.0-14/r3417. Available online: https://R-Forge.R-project.org/projects/raster/ (accessed on 10 December 2020).
- Niklfeld, H. Bericht über die Kartierung der Flora Mitteleuropas. Taxon 1971, 20, 545–571. [Google Scholar] [CrossRef]
- Šmilauer, P.; Lepš, J. Multivariate Analysis of Ecological Data using CANOCO 5, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Phillips, S.J.; Dudík, M. Modeling of species distribution with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Kass, J.M.; Vilela, B.; Aiello-Lammens, M.E.; Muscarella, R.; Merow, C.; Anderson, R.P. Wallace: A flexible platform for reproducible modeling of species niches and distributions built for community expansion. Methods Ecol. Evol. 2018, 9, 1151–1156. [Google Scholar] [CrossRef] [Green Version]
- Fielding, A.; Bell, J. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Pietsch, W. Beitrag zur Gliederung der europäischen Zwergbinsengesellschaften (Isoëto-Nanojuncetea Br.-BI. & Tx. 1943). Vegetatio 1973, 28, 401–438. [Google Scholar]
- Šumberová, K.; Lososová, Z.; Horáková, V. Vegetation dynamics on exposed pond bottoms in the Českobudějovická basin (Czech Republic). Phytocoenologia 2005, 35, 421–448. [Google Scholar] [CrossRef]
- Aichinger, E. Vegetationskunde der Karawanken; Gustav Fischer: Jena, Germany, 1933. [Google Scholar]
- Poschlod, P.; Rosbakh, S. Mudflat species: Threatened or hidden? An extensive seed bank survey of 108 fish ponds in Southern Germany. Biol. Conserv. 2018, 225, 154–163. [Google Scholar]
- Phartyal, S.S.; Rosbakh, S.; Ritz, C.; Poschlod, P. Ready for change: Seed traits contribute to the high adaptability of mudflat species to their unpredictable habitat. J. Veg. Sci. 2020, 31, 331–342. [Google Scholar] [CrossRef] [Green Version]
- Tolasz, R. (Ed.) Climate Atlas of Czechia; Czech Hydrometeorological Institute & Palacký University: Prague & Olomouc, Czech Republic, 2007. [Google Scholar]
- Hejný, S. Ökologische Charakteristik der Wasser- und Sumpfpflanzen in den slowakischen Tiefebenen (Donau- und Theissgebiet); Verlag der Slowakischen Akademie der Wissenschaften: Bratislava, Slovakia, 1960. [Google Scholar]
- Müller-Stoll, W.R.; Pietsch, W. Ökologische Untersuchungen über die Gesellschaft des Eleocharito-Caricetum bohemica auf wasserfrei gewordenen Teichböden in Zentraleuropa. Verh. Zool. Bot. Ges. Österreich 1985, 123, 51–70. [Google Scholar]
- Šumberová, K.; Lososová, Z.; Fabšicová, M.; Horáková, V. Variability of vegetation of exposed pond bottoms in relation to management and environmental factors. Preslia 2006, 78, 235–252. [Google Scholar]
- Francová, K.; Šumberová, K.; Janauer, G.A.; Adámek, Z. Effects of fish farming on macrophytes in temperate carp ponds. Aquac. Int. 2019, 27, 413–436. [Google Scholar] [CrossRef]
- Chytrý, M.; Pyšek, P.; Tichý, L.; Knollová, I.; Danihelka, J. Invasions by alien plants in the Czech Republic: A quantitative assessment across habitats. Preslia 2005, 77, 339–354. [Google Scholar]
- Farmer, J.A.; Webb, E.B.; Pierce, R.A.; Bradley, K.W. Evaluating the potential for weed seed dispersal based on waterfowl consumption and seed viability. Pest Manag. Sci. 2016, 73, 2592–2603. [Google Scholar] [CrossRef]
- Šumberová, K.; Lososová, Z.; Ducháček, M.; Horáková, V.; Fabšičová, M. Distribution, habitat ecology, soil seed bank and seed dispersal of threatened Lindernia procumbens and alien Lindernia dubia (Antirrhinaceae) in the Czech Republic. Phyton 2012, 52, 39–72. [Google Scholar]
- Zarzycki, K.; Szelag, Z. Red list of the vascular plants in Poland. In Red List of Plants and Fungi in Poland; Mirek, Z., Zarzycki, K., Wojewoda, W., Szelag, Z., Eds.; Polish Academy of Sciences & W. Szafer Institute of Botany: Kraków, Poland, 2006; pp. 11–20. [Google Scholar]
- Eliáš, J.P.; Dítě, D.; Kliment, J.; Hrivnák, R.; Feráková, V. Red list of ferns and flowering plants of Slovakia, 5th edition (October 2014). Biologia 2015, 70, 218–228. [Google Scholar] [CrossRef]
- Böckelmann, J.; Wieser, D.; Tremetsberger, K.; Šumberová, K.; Bernhardt, K.G. Isolation of nuclear microsatellite markers for Cyperus fuscus (Cyperaceae). Appl. Plant Sci. 2015, 3, 1500071. [Google Scholar] [CrossRef]
- Roalson, E.H. A synopsis of chromosome number variation in the Cyperaceae. Bot. Rev. 2008, 74, 209–393. [Google Scholar] [CrossRef]
- Landolt, E.; Bäumler, B.; Erhardt, A.; Hegg, O.; Klötzli, F.; Lämmler, W.; Nobis, M.; Rudmann-Maurer, K.; Schweingruber, F.H.; Theurillat, J.P.; et al. Flora Indicativa, 2nd ed.; Haupt: Bern, Switzerland, 2010. [Google Scholar]
- Rydlo, J. Vodní makrofyta v oblasti soutoku Labe a Vltavy [Aquatic macrophytes in the area of the Labe and Vltava confluence]. Muz. Souč. Ser. Natur. 2006, 21, 25–70. (In Czech) [Google Scholar]
- Rydlo, J. Vodní makrofyta ve stojatých vodách na Poděbradsku a Nymbursku [Aquatic macrophytes of stagnant waters in the Poděbrady and Nymburk regions]. Muz. Souč. Ser. Natur. 2005, 20, 11–134. (In Czech) [Google Scholar]
- Bravencová, L.; Musil, Z.; Reiter, A. Flóra a vegetace obnaženého dna Znojemské a Vranovské údolí nádrže (střední Podyjí) [Flora and vegetation of the exposed bottom of the Znojmo and Vranov water reservoirs (middle Dyje basin)]. Thayensia 2007, 7, 153–173. (In Czech) [Google Scholar]
- Malíková, L. Vliv Obhospodařování na Strukturu Makrovegetace. Vliv Obhospodařování Rybníků na Strukturu Vegetace a Flóry Obnažených den [Influence of Management on the Structure of Macrovegetation. Influence of Pond Management on the Structure of Vegetation and Flora of Exposed Bottoms]. Bachelor’s Thesis, University of South Bohemia, České, Budějovice, 2000. [Google Scholar]
- Vicherek, J. Poznámky k syntaxonomii asociace Cyperus fuscus-Chenopodium glaucum as. Klika 1935. Spisy Přírod. Fak. Univ. J. E. Purkyně Brno 1969, 501, 63–73. (In Czech) [Google Scholar]
- Vicherek, J. Poznámky k cenologické afinitě Myosurus minimus L. [Remarks on the coenological affinity of Myosurus minimus L.]. Preslia 1968, 40, 387–396. (In Czech) [Google Scholar]
- Borysiak, J. Struktura Aluwialnej Roślinności Lądowej Środkowego i Dolnego Biegu Warty [The Structure of Alluvial Vegetation of the Middle and Lower Warta River]; Wydawnictwo Naukowe UAM: Poznan, Poland, 1994. (In Polish) [Google Scholar]
- Krawczyk, R.; Cwener, A.; Michalczuk, W.; Zubel, R. Ephemeral wetland communities of Isoëto-Nano-Juncetea class—New data from south-eastern Poland. Biodiv. Res. Conserv. 2016, 42, 41–54. [Google Scholar] [CrossRef]
- Macicka-Pawlik, T.; Wilczyńska, W. Zbiorowiska roślinne starorzeczy w dolinie środkowego biegu Odry [Plant communities of oxbow lakes in the middle Odra river valley]. Acta Univ. Wratislav. Pr. Bot. 1996, 64, 73–120. (In Polish) [Google Scholar]
- Podbielkowski, Z. Roślinność stawów rybnych woj. Warszawskiego [Vegetation of fishponds in the province of Warsaw]. Monogr. Bot. 1968, 27, 3–123. (In Polish) [Google Scholar] [CrossRef] [Green Version]
- Popiela, A. Zbiorowiska z klasy Isoëto-Nanojuncetea na terenie Polski Zachodniej [Communities of the Isoëto-Nanojuncetea class in western Poland]. Fragm. Flor. Geobot. Ser. Polon. 1996, 3, 289–310. (In Polish) [Google Scholar]
- Spałek, K. Bidentetum cernuae (Kobendza 1948) Slavnic 1951 w zbiornikach zaporowych na Śląsku Opolskim [Bidentetum cernuae (Kobendza 1948) Slavnic 1951 in dam reservoirs in Opole Silesia]. Acta Bot. Siles. 2008, 3, 137–144. (In Polish) [Google Scholar]
- Spałek, K.; Nowak, A. Zbiorowiska namułkowe z klasy Isoëto-Nanojuncetea w zbiornikach zaporowych na Śląsku Opolskim [Mudflat communities of the class Isoëto-Nanojuncetea in dam reservoirs in Opole Silesia]. Fragm. Flor. Geobot. Polon. 2006, 13, 361–368. (In Polish) [Google Scholar]
- Zając, M.; Zając, A. Zbiorowiska z klasy Isoëto-Nanojuncetea na dnach wysychających stawów w południowej części Kotliny Oświęcimskiej [Communities of the class Isoëto-Nanojuncetea on the bottoms of drying-up ponds in the southern part of the Oświęcim basin]. Zesz. Nauk. Uniw. Jagiell., Pr. Bot. 1988, 17, 155–160. (In Polish) [Google Scholar]
- Traxler, A. Zwergbinsengesellschaften in Österreich. Diploma Thesis, University of Vienna, Vienna, Austria, 1990. [Google Scholar]



| Vegetation Units | Layer | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of relevés | 622 | 109 | 73 | 9 | 5 | 5 | 4 | 4 | 2 | 2 | 1 | 1 | |
| Isoëto-Nanojuncetea | |||||||||||||
| Cyperus fuscus | 6 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Plantago major subsp. intermedia | 6 | 49 | 54 | 16 | 11 | 40 | 60 | 25 | 100 | ||||
| Gnaphalium uliginosum | 6 | 50 | 38 | 16 | 50 | ||||||||
| Juncus bufonius | 6 | 42 | 28 | 12 | 22 | 50 | |||||||
| Limosella aquatica | 6 | 36 | 16 | 12 | 11 | 50 | |||||||
| Eleocharis acicularis | 6 | 28 | 3 | 8 | 25 | ||||||||
| Potentilla supina | 6 | 21 | 25 | 7 | 20 | 60 | 50 | 50 | |||||
| Eleocharis ovata | 6 | 23 | 4 | 5 | 100 | ||||||||
| Carex bohemica | 6 | 19 | 7 | 4 | 50 | ||||||||
| Lythrum portula | 6 | 21 | 1 | 3 | 50 | ||||||||
| Riccia cavernosa/crystallina | 9 | 14 | 1 | 4 | |||||||||
| Lindernia procumbens | 6 | 7 | 3 | ||||||||||
| Pulicaria vulgaris | 6 | 5 | 6 | 11 | 25 | ||||||||
| Lythrum hyssopifolia | 6 | 4 | 3 | 4 | |||||||||
| Bidentetea tripartitae | |||||||||||||
| Persicaria lapathifolia s. l. (excl. P. lapathifolia subsp. brittingeri) | 6 | 51 | 61 | 42 | 33 | 40 | 20 | 25 | 25 | 100 | 100 | 100 | |
| Rumex maritimus | 6 | 41 | 67 | 47 | 33 | 60 | 40 | 25 | 50 | 50 | 100 | ||
| Rorippa palustris | 6 | 47 | 51 | 29 | 22 | 20 | 50 | 50 | 100 | ||||
| Ranunculus sceleratus | 6 | 33 | 36 | 22 | 11 | 20 | 50 | 25 | 100 | 100 | |||
| Persicaria hydropiper | 6 | 26 | 39 | 34 | 22 | 50 | 50 | 100 | |||||
| Bidens tripartitus | 6 | 26 | 36 | 23 | 11 | 25 | 50 | 100 | 100 | ||||
| Alopecurus aequalis | 6 | 27 | 21 | 19 | 22 | 60 | 25 | 50 | |||||
| Oxybasis rubra | 6 | 20 | 48 | 26 | 20 | 40 | 25 | ||||||
| Bidens frondosus | 6 | 15 | 40 | 23 | 11 | 20 | 50 | 100 | |||||
| Bidens radiatus | 6 | 13 | 10 | 16 | |||||||||
| Oxybasis glauca | 6 | 11 | 23 | 3 | 40 | 25 | |||||||
| Bidens cernuus | 6 | 10 | 15 | 7 | 22 | 50 | |||||||
| Persicaria maculosa | 6 | 9 | 14 | 8 | 20 | 25 | 100 | ||||||
| Persicaria minor | 6 | 11 | 4 | 5 | 50 | ||||||||
| Lipandra polysperma | 6 | 6 | 24 | 8 | 25 | 50 | |||||||
| Atriplex prostrata | 6 | 5 | 25 | 5 | 60 | 25 | 25 | ||||||
| Persicaria dubia | 6 | 5 | 5 | 1 | 20 | ||||||||
| Xanthium orientale agg. | 6 | 3 | 9 | 5 | |||||||||
| Chenopodium ficifolium | 6 | 4 | 7 | 1 | 40 | ||||||||
| Phragmito-Magnocaricetea | |||||||||||||
| Veronica anagallis-aquatica | 6 | 31 | 32 | 22 | 11 | 40 | 20 | 25 | 25 | 50 | 100 | ||
| Alisma plantago-aquatica | 6 | 27 | 13 | 52 | 22 | 20 | 50 | 50 | 100 | 100 | |||
| Leersia oryzoides | 6 | 26 | 8 | 30 | 20 | 50 | |||||||
| Oenanthe aquatica | 6 | 20 | 32 | 27 | 11 | 60 | 50 | 50 | 100 | ||||
| Rorippa amphibia | 6 | 14 | 34 | 26 | 22 | 25 | |||||||
| Bolboschoenus maritimus agg. | 6 | 12 | 12 | 34 | 11 | 40 | 60 | 100 | 50 | ||||
| Phalaroides arundinacea | 6 | 10 | 31 | 21 | 22 | 20 | 25 | 100 | |||||
| Eleocharis palustris agg. | 6 | 15 | 8 | 11 | 25 | 100 | |||||||
| Typha latifolia | 6 | 12 | 15 | 19 | 11 | 20 | 25 | ||||||
| Phragmites australis | 6 | 9 | 17 | 12 | 22 | 20 | 40 | 25 | 100 | ||||
| Typha angustifolia | 6 | 7 | 7 | 8 | 11 | 20 | 25 | 50 | |||||
| Poa palustris | 6 | 6 | 11 | 5 | 11 | 20 | |||||||
| Veronica beccabunga | 6 | 5 | 5 | 5 | 11 | 100 | 100 | ||||||
| Butomus umbellatus | 6 | 2 | 12 | 11 | 25 | ||||||||
| Molinio-Arrhenatheretea | |||||||||||||
| Lythrum salicaria | 6 | 27 | 22 | 25 | 56 | 20 | 100 | ||||||
| Taraxacum sect. Ruderalia | 6 | 19 | 20 | 8 | 33 | 20 | 20 | 75 | 25 | ||||
| Agrostis stolonifera | 6 | 19 | 17 | 12 | 44 | 20 | 40 | 25 | |||||
| Juncus effusus | 6 | 10 | 11 | 5 | 22 | ||||||||
| Trifolium hybridum | 6 | 12 | 5 | 4 | 11 | 25 | |||||||
| Myosotis palustris agg. | 6 | 10 | 8 | 11 | 11 | ||||||||
| Trifolium repens | 6 | 9 | 12 | 1 | 33 | 50 | |||||||
| Juncus compressus | 6 | 8 | 6 | 7 | 22 | 20 | 40 | 50 | |||||
| Argentina anserina | 6 | 7 | 10 | 7 | |||||||||
| Rorippa sylvestris | 6 | 7 | 6 | 8 | 22 | 20 | 25 | ||||||
| Ranunculus repens | 6 | 6 | 9 | 4 | 33 | 20 | |||||||
| Alopecurus geniculatus | 6 | 7 | 4 | 5 | |||||||||
| Poa trivialis | 6 | 2 | 11 | 3 | 33 | 20 | 25 | ||||||
| Juncus inflexus | 6 | 3 | 4 | 1 | 44 | 20 | |||||||
| Scheuchzerio-Caricetea nigrae | |||||||||||||
| Juncus articulatus | 6 | 34 | 15 | 29 | 56 | 100 | 50 | 50 | 50 | ||||
| Potamogetonetea | |||||||||||||
| Callitriche palustris agg. | 6 | 18 | 6 | 7 | |||||||||
| Papaveretea rhoeadis | |||||||||||||
| Echinochloa crus-galli | 6 | 41 | 43 | 36 | 22 | 20 | 60 | 25 | 75 | 100 | 100 | ||
| Tripleurospermum inodorum | 6 | 16 | 23 | 10 | 20 | 20 | 25 | 50 | |||||
| Epilobietea angustifolii | |||||||||||||
| Urtica dioica | 6 | 8 | 28 | 5 | 22 | 20 | 25 | 50 | |||||
| Myosoton aquaticum | 6 | 9 | 19 | 5 | 11 | 20 | 25 | 25 | 50 | ||||
| Epilobium hirsutum | 6 | 6 | 6 | 8 | 100 | ||||||||
| Others | |||||||||||||
| Salix sp. | 7 | 34 | 38 | 18 | 22 | 75 | 25 | 100 | |||||
| Lycopus europaeus | 6 | 16 | 18 | 18 | 22 | 20 | 25 | ||||||
| Nostoc sp. | 9 | 14 | 4 | 3 | 20 | ||||||||
| Polygonum aviculare agg. | 6 | 9 | 19 | 8 | |||||||||
| Epilobium ciliatum | 6 | 11 | 5 | 5 | 11 | ||||||||
| Plantago major | 6 | 9 | 6 | 10 | 11 | 25 | |||||||
| Ochlopoa annua | 6 | 8 | 9 | 3 | 22 | 50 | |||||||
| Epilobium sp. | 6 | 8 | 4 | 5 | 22 | 25 | 50 | ||||||
| Sagina procumbens | 6 | 9 | 5 | 3 | 11 | 50 | |||||||
| Bryum argenteum | 9 | 9 | 6 | 11 | 50 | ||||||||
| Variable | Explained Variation [%] | F | p (adj.) |
|---|---|---|---|
| Nutrients | 1.94 | 16.0 | <0.001 |
| Moisture | 1.75 | 14.5 | <0.001 |
| Soil reaction | 1.59 | 13.1 | <0.001 |
| Continentality | 1.50 | 12.4 | <0.001 |
| Temperature | 1.11 | 9.1 | <0.001 |
| Number of archaeophytes | 1.08 | 8.9 | <0.001 |
| Number of neophytes | 0.92 | 7.6 | <0.001 |
| Light | 0.88 | 7.2 | <0.001 |
| Year | 0.85 | 6.9 | <0.001 |
| Total cover of archaeophytes | 0.63 | 5.2 | <0.001 |
| Total cover of neophytes | 0.49 | 4.0 | <0.001 |
| Cyperus fuscus cover | 0.27 | 2.2 | <0.001 |
| Coefficient | Estimate | t Value | p Value | |
|---|---|---|---|---|
| Isoëto-Nanojuncetea and Crypsietea (intercept) | 0.509 | 9.53 | 0.000 | *** |
| Bidentetea | −0.086 | −1.16 | 0.251 | |
| Phragmito-Magnocaricetea and Scheuchzerio-Caricetea | −0.216 | −2.86 | 0.005 | ** |
| Molinio-Arrhenatheretea and other grassland classes | −0.294 | −3.77 | 0.000 | *** |
| Potamogetonetea and Lemnetea | 0.045 | 0.36 | 0.717 | |
| Papaveretea and other classes of annual ruderal vegetation | −0.179 | −2.10 | 0.038 | * |
| Epilobietea and other classes of perennial ruderal vegetation | −0.345 | −4.05 | 0.000 | *** |
| Climatic Variable | Description | Explained Variation [%] | p (adj.) | Biplot Score |
|---|---|---|---|---|
| bio10 | Mean temperature of warmest quarter | 19.1 | <0.001 | 0.92 |
| bio08 | Mean temperature of wettest quarter | 18.9 | <0.001 | 0.91 |
| bio05 | Maximum temperature of warmest month | 18.9 | <0.001 | 0.91 |
| bio01 | Annual mean temperature | 18.7 | <0.001 | 0.91 |
| bio11 | Mean temperature of coldest quarter | 15.2 | <0.001 | 0.82 |
| bio04 | Temperature seasonality | 13.6 | <0.001 | 0.77 |
| bio07 | Temperature annual range | 12.4 | <0.001 | 0.74 |
| bio06 | Minimum temperature of coldest month | 9.4 | <0.001 | 0.65 |
| bio02 | Mean diurnal range | 9.3 | <0.001 | 0.64 |
| bio12 | Annual precipitation | 6.7 | <0.001 | −0.54 |
| bio14 | Precipitation of driest month | 6.6 | <0.001 | −0.54 |
| bio17 | Precipitation of driest quarter | 6.4 | <0.001 | −0.53 |
| bio19 | Precipitation of coldest quarter | 6.3 | <0.001 | −0.53 |
| bio16 | Precipitation of wettest quarter | 5.0 | <0.001 | −0.47 |
| bio18 | Precipitation of warmest quarter | 5.0 | <0.001 | −0.47 |
| bio15 | Precipitation seasonality | 4.5 | <0.001 | 0.44 |
| bio13 | Precipitation of wettest month | 4.3 | <0.001 | −0.44 |
| bio09 | Mean temperature of driest quarter | 4.2 | <0.001 | 0.43 |
| bio03 | Isothermality | 3.4 | <0.001 | 0.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kúr, P.; Píšová, S.; Tremetsberger, K.; Dřevojan, P.; Kącki, Z.; Böckelmann, J.; Bernhardt, K.-G.; Hroudová, Z.; Mesterházy, A.; Šumberová, K. Ecology and Genetics of Cyperus fuscus in Central Europe—A Model for Ephemeral Wetland Plant Research and Conservation. Water 2021, 13, 1277. https://doi.org/10.3390/w13091277
Kúr P, Píšová S, Tremetsberger K, Dřevojan P, Kącki Z, Böckelmann J, Bernhardt K-G, Hroudová Z, Mesterházy A, Šumberová K. Ecology and Genetics of Cyperus fuscus in Central Europe—A Model for Ephemeral Wetland Plant Research and Conservation. Water. 2021; 13(9):1277. https://doi.org/10.3390/w13091277
Chicago/Turabian StyleKúr, Pavel, Soňa Píšová, Karin Tremetsberger, Pavel Dřevojan, Zygmunt Kącki, Jörg Böckelmann, Karl-Georg Bernhardt, Zdenka Hroudová, Attila Mesterházy, and Kateřina Šumberová. 2021. "Ecology and Genetics of Cyperus fuscus in Central Europe—A Model for Ephemeral Wetland Plant Research and Conservation" Water 13, no. 9: 1277. https://doi.org/10.3390/w13091277







