Daily Samples Revealing Shift in Phytoplankton Community and Its Environmental Drivers during Summer in Qinhuangdao Coastal Area, China
Abstract
:1. Introduction
2. Materials and Methods
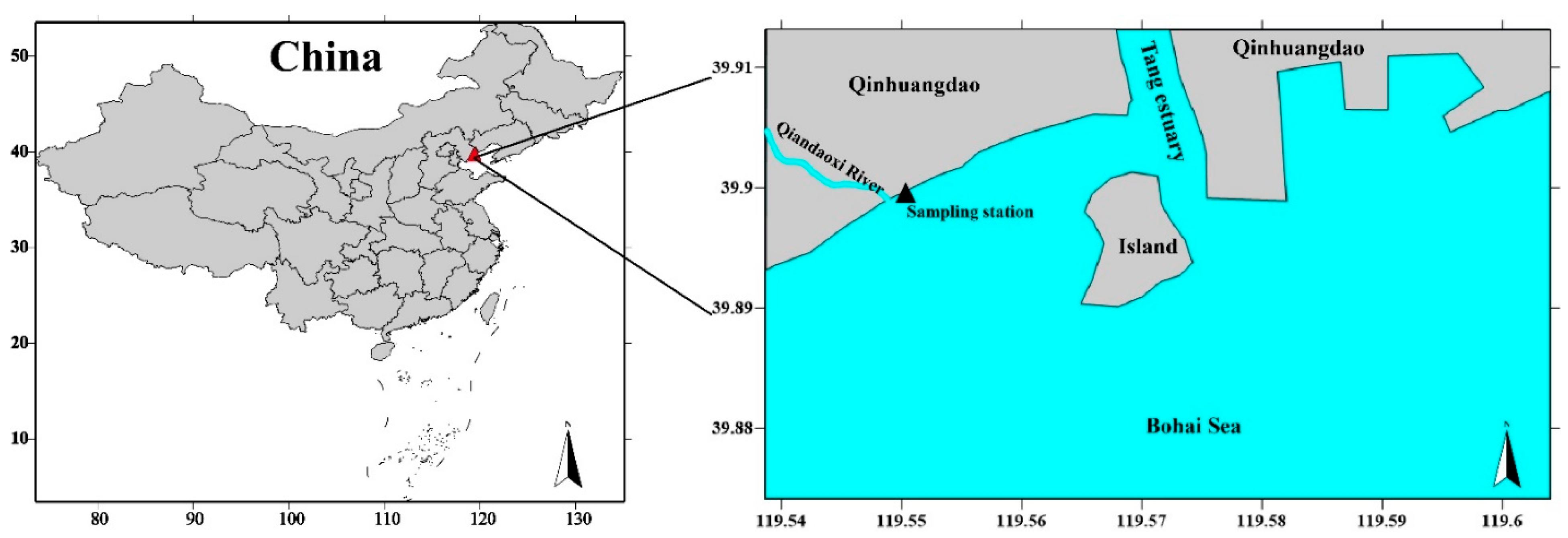
2.1. Study Area and Water Sampling
2.2. Environmental Parameters
2.3. Phytoplankton Identification
2.4. Data Analysis
3. Results
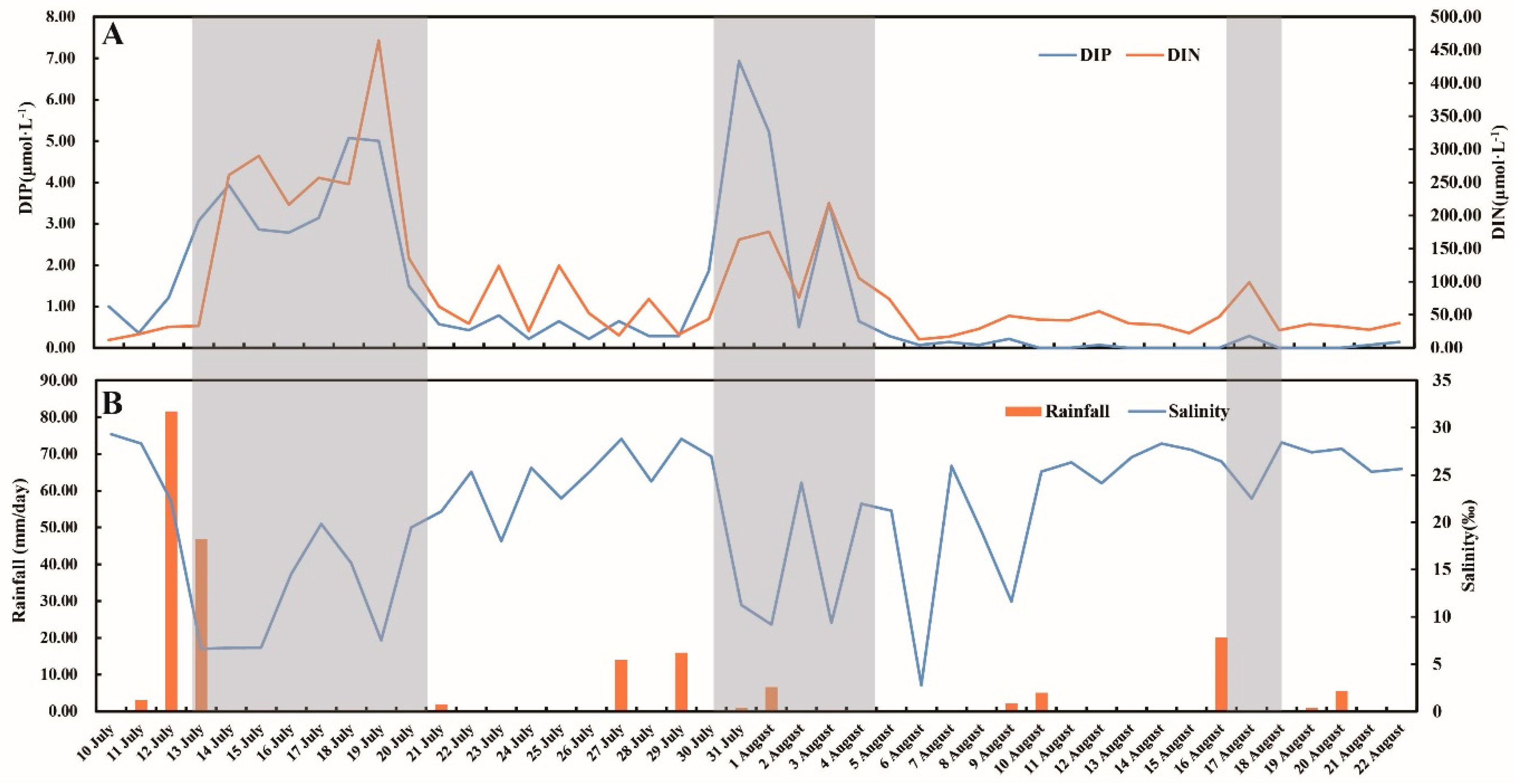
3.1. Environmental Factors
3.2. Total Abundance and Diversity of the Phytoplankton
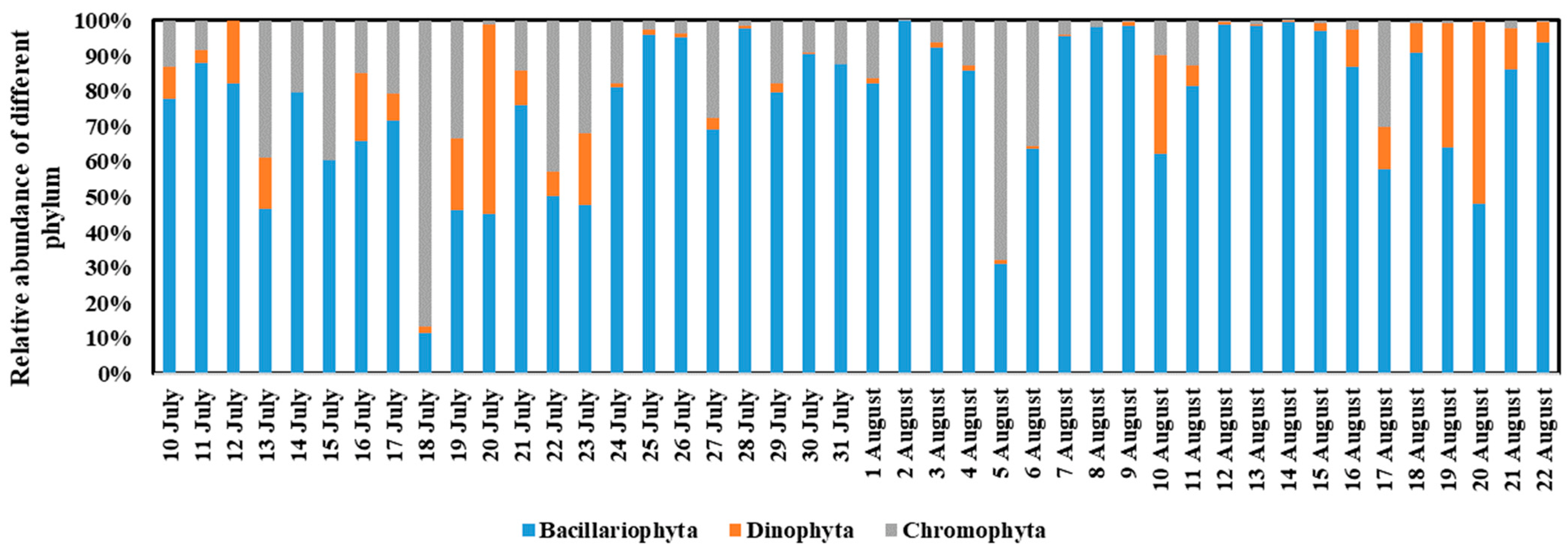
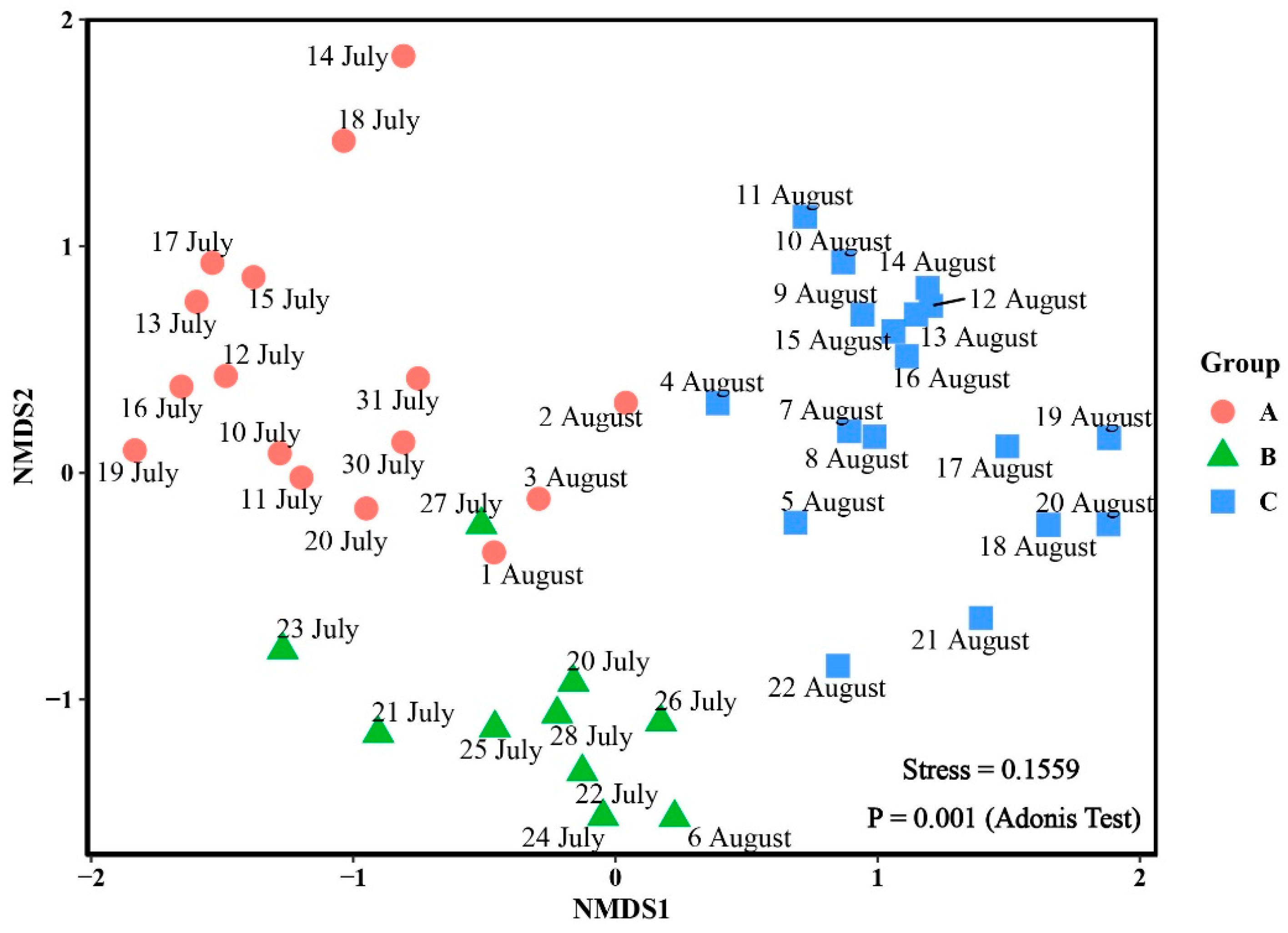
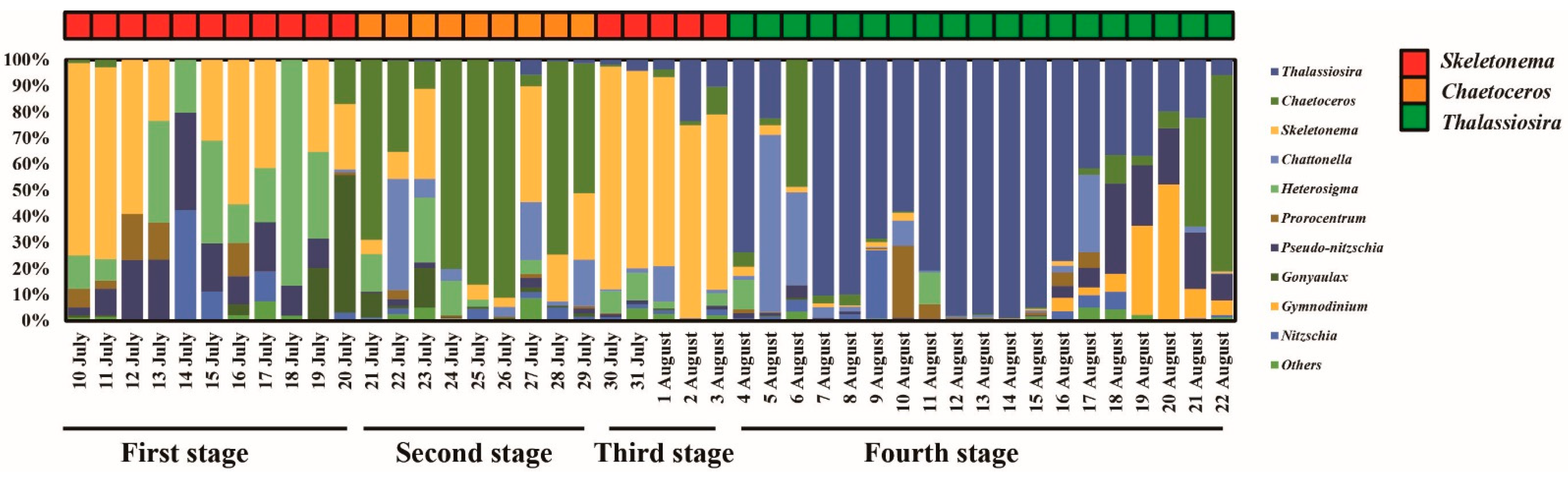
3.3. Phytoplankton Community
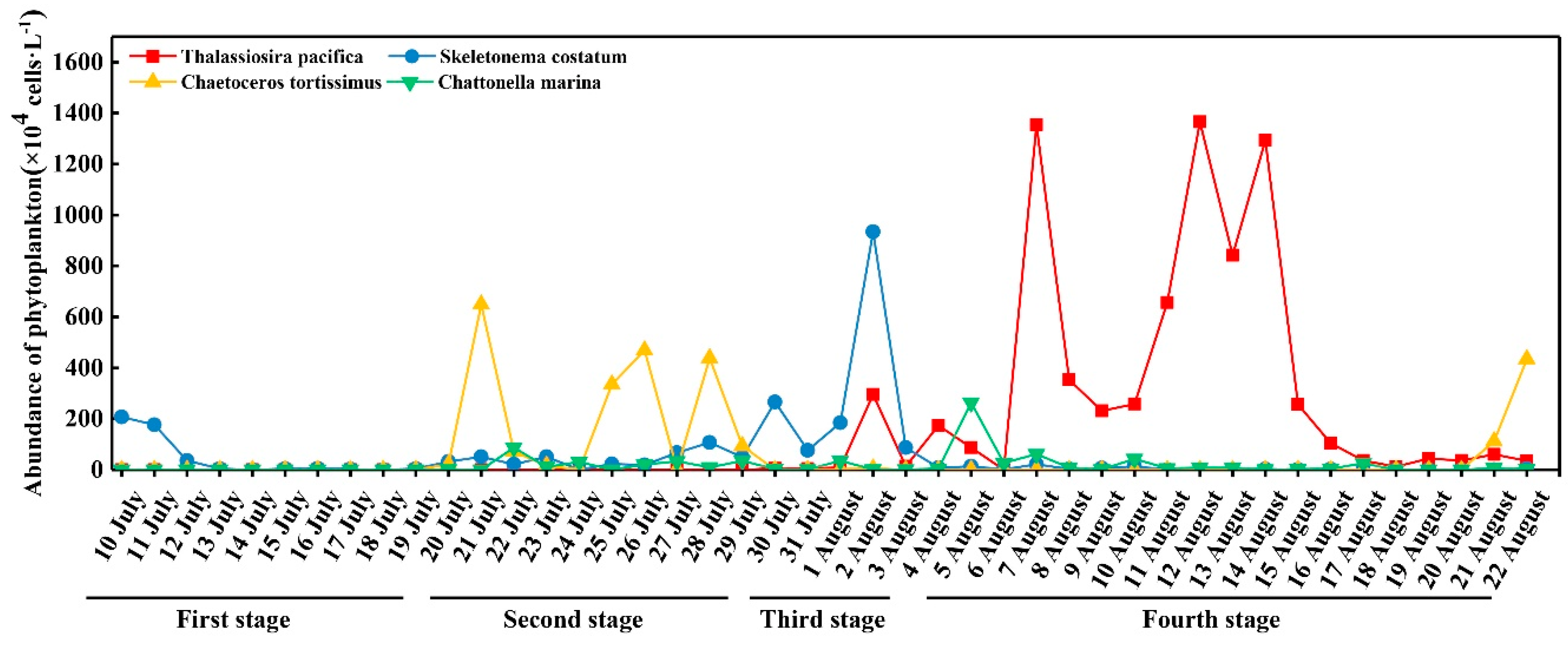
3.4. Abundance of Dominant Species
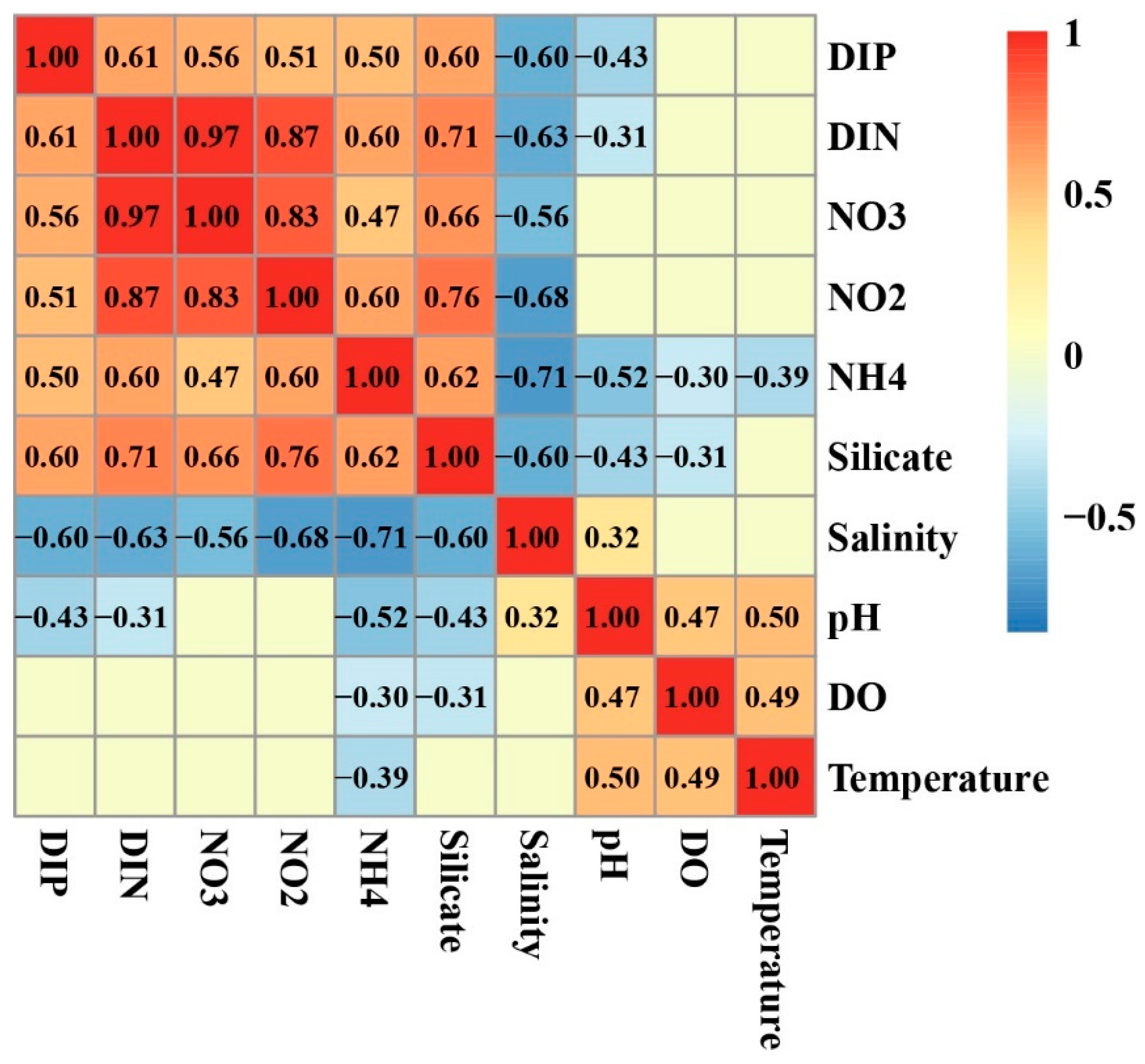
3.5. Relationship of Environmental Factors with Phytoplankton Abundance and Community Structure
4. Discussion
4.1. Inflows Facilitating the Rapid Succession of Phytoplankton
4.2. DIP and the Bloom of Phytoplankton
4.3. The Potential Risk of Harmful Algal Blooms
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Environmental Factors | Minimum Value | Maximum Value | Mean ± SD |
|---|---|---|---|
| NH4 (μmol·L−1) | 0.57 | 61.14 | 11.35 ± 11.94 |
| NO3 (μmol·L−1) | 4.00 | 435.00 | 77.26 ± 89.46 |
| NO2 (μmol·L−1) | 0.21 | 8.00 | 2.48 ± 1.84 |
| DIP (μmol·L−1) | ND | 6.93 | 1.23 ± 1.77 |
| Silicate (μmol·L−1) | 27.5 | 871.43 | 242.94 ± 185.71 |
| DO (%) | 6.05 | 13.81 | 8.84 ± 1.75 |
| Salinity (‰) | 2.77 | 29.31 | 20.97 ± 7.58 |
| pH | 7.64 | 8.62 | 8.10 ± 0.21 |
| Temperature (°C) | 24.40 | 31.10 | 28.05 ± 1.76 |
| DIN (μmol·L−1) | 27.50 | 871.42 | 91.09 ± 96.81 |
| N/P | 23.894 | ∞ | -- |
| Phylum | Species | Detectable Rate |
|---|---|---|
| Bacillariophyta | Thalassiosira pacifica | 0.52 |
| Skeletonema costatum | 0.75 | |
| Chaetoceros tortissimus | 0.30 | |
| Chaetoceros spp. | 0.41 | |
| Pseudo-nitzschia delicatissima | 0.84 | |
| Nitzschia spp. | 0.73 | |
| Leptocylindrus danicus | 0.05 | |
| Chaetoceros curvisetus | 0.34 | |
| Pseudo-nitzschia pungens | 0.16 | |
| Thalassionema nitzschioides | 0.20 | |
| Chaetoceros diadema | 0.11 | |
| Chaetoceros decipiens | 0.05 | |
| Thalassiosira spp. | 0.14 | |
| Licmophora abbreviata | 0.11 | |
| Chaetoceros lorenzianus | 0.14 | |
| Pinnularia spp. | 0.16 | |
| Thalassiothrix frauenfeldii | 0.02 | |
| Coscinodiscus spp. | 0.09 | |
| Detonula pumila | 0.09 | |
| Guinardia delicatula | 0.05 | |
| Thalassiosira nordenskioeldii | 0.02 | |
| Chaetoceros peruvianus | 0.02 | |
| Chaetoceros affinis | 0.07 | |
| Chaetoceros abnormis | 0.02 | |
| Hemiaulus sinensis | 0.02 | |
| Guinardia flaccida | 0.05 | |
| Nitzschia longissima | 0.02 | |
| Dinophyta | Prorocentrum sigmoides | 0.57 |
| Gymnodinium catenatum | 0.25 | |
| Gonyaulax verior | 0.18 | |
| Gonyaulax spp. | 0.20 | |
| Scrippsiella trochoidea | 0.27 | |
| Protoperidinium pellucidum | 0.27 | |
| Prorocentrum minimum | 0.11 | |
| Ceratium fusus | 0.11 | |
| Alexandrium catenella | 0.02 | |
| Ceratium tripos | 0.11 | |
| Karenia mikimotoi | 0.02 | |
| Akashiwo sanguinea | 0.02 | |
| Protoperidinium spp. | 0.02 | |
| Chromophyta | Chattonella marina | 0.66 |
| Heterosigma akashiwo | 0.48 | |
| Dictyocha fibula | 0.11 |
| Groups | A | B |
|---|---|---|
| B | 0.001 | - |
| C | 0.001 | 0.001 |
| Environmental Factors | r | p Value |
|---|---|---|
| NH4 | 0.099 * | 0.027 |
| NO3 | 0.106 * | 0.01 |
| NO2 | 0.007 | 0.406 |
| DIP | 0.287 ** | 0.001 |
| Silicate | 0.047 | 0.133 |
| DO | 0.047 | 0.137 |
| Salinity | 0.092 * | 0.022 |
| pH | 0.085 * | 0.026 |
| Temperature | 0.148 ** | 0.001 |
| DIN | 0.099 * | 0.022 |
| Environmental Factors | Thalassiosira pacifica | Skeletonema costatum | Chaetoceros tortissimus | Chattonella marina |
|---|---|---|---|---|
| NH4 | −0.224 | −0.179 | −0.169 | −0.274 |
| NO3 | −0.160 | 0.109 | 0.077 | −0.207 |
| NO2 | 0.039 | 0.034 | −0.004 | −0.117 |
| DIP | −0.620 ** | 0.472 ** | 0.070 | −0.306 * |
| Silicate | −0.106 | 0.101 | −0.189 | −0.305 * |
| DO | 0.172 | 0.175 | 0.257 | 0.231 |
| Salinity | 0.263 | −0.010 | 0.067 | 0.144 |
| pH | 0.255 | −0.081 | 0.284 | 0.075 |
| Temperature | 0.028 | 0.182 | 0.476 ** | 0.288 |
| DIN | −0.189 | 0.072 | 0.058 | −0.259 |
References
- Falkowski, P.G.; Barber, R.T.; Smetacek, V. Biogeochemical controls and feedbacks on ocean primary production. Science 1998, 281, 200–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arrigo, K. Marine microorganisms and global nutrient cycles. Nature 2005, 437, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Hilligsøe, K.M.; Richardson, K.; Bendtsen, J.; SøRensen, L.L.; Nielsen, T.G.; Lyngsgaard, M.M. Linking phytoplankton community size composition with temperature, plankton food web structure and sea–air CO2 flux. Deep-Sea Res. Part I 2011, 58, 826–838. [Google Scholar] [CrossRef]
- Kroeze, C.; Hofstra, N.; Ivens, W.; Loehr, A.; Strokal, M.; Wijnen, J.V. The links between global carbon, water and nutrient cycles in an urbanizing world—The case of coastal eutrophication. Curr. Opin. Environ. Sustain. 2013, 5, 566–572. [Google Scholar] [CrossRef]
- Genitsaris, S.; Sommer, M.-G. Phytoplankton Blooms, Red Tides and Mucilaginous Aggregates in the Urban Thessaloniki Bay, Eastern Mediterranean. Diversity 2019, 11, 136. [Google Scholar] [CrossRef] [Green Version]
- Kouakou, C.R.; Poder, T.G. Economic impact of harmful algal blooms on human health: A systematic review. J. Water Health 2019, 17, 499–516. [Google Scholar] [CrossRef] [Green Version]
- Cira, E.K.; Palmer, T.A.; Wetz, M.S. Phytoplankton dynamics in a low-inflow estuary (Baffin Bay, TX) during drought and high-rainfall conditions associated with an El Niño event. Estuaries Coasts 2021, 44, 1752–1764. [Google Scholar] [CrossRef]
- Kotsedi, D.; Adams, J.B.; Snow, G.C. The response of microalgal biomass and community composition to environmental factors in the Sundays Estuary. Water 2012, 38, 177–190. [Google Scholar] [CrossRef] [Green Version]
- Kaselowski, T. Not so pristine-characterising the physico-chemical conditions of an undescribed temporarily open/closed estuary. Water 2013, 39, 627–635. [Google Scholar] [CrossRef] [Green Version]
- Buyukates, Y.; Roelke, D. Influence of pulsed inflows and nutrient loading on zooplankton and phytoplankton community structure and biomass in microcosm experiments using estuarine assemblages. Hydrobiologia 2005, 548, 233–249. [Google Scholar] [CrossRef]
- Hylén, A.; van de Velde, S.J.; Kononets, M.; Luo, M.; Almroth-Rosell, E.; Hall, P.O. Deep-water inflow event increases sedimentary phosphorus release on a multi-year scale. Biogeosciences 2021, 18, 2981–3004. [Google Scholar] [CrossRef]
- Wachnicka, A.; Browder, J.; Jackson, T.; Louda, W.; Kelble, C.; Abdelrahman, O.; Stabenau, E.; Avila, C. Hurricane Irma’s impact on water quality and phytoplankton communities in Biscayne Bay (Florida, USA). Estuaries Coasts 2020, 43, 1217–1234. [Google Scholar] [CrossRef]
- Cui, L.; Lu, X.; Dong, Y.; Cen, J.; Cao, R.; Pan, L.; Lu, S.; Ou, L. Relationship between phytoplankton community succession and environmental parameters in Qinhuangdao coastal areas, China: A region with recurrent brown tide outbreaks. Ecotoxicol. Environ. Saf. 2018, 159, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Sin, Y.; Yu, H. Phytoplankton community and surrounding water conditions in the Youngsan River estuary: Weekly variation in the saltwater zone. Ocean Polar Res. 2018, 40, 191–202. [Google Scholar]
- Nunes, M.; Adams, J.B.; Rishworth, G.M. Shifts in phytoplankton community structure in response to hydrological changes in the shallow St Lucia Estuary. Mar. Pollut. Bull. 2018, 128, 275–286. [Google Scholar] [CrossRef]
- Tao, J.-H. Numerical simulation of aquatic eco-environment of Bohai Bay. J. Hydrodyn. Ser. B 2006, 18, 34–42. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Zhang, W.; Zhang, J. Spatial and temporal distribution of silicate and chlorophyll a in the coastal waters with picophytoplankton algal bloom. Ecol. Sci. 2013, 32, 509–513. [Google Scholar]
- He, Y.; He, Y.; Sen, B.; Li, H.; Li, J.; Zhang, Y.; Zhang, J.; Jiang, S.C.; Wang, G. Storm runoff differentially influences the nutrient concentrations and microbial contamination at two distinct beaches in northern China. Sci. Total Environ. 2019, 663, 400–407. [Google Scholar] [CrossRef]
- GB/T 12763-2007; General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China. Specification for the Oceanographic Survey. China National Standardization Management Committee: Beijing, China, 2007.
- HY/T 069-2005; State Oceanic Administration (SOA). Technical Specification for Red Tide Monitoring in China. China National Standardization Management Committee: Beijing, China, 2005.
- Utermöhl, H. Zur vervollkommnung der quantitativen phytoplankton-methodik: Mit 1 Tabelle und 15 abbildungen im Text und auf 1 Tafel. Int. Ver. Für Theor. Und Angew. Limnol. Mitt. 1958, 9, 1–38. [Google Scholar] [CrossRef]
- Team, R.C. R: A language and environment for statistical computing. Computing 2011, 1, 12–21. [Google Scholar]
- Ji, F.; Sun, Y.; Ma, Q.; Feng, X.; Mi, D. Response of planktonic communities to environmental stress in the eutrophic waters of Xiaoping Island in China. Chemosphere 2021, 275, 130107. [Google Scholar] [CrossRef] [PubMed]
- Redfield, A. The biological control of chemical factors in the environment. Am. Sci. 1958, 46, 205–221. [Google Scholar]
- Hemraj, D.A.; Hossain, A.; Ye, Q.; Qin, J.G.; Leterme, S.C. Anthropogenic shift of planktonic food web structure in a coastal lagoon by freshwater flow regulation. Sci. Rep. 2017, 7, 44441. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Zhu, B.; Chen, H.; Liu, Z.; Cui, B.; Wu, J.; Li, B.; Yu, H.; Peng, M. The relationship between the Skeletonema costatum red tide and environmental factors in Hongsha Bay of Sanya, South China Sea. J. Coast. Res. 2009, 25, 651–658. [Google Scholar] [CrossRef]
- Samuels, W.B.; Kleppel, G.S.; McLaughlin, J.J. Population growth patterns of Skeletonema costatum and nutrient levels in the lower East River, New York, USA. Hydrobiologia 1983, 98, 35–43. [Google Scholar] [CrossRef]
- Cleve, P.T. Examination of diatoms found on the surface of the Sea of Java. Bih. Kongl. Sven. Vet-Akad. Handl. 1873, 1, 1–13. [Google Scholar]
- Roelke, D.L.; Spatharis, S. Phytoplankton succession in recurrently fluctuating environments. PLoS ONE 2015, 10, e0121392. [Google Scholar] [CrossRef] [Green Version]
- Chong, B.; Leong, S.; Kuwahara, V.S.; Yoshida, T. Monsoonal variation of the marine phytoplankton community in Kota Kinabalu, Sabah. Reg. Stud. Mar. Sci. 2020, 37, 101326. [Google Scholar] [CrossRef]
- Zhang, C.; Yao, X.; Chen, Y.; Chu, Q.; Yu, Y.; Shi, J.; Gao, H. Variations in the phytoplankton community due to dust additions in eutrophication, LNLC and HNLC oceanic zones. Sci. Total Environ. 2019, 669, 282–293. [Google Scholar] [CrossRef]
- Zohdi, E.; Abbaspour, M. Harmful algal blooms (red tide): A review of causes, impacts and approaches to monitoring and prediction. Int. J. Environ. Sci. Technol. 2019, 16, 1789–1806. [Google Scholar] [CrossRef]
- Sengupta, A.; Carrara, F.; Stocker, R. Phytoplankton can actively diversify their migration strategy in response to turbulent cues. Nature 2017, 543, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, H.; Zhu, Y.; Xiao, Y.; Chen, L. Effect of flow velocity on phytoplankton biomass and composition in a freshwater lake. Sci. Total Environ. 2013, 447, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Livingston, R.J. Phytoplankton bloom effects on a gulf estuary: Water quality changes and biological response. Ecol. Appl. 2007, 17, S110–S128. [Google Scholar] [CrossRef]
- Imai, I. Ecophysiology, life cycle, and bloom dynamics of Chattonella in the Seto Inland Sea, Japan. Physiol. Ecol. Harmful Algal Bloom. 1998, 2, 71–84. [Google Scholar]
- Al Gheilani, H.M.; Matsuoka, K.; AlKindi, A.Y.; Amer, S.; Waring, C. Fish kill incidents and harmful algal blooms in Omani waters. J. Agric. Mar. Sci. 2011, 16, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Mühlenbruch, M.; Grossart, H.P.; Eigemann, F.; Voss, M. Mini-review: Phytoplankton-derived polysaccharides in the marine environment and their interactions with heterotrophic bacteria. Environ. Microbiol. 2018, 20, 2671–2685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benitez-Nelson, C.R. The biogeochemical cycling of phosphorus in marine systems. Earth-Sci. Rev. 2000, 51, 109–135. [Google Scholar] [CrossRef]
- Ammerman, J.W.; Hood, R.R.; Case, D.A.; Cotner, J.B. Phosphorus deficiency in the Atlantic: An emerging paradigm in oceanography. Eos Trans. Am. Geophys. Union 2003, 84, 165. [Google Scholar] [CrossRef]
- Dyhrman, S.T.; Jenkins, B.D.; Rynearson, T.A.; Saito, M.A.; Mercier, M.L.; Harriet, A.; Whitney, L.P.; Andrea, D.; Bulygin, V.V.; Bertrand, E.M. The Transcriptome and Proteome of the Diatom Thalassiosira pseudonana Reveal a Diverse Phosphorus Stress Response. PLoS ONE 2012, 7, e33768. [Google Scholar] [CrossRef] [Green Version]
- Mather, R.L.; Reynolds, S.E.; Wolff, G.A.; Williams, R.G.; Torres-Valdes, S.; Woodward, E.; Landolfi, A.; Pan, X.; Sanders, R.; Achterberg, E.P. Phosphorus cycling in the North and South Atlantic Ocean subtropical gyres. Nat. Geosci. 2008, 1, 439–443. [Google Scholar] [CrossRef]
- Lomas, M.W.; Burke, A.; Lomas, D.; Bell, D.; Shen, C.; Dyhrman, S.T.; Ammerman, J.W. Sargasso Sea phosphorus biogeochemistry: An important role for dissolved organic phosphorus (DOP). Biogeosciences 2010, 7, 695–710. [Google Scholar] [CrossRef] [Green Version]
- Whitney, L.A.P.; Lomas, M.W. Phosphonate utilization by eukaryotic phytoplankton. Limnol. Oceanogr. Lett. 2019, 4, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.L.; Wang, Q.Y.; Zhang, Y.F.; Zhang, W.L.; Li, L. Characteristics of seawater nutrients during the occurrence of brown tide in the coastal area of Qinhuangdao, China. Ying Yong Sheng Tai Xue Bao J. Appl. Ecol. 2020, 31, 282–292. [Google Scholar]
- Wang, Y.; Sun, Y.; Wang, C.; Chen, W.; Hu, X. Net-phytoplankton community structure and its environmental correlations in central Bohai Sea and the Bohai Strait. Aquat. Ecosyst. Health Manag. 2019, 22, 481. [Google Scholar] [CrossRef]
- Zhuming; Lian, Y. Research on the Nitrogen and Phosphorus Requirement of Thalassiosira sp. J. Aquac. 2004, 1, 33–36. [Google Scholar]
- Chen, X.-H.; Li, Y.-Y.; Zhang, H.; Liu, J.-L.; Xie, Z.-X.; Lin, L.; Wang, D.-Z. Quantitative proteomics reveals common and specific responses of a marine diatom Thalassiosira pseudonana to different macronutrient deficiencies. Front. Microbiol. 2018, 9, 2761. [Google Scholar] [CrossRef]
- Mooy, B.V.; Fredricks, H.F.; Pedler, B.E.; Dyhrman, S.T.; Karl, D.M.; Koblížek, M.; Lomas, M.W.; Mincer, T.J.; Moore, L.R.; Moutin, T. Phytoplankton in the ocean use non-phosphorus lipids in response to phosphorus scarcity. Nature 2009, 458, 69. [Google Scholar] [CrossRef]
- Yu, L.; Rong-cheng, L. Research on red tide occurrences using enclosed experimental ecosystem in West Xiamen Harbor, China—Relationship between nutrients and red tide occurrence. Chin. J. Oceanol. Limnol. 2000, 18, 253–259. [Google Scholar] [CrossRef]
- Gao, G.; Xia, J.; Yu, J.; Fan, J.; Zeng, X. Regulation of inorganic carbon acquisition in a red tide alga (Skeletonema costatum): The importance of phosphorus availability. Biogeosciences 2018, 15, 4871–4882. [Google Scholar] [CrossRef] [Green Version]
- Anderson, D.M.; Fensin, E.; Gobler, C.J.; Hoeglund, A.E.; Hubbard, K.A.; Kulis, D.M.; Landsberg, J.H.; Lefebvre, K.A.; Provoost, P.; Richlen, M.L. Marine harmful algal blooms (HABs) in the United States: History, current status and future trends. Harmful Algae 2021, 102, 101975. [Google Scholar] [CrossRef]
- Shetye, S.S.; Bandekar, M.; Nandakumar, K.; Kurian, S.; Gauns, M.; Jawak, S.; Pratihary, A.; Elangovan, S.S.; Naik, B.R.; Lakshmi, S. Sea foam-associated pathogenic bacteria along the west coast of India. Environ. Monit. Assess. 2021, 193, 27. [Google Scholar] [CrossRef] [PubMed]
- Halsband-Lenk, C.; Pierson, J.J.; Leising, A.W. Reproduction of Pseudocalanus newmani (Copepoda: Calanoida) is deleteriously affected by diatom blooms—A field study. Prog. Oceanogr. 2005, 67, 332–348. [Google Scholar] [CrossRef]
- Yuzao, Q.; Changjiang, H. The causative mechanism of Chatonella marina bloom in Dapeng Bay, the South China Sea. Oceanol. Limnol. Sin. 1997, 28, 337–342. [Google Scholar]
- Hallegraeff, G. Chattonella marina raphidophyte bloom associated with mortality of cultured bluefin tuna (Thunnus maccoyii) in South Australia. Harmful Algae 1998, 105, 414–417. [Google Scholar]
- Landsberg, J.H. The effects of harmful algal blooms on aquatic organisms. Rev. Fish. Sci. 2002, 10, 113–390. [Google Scholar] [CrossRef]
- Ishimatsu, A.; Maruta, H.; Oda, T.; Ozaki, M. A comparison of physiological responses in yellowtail to fatal environmental hypoxia and exposure to Chattonella marina. Fish. Sci. 1997, 63, 557–562. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.; Ishimatsu, A.; Sakaguchi, H.; Oda, T. Cardiac output during exposure to Chattonella marina and environmental hypoxia in yellowtail (Seriola quinqueradiata). Mar. Biol. 2003, 142, 391–397. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Chen, Z.; Feng, X.; Wang, G.; Wang, G.; Zhang, J. Daily Samples Revealing Shift in Phytoplankton Community and Its Environmental Drivers during Summer in Qinhuangdao Coastal Area, China. Water 2022, 14, 1625. https://doi.org/10.3390/w14101625
He Y, Chen Z, Feng X, Wang G, Wang G, Zhang J. Daily Samples Revealing Shift in Phytoplankton Community and Its Environmental Drivers during Summer in Qinhuangdao Coastal Area, China. Water. 2022; 14(10):1625. https://doi.org/10.3390/w14101625
Chicago/Turabian StyleHe, Yike, Zuoyi Chen, Xin Feng, Guangyi Wang, Gang Wang, and Jiabo Zhang. 2022. "Daily Samples Revealing Shift in Phytoplankton Community and Its Environmental Drivers during Summer in Qinhuangdao Coastal Area, China" Water 14, no. 10: 1625. https://doi.org/10.3390/w14101625
APA StyleHe, Y., Chen, Z., Feng, X., Wang, G., Wang, G., & Zhang, J. (2022). Daily Samples Revealing Shift in Phytoplankton Community and Its Environmental Drivers during Summer in Qinhuangdao Coastal Area, China. Water, 14(10), 1625. https://doi.org/10.3390/w14101625







