Dynamic Adsorption of As(V) onto the Porous α-Fe2O3/Fe3O4/C Composite Prepared with Bamboo Bio-Template
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization
2.1.1. Materials and Reagents
2.1.2. Sample Preparation
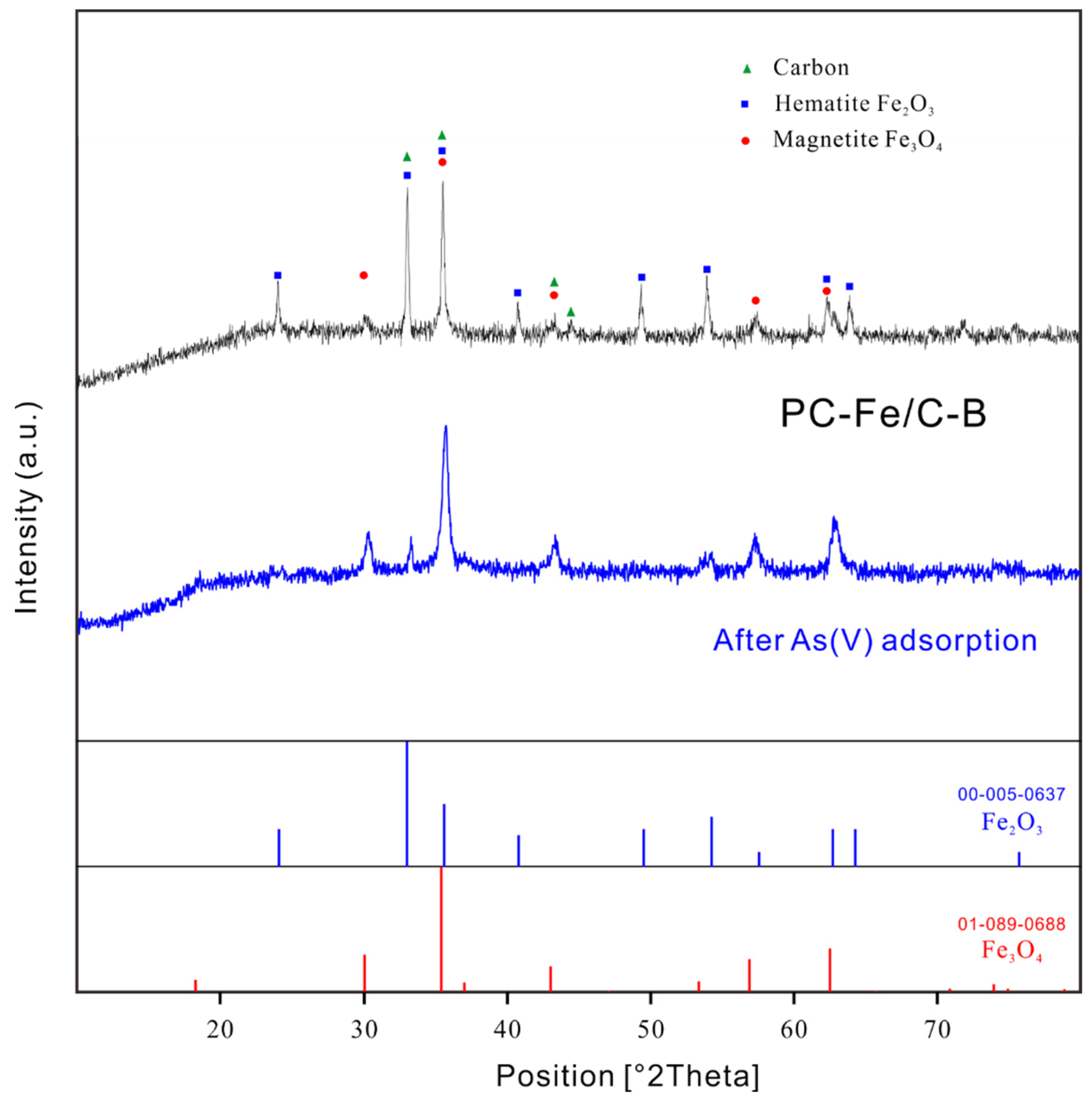
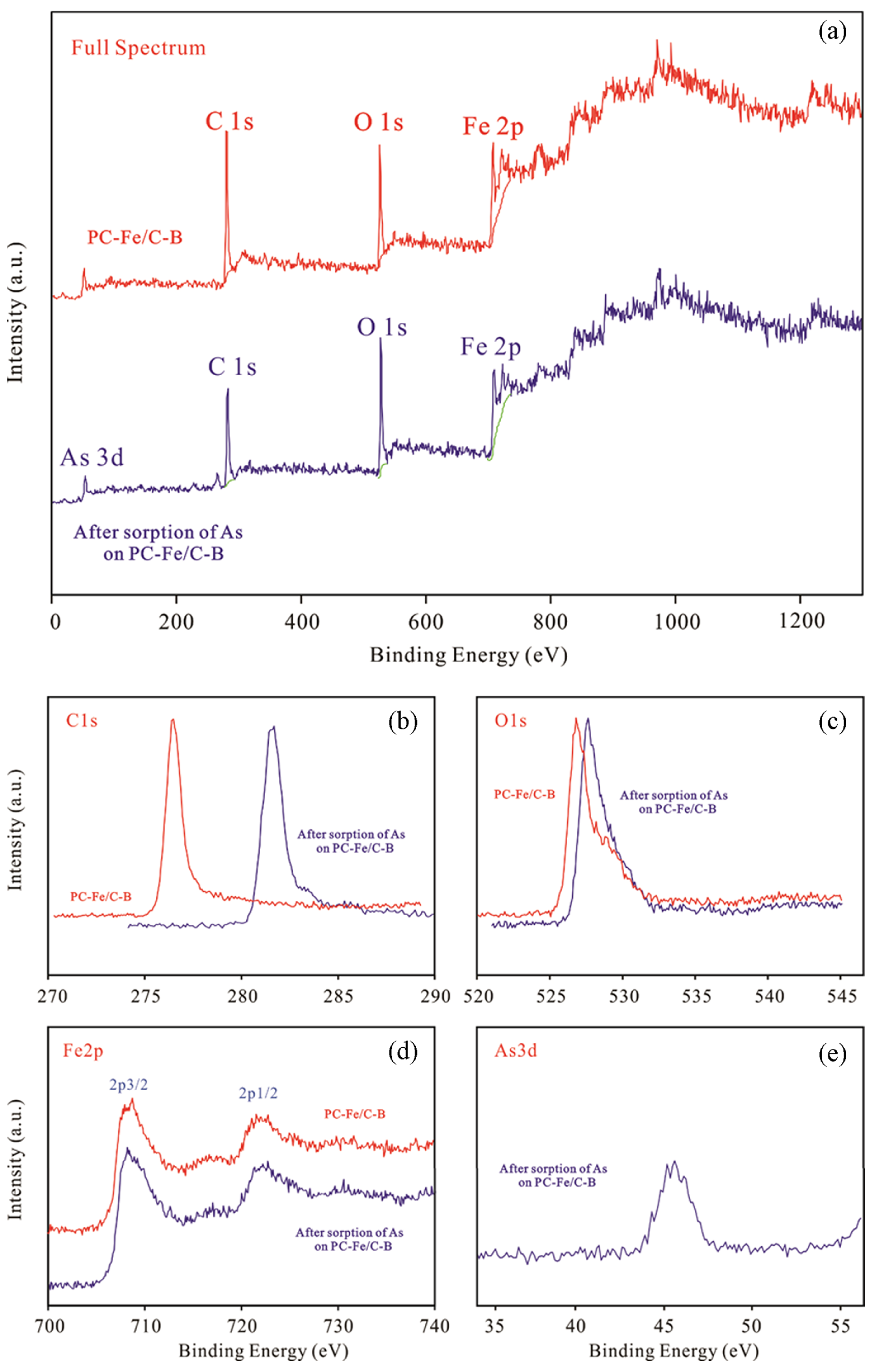
2.1.3. Characterization of PC-Fe/C-B Sorbent
2.2. Arsenate Adsorption to PC-Fe/C-B
2.3. Modeling
3. Results and Discussion
3.1. Characterization of PC-Fe/C-B
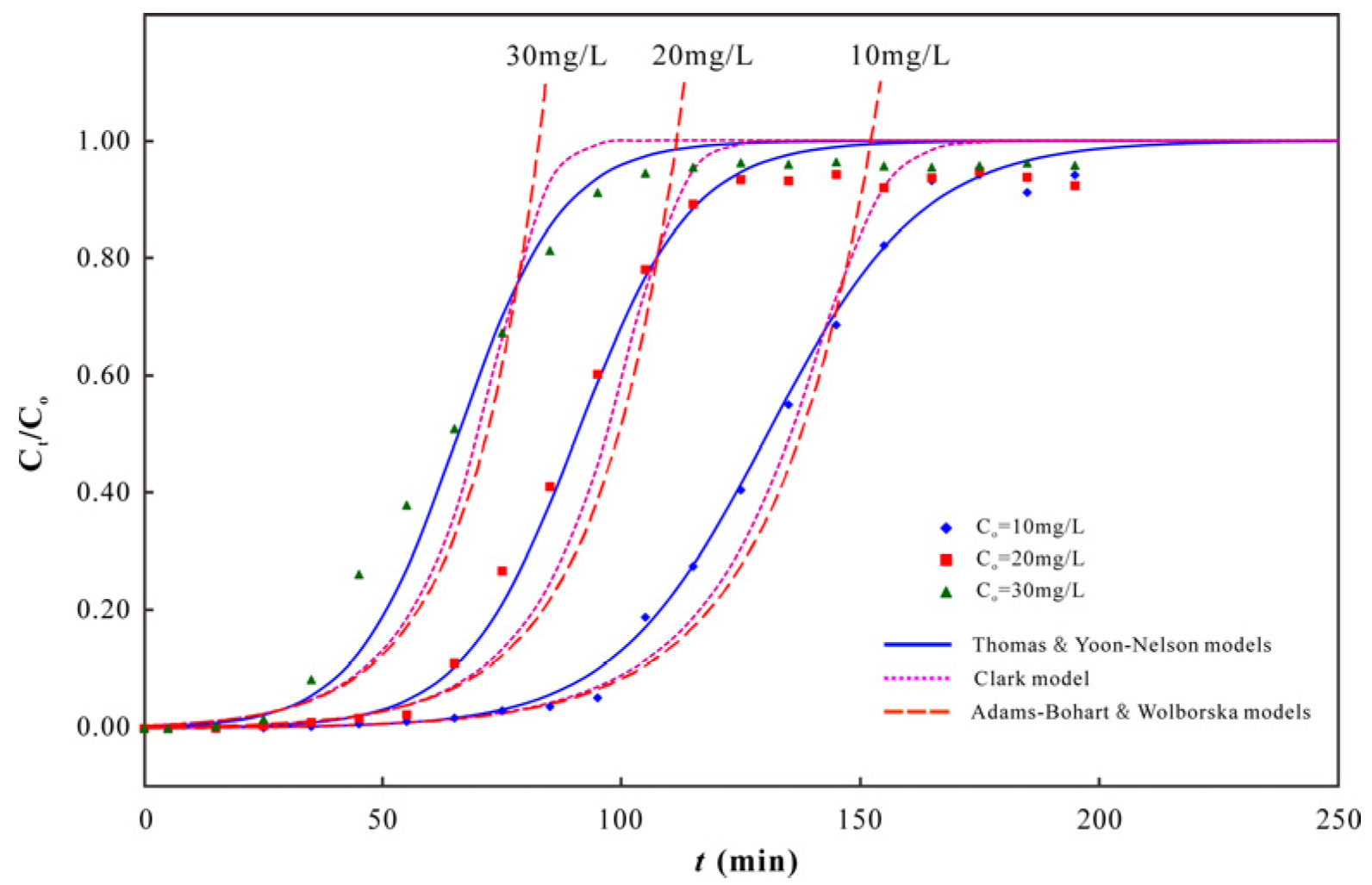
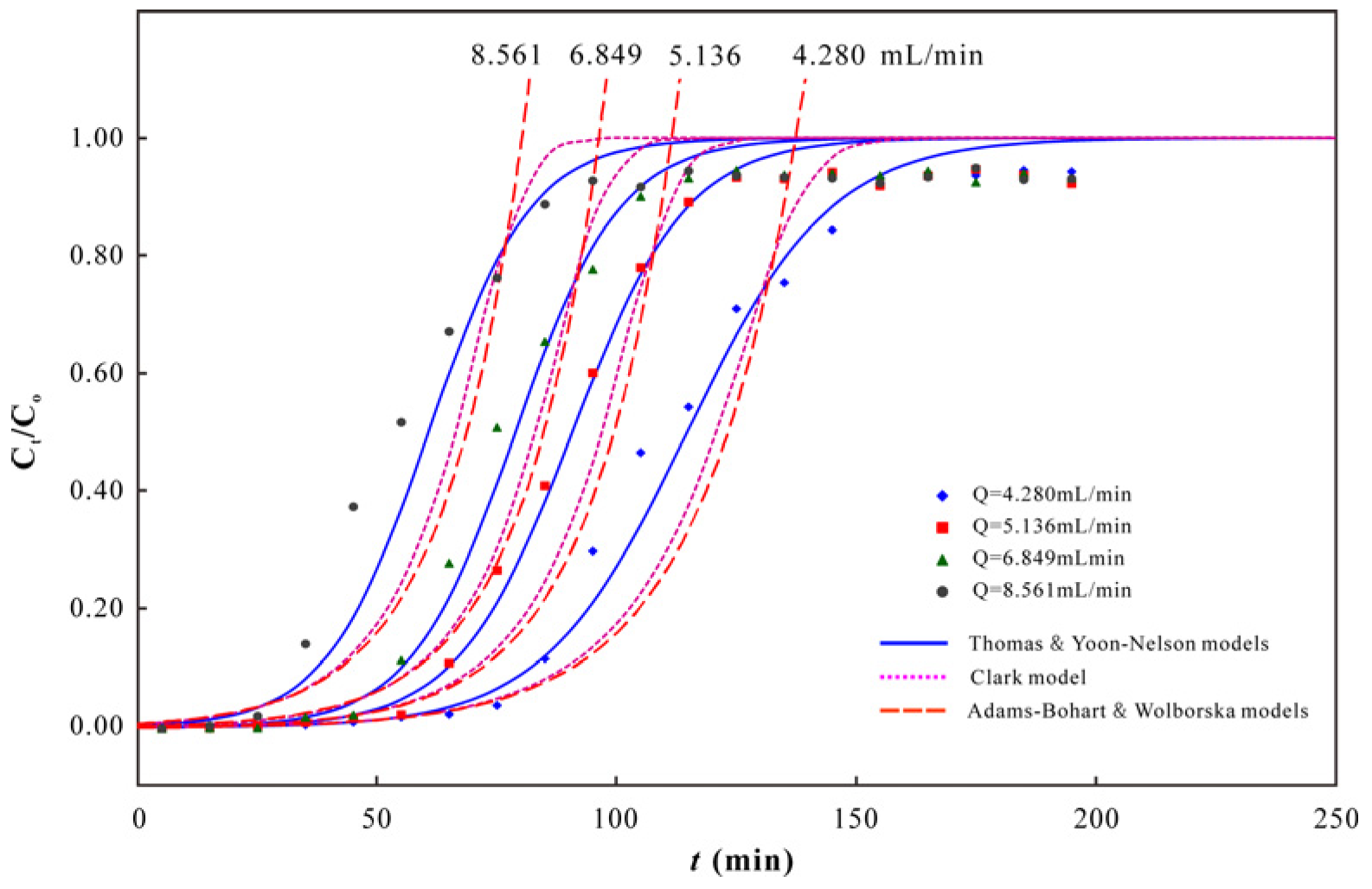
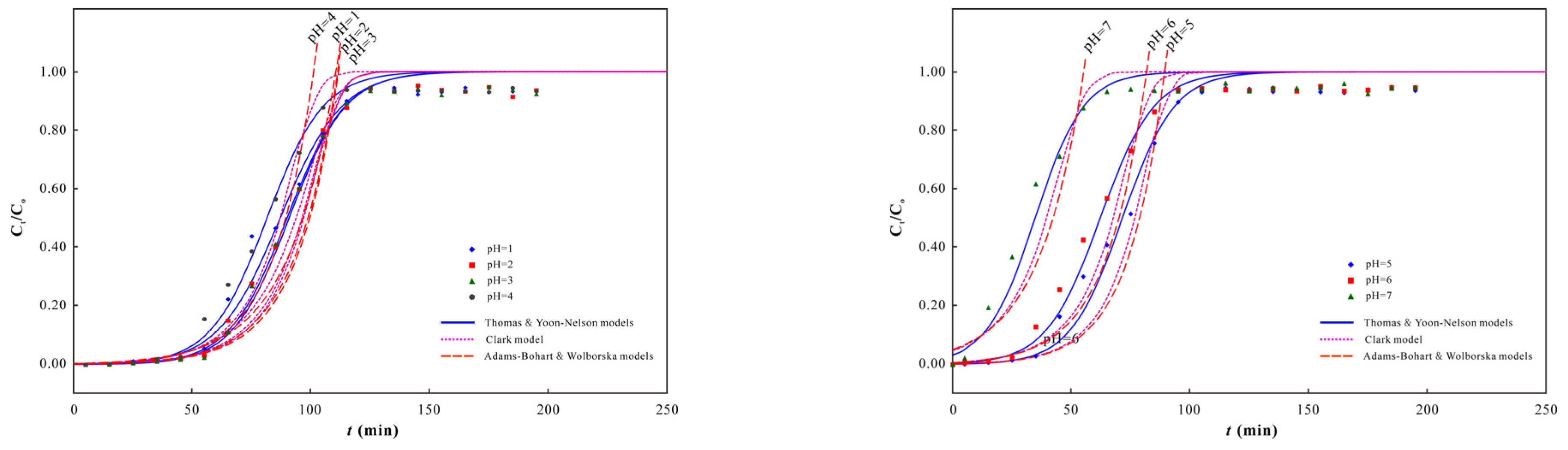
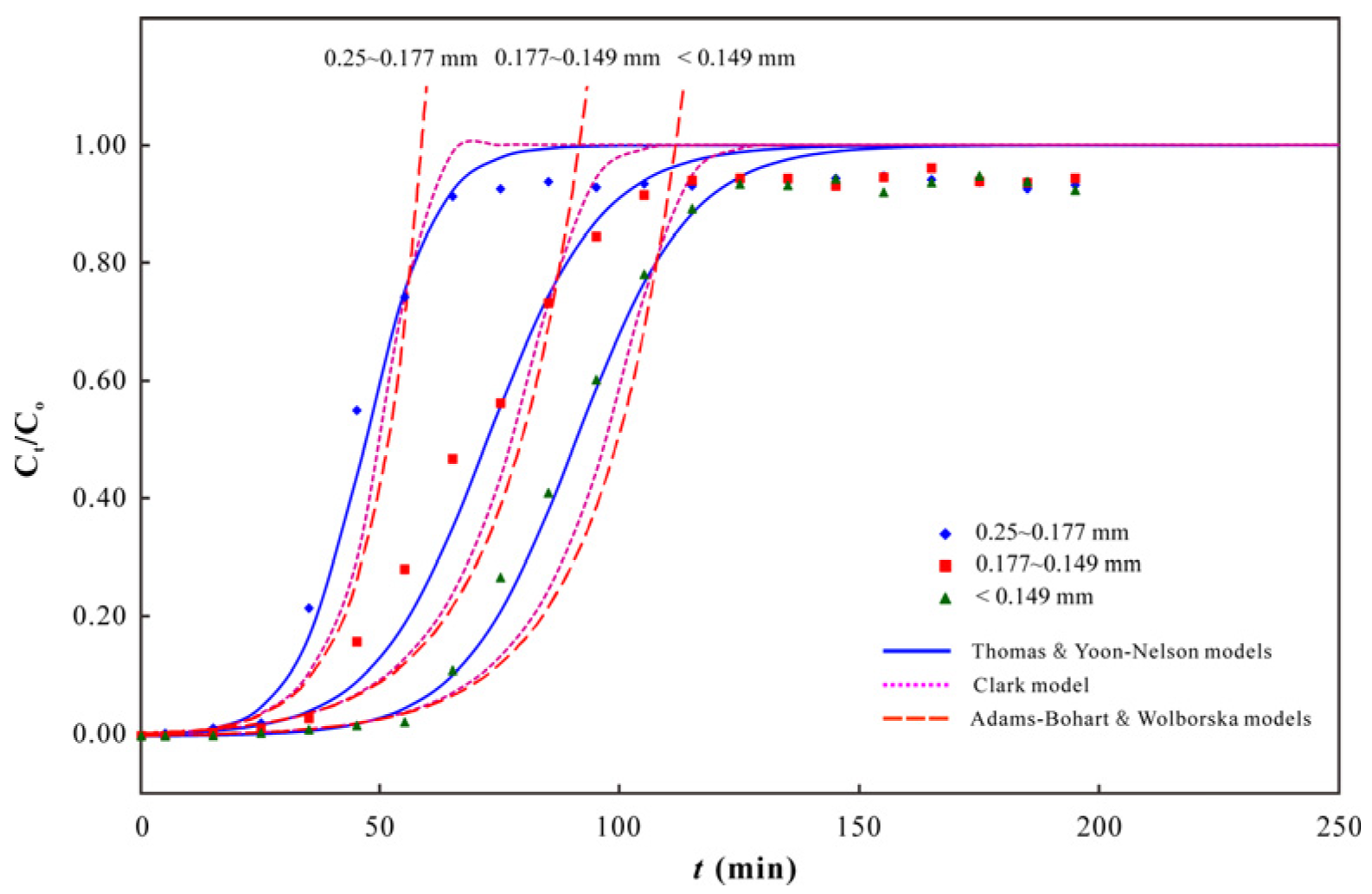
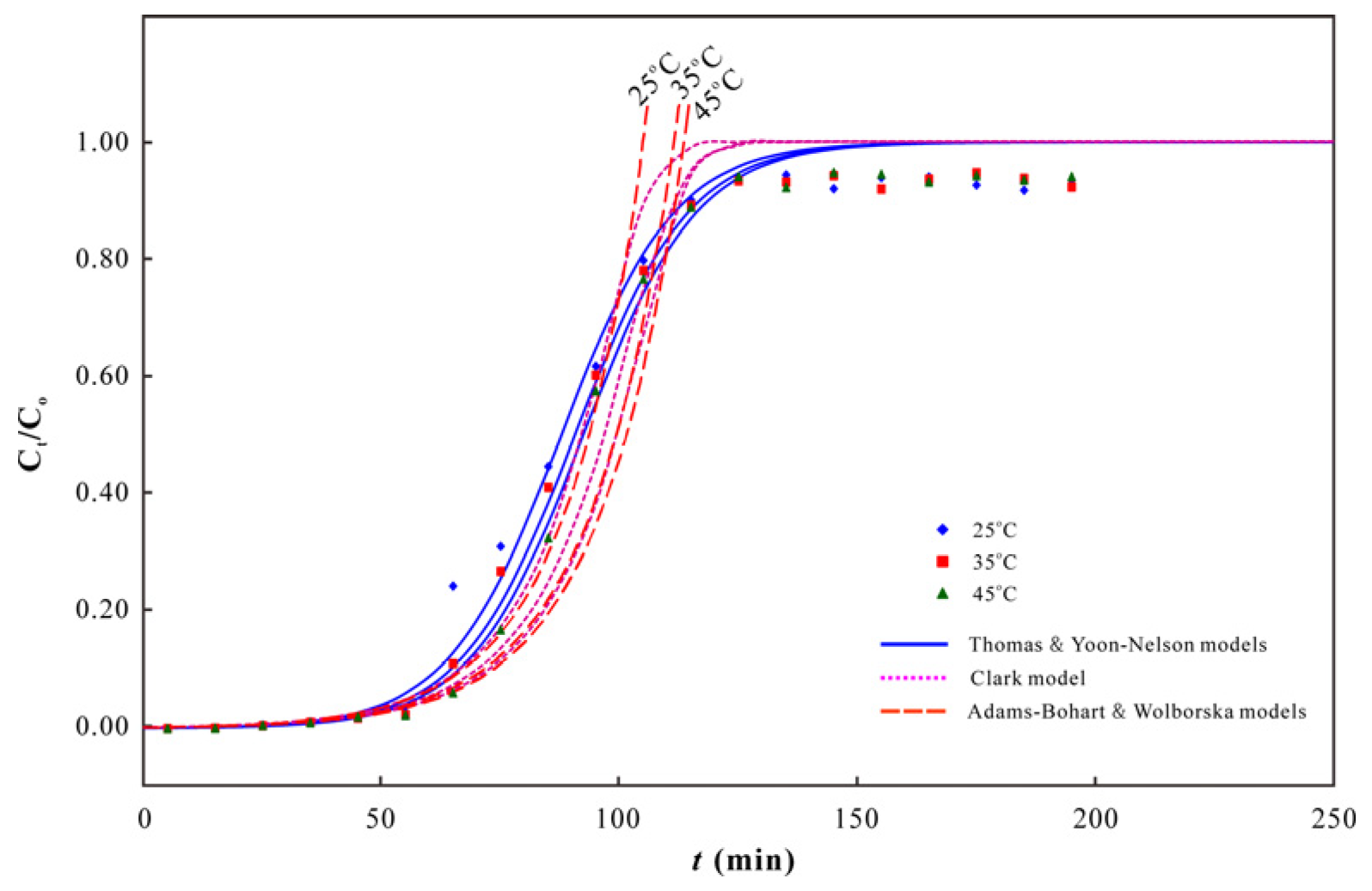
3.2. As(V) Dynamic Adsorption
3.3. As(V) Adsorption Capacity of PC-Fe/C-B
3.4. CD-MUSIC Modeling
3.4.1. Simulation Parameters of CD-MUSIC Model with PHREEQC
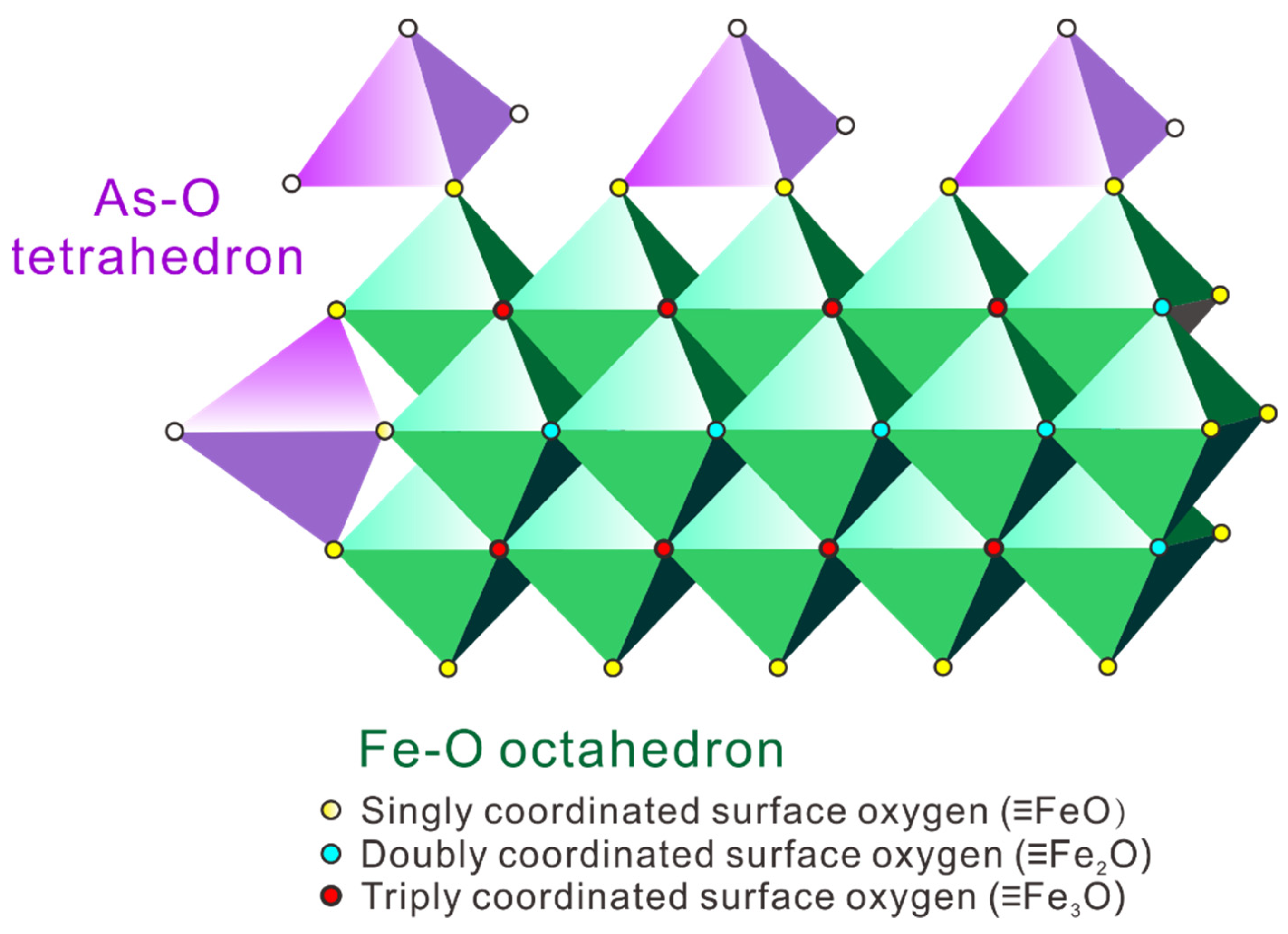
3.4.2. Simulation Calculation of Solid-Surface-Complexing State
3.4.3. Simulation of Reactive Transportation in Dynamic-Adsorption Process Using PHREEQC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Samuel, M.S.; Selvarajan, E.; Sarswat, A.; Muthukumar, H.; Jacob, J.M.; Mukesh, M.; Pugazhendhi, A. Nanomaterials as adsorbents for As (III) and As (V) removal from water: A review. J. Hazard. Mater. 2022, 424, 127572. [Google Scholar] [CrossRef] [PubMed]
- Purohit, S.; Chini, M.K.; Chakraborty, T.; Yadav, K.L.; Satapathi, S. Rapid removal of arsenic from water using metal oxide doped recyclable cross-linked chitosan cryogel. SN Appl. Sci. 2020, 2, 768. [Google Scholar] [CrossRef]
- Carneiro, M.A.; Pintor, A.; Boaventura, R.A.; Botelho, C. Current trends of arsenic adsorption in continuous mode: Literature review and future perspectives. Sustainability 2021, 13, 1186. [Google Scholar] [CrossRef]
- Liao, T.; Xi, Y.; Zhang, L.; Li, J.; Cui, K. Removal of toxic arsenic (As (Ⅲ)) from industrial wastewater by ultrasonic enhanced zero-valent lead combined with CuSO4. J. Hazard. Mater. 2021, 408, 124464. [Google Scholar] [CrossRef]
- Kumar, A.; Bhattacharya, T.; Shaikh, W.A.; Roy, A.; Mukherjee, S.; Kumar, M. Performance evaluation of crop residue and kitchen waste-derived biochar for eco-efficient removal of arsenic from soils of the Indo-Gangetic plain: A step towards sustainable pollution management. Environ. Res. 2021, 200, 111758. [Google Scholar] [CrossRef]
- Yang, X.; Li, J.; Liang, T.; Yan, X.; Zhong, L.; Shao, J.; El-Naggar, A.; Guan, C.; Liu, J.; Zhou, Y. A combined management scheme to simultaneously mitigate As and Cd concentrations in rice cultivated in contaminated paddy soil. J. Hazard. Mater. 2021, 416, 125837. [Google Scholar] [CrossRef]
- Lee, Y.; Ren, Y.; Cui, M.; Zhou, Y.; Kwon, O.; Ko, J.; Khim, J. Arsenic adsorption study in acid mine drainage using fixed bed column by novel beaded adsorbent. Chemosphere 2022, 291, 132894. [Google Scholar] [CrossRef]
- Glass, S.; Mantel, T.; Appold, M.; Sen, S.; Usman, M.; Ernst, M.; Filiz, V. Amine-terminated PAN membranes as anion-adsorber materials. Chem. Ing. Tech. 2021, 93, 1396–1400. [Google Scholar] [CrossRef]
- Asere, T.G.; Stevens, C.V.; Du Laing, G. Use of (modified) natural adsorbents for arsenic remediation: A review. Sci. Total Environ. 2019, 676, 706–720. [Google Scholar] [CrossRef]
- Gu, Y.; Xie, D.; Wang, Y.; Qin, W.; Zhang, H.; Wang, G.; Zhang, Y.; Zhao, H. Facile fabrication of composition-tunable Fe/Mg bimetal-organic frameworks for exceptional arsenate removal. Chem. Eng. J. 2019, 357, 579–588. [Google Scholar] [CrossRef]
- Ali, S.; Rizwan, M.; Shakoor, M.B.; Jilani, A.; Anjum, R. High sorption efficiency for As (III) and As (V) from aqueous solutions using novel almond shell biochar. Chemosphere 2020, 243, 125330. [Google Scholar] [CrossRef]
- Lan, B.; Wang, Y.; Wang, X.; Zhou, X.; Kang, Y.; Li, L. Aqueous arsenic (As) and antimony (Sb) removal by potassium ferrate. Chem. Eng. J. 2016, 292, 389–397. [Google Scholar] [CrossRef]
- Ramirez-Muñiz, K.; Perez-Rodriguez, F.; Rangel-Mendez, R. Adsorption of arsenic onto an environmental friendly goethite-polyacrylamide composite. J. Mol. Liq. 2018, 264, 253–260. [Google Scholar] [CrossRef]
- Navarathna, C.M.; Karunanayake, A.G.; Gunatilake, S.R.; Pittman, C.U., Jr.; Perez, F.; Mohan, D.; Mlsna, T. Removal of Arsenic (III) from water using magnetite precipitated onto Douglas fir biochar. J. Environ. Manag. 2019, 250, 109429. [Google Scholar] [CrossRef]
- Chen, M.L.; Sun, Y.; Huo, C.B.; Liu, C.; Wang, J.H. Akaganeite decorated graphene oxide composite for arsenic adsorption/removal and its proconcentration at ultra-trace level. Chemosphere 2015, 130, 52–58. [Google Scholar] [CrossRef]
- Usman, M.; Zarebanadkouki, M.; Waseem, M.; Katsoyiannis, I.A.; Ernst, M. Mathematical modeling of arsenic (V) adsorption onto iron oxyhydroxides in an adsorption-submerged membrane hybrid system. J. Hazard. Mater. 2020, 400, 123221. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, T.; Zhu, Y.; Abass, O.K.; Liu, L.; Su, C.; Shan, H. Application of siderite tailings in water-supply well for As removal: Experiments and field tests. Int. Biodeterior. Biodegrad. 2018, 128, 85–93. [Google Scholar] [CrossRef]
- Stachowicz, M.; Hiemstra, T.; Riemsdijk, W. Surface speciation of As(III) and As(V) in relation to charge distribution. J. Colloid Interface Sci. 2006, 302, 62–75. [Google Scholar] [CrossRef]
- Huang, W.; Xu, H.; Liu, X.; Wang, L.; Li, S.; Ji, L.; Qu, Z.; Yan, N. Surface protection method for the magnetic core using covalent organic framework shells and its application in As (III) depth removal from acid wastewater. J. Environ. Sci. 2022, 115, 1–9. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, Z.; Chen, Y.; Yang, F.; Hui, Q. Kinetics and thermodynamics of sorption for As(V) on the porous biomorph-genetic composite of α-Fe2O3/Fe3O4/C with eucalyptus wood hierarchical microstructure. Water Air Soil Pollut. 2013, 224, 1589. [Google Scholar] [CrossRef]
- Hiemstra, T.; Van Riemsdijk, W.H. A surface structural approach to ion adsorption: The charge distribution (CD) model. J. Colloid Interface Sci. 1996, 179, 488–508. [Google Scholar] [CrossRef]
- Deng, Y.; Li, Y.; Li, X.; Wang, L.; Ma, J.; Chen, Y.; Weng, L. Development and application of chemical speciation models for heavy metals in environmental soil samples. J. Agro-Environ. Sci. 2018, 37, 1350–1361. [Google Scholar]
- Salazar-Camacho, C.; Villalobos, M. Goethite surface reactivity: III. Unifying arsenate adsorption behavior through a variable crystal face-site density model. Geochim. Cosmochim. Acta 2010, 74, 2257–2280. [Google Scholar] [CrossRef]
- Gustafsson, J.P. Modelling competitive anion adsorption on oxide minerals and an allophane-containing soil. Eur. J. Soil Sci. 2001, 52, 639–653. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, J.; Koopal, L.K.; Wang, M.; Xiong, J.; Hou, J.; Tan, W. Facet-dependent surface charge and Pb2+ adsorption characteristics of hematite nanoparticles: CD-MUSIC-eSGC modeling. Environ. Res. 2021, 196, 110383. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C. Description of Input and Examples for PHREEQC Version 3: A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; US Geological Survey: Reston, VA, USA, 2013; pp. 2328–7055. [Google Scholar]
- Thomas, H.C. Heterogeneous ion exchange in a flowing system. J. Am. Chem. Soc. 1944, 66, 1664–1666. [Google Scholar] [CrossRef]
- Yoon, Y.H.; Nelson, J.H. Application of gas adsorption kinetics I. A theoretical model for respirator cartridge service life. Am. Ind. Hyg. Assoc. J. 1984, 45, 509–516. [Google Scholar] [CrossRef]
- Yoon, Y.H.; Nelson, J.H. Application of gas adsorption kinetics—II. A theoretical model for respirator cartridge service life and its practical applications. Am. Ind. Hyg. Assoc. J. 1984, 45, 517–524. [Google Scholar] [CrossRef]
- Bohart, G.; Adams, E. Some aspects of the behavior of charcoal with respect to chlorine. J. Am. Chem. Soc. 1920, 42, 523–544. [Google Scholar] [CrossRef]
- Clark, R.M. Evaluating the cost and performance of field-scale granular activated carbon systems. Environ. Sci. Technol. 1987, 21, 573–580. [Google Scholar] [CrossRef]
- Wolborska, A.; Pustelnik, P. A simplified method for determination of the break-through time of an adsorbent layer. Water Res. 1996, 30, 2643–2650. [Google Scholar] [CrossRef]
- Vaughan, R.L., Jr.; Reed, B.E. Modeling As (V) removal by a iron oxide impregnated activated carbon using the surface complexation approach. Water Res. 2005, 39, 1005–1014. [Google Scholar] [CrossRef]
- Vaughan, R.L., Jr.; Reed, B.E.; Smith, E.H. Modeling As (V) removal in iron oxide impregnated activated carbon columns. J. Environ. Eng. 2007, 133, 121–124. [Google Scholar] [CrossRef]
- Vithanage, M.; Rajapaksha, A.U.; Dou, X.; Bolan, N.S.; Yang, J.E.; Ok, Y.S. Surface complexation modeling and spectroscopic evidence of antimony adsorption on iron-oxide-rich red earth soils. J. Colloid Interface Sci. 2013, 406, 217–224. [Google Scholar] [CrossRef]
- Moulder, J.; Stickle, W.; Sobol, P.; Bomben, K. Handbook of X-ray Photoelectron Spectroscopy; Chastain, J., Ed.; Perkin-Elmer Corporation: Waltham, MA, USA, 1992. [Google Scholar]
- Costa, E.; Guilherme, L.; Lopes, G.; Lima, J.; Curi, N. Competitive sorption of arsenate and phosphate on aluminum mining by-product. Water Air Soil Pollut. 2012, 223, 5433–5444. [Google Scholar] [CrossRef]
- Misaelides, P.; Nikashina, V.; Godelitsas, A.; Gembitskii, P.; Kats, E. Sorption of As(V)-anions from aqueous solutions by organo-modified natural zeolitic materials. J. Radioanal. Nucl. Chem. 1998, 227, 183–186. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, S.; Shan, X.; Zhu, Y. Effects of oxalate and humic acid on arsenate sorption by and desorption from a Chinese red soil. Water Air Soil Pollut. 2006, 176, 269–283. [Google Scholar] [CrossRef][Green Version]
- Vieira, B.R.; Pintor, A.M.; Boaventura, R.A.; Botelho, C.M.; Santos, S.C. Arsenic removal from water using iron-coated seaweeds. J. Environ. Manag. 2017, 192, 224–233. [Google Scholar] [CrossRef]
- Usman, M.; Belkasmi, A.I.; Kastoyiannis, I.A.; Ernst, M. Pre-deposited dynamic membrane adsorber formed of microscale conventional iron oxide-based adsorbents to remove arsenic from water: Application study and mathematical modeling. J. Chem. Technol. Biotechnol. 2021, 96, 1504–1514. [Google Scholar] [CrossRef]
- Lee, C.; Jung, J.; Pawar, R.R.; Kim, M.; Lee, S.M. Arsenate and phosphate removal from water using Fe-sericite composite beads in batch and fixed-bed systems. J. Ind. Eng. Chem. 2017, 47, 375–383. [Google Scholar] [CrossRef]
- Wu, C.; Huang, L.; Xue, S.; Huang, Y.; Hartley, W.; Cui, M.; Wong, M. Arsenic sorption by red mud-modified biochar produced from rice straw. Environ. Sci. Pollut. Res. 2017, 24, 18168–18178. [Google Scholar] [CrossRef]
- Qian, W.; Zhao, A.; Xu, R. Sorption of As (V) by aluminum-modified crop straw-derived biochars. Water Air Soil Pollut. 2013, 224, 1610. [Google Scholar] [CrossRef]
- Yusof, A.M.; Malek, N.A.N.N. Removal of Cr (VI) and As (V) from aqueous solutions by HDTMA-modified zeolite Y. J. Hazard. Mater. 2009, 162, 1019–1024. [Google Scholar] [CrossRef]
- Alijani, H.; Shariatinia, Z. Effective aqueous arsenic removal using zero valent iron doped MWCNT synthesized by in situ CVD method using natural α-Fe2O3 as a precursor. Chemosphere 2017, 171, 502–511. [Google Scholar] [CrossRef]
- Han, L.; Sun, H.; Ro, K.S.; Sun, K.; Libra, J.A.; Xing, B. Removal of antimony (III) and cadmium (II) from aqueous solution using animal manure-derived hydrochars and pyrochars. Bioresour. Technol. 2017, 234, 77–85. [Google Scholar] [CrossRef]
- Jiang, W.; Chen, X.; Niu, Y.; Pan, B. Spherical polystyrene-supported nano-Fe3O4 of high capacity and low-field separation for arsenate removal from water. J. Hazard. Mater. 2012, 243, 319–325. [Google Scholar] [CrossRef]
- Luo, T.; Cui, J.; Hu, S.; Huang, Y.; Jing, C. Arsenic removal and recovery from copper smelting wastewater using TiO2. Environ. Sci. Technol. 2010, 44, 9094–9098. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, Y.; Cheng, Z. Removal of heavy metal ions using chitosan and modified chitosan: A review. J. Mol. Liq. 2016, 214, 175–191. [Google Scholar] [CrossRef]
- Mangold, J.E.; Park, C.M.; Liljestrand, H.M.; Katz, L.E. Surface complexation modeling of Hg (II) adsorption at the goethite/water interface using the Charge Distribution Multi-Site Complexation (CD-MUSIC) model. J. Colloid Interface Sci. 2014, 418, 147–161. [Google Scholar] [CrossRef]
- Sø, H.U.; Postma, D.; Jakobsen, R.; Larsen, F. Competitive adsorption of arsenate and phosphate onto calcite; experimental results and modeling with CCM and CD-MUSIC. Geochim. Cosmochim. Acta 2012, 93, 1–13. [Google Scholar] [CrossRef]
- Antelo, J.; Avena, M.; Fiol, S.; López, R.; Arce, F. Effects of pH and ionic strength on the adsorption of phosphate and arsenate at the goethite–water interface. J. Colloid Interface Sci. 2005, 285, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Antelo, J.; Arce, F.; Fiol, S. Arsenate and phosphate adsorption on ferrihydrite nanoparticles. Synergetic interaction with calcium ions. Chem. Geol. 2015, 410, 53–62. [Google Scholar] [CrossRef]
- Hayes, K.F.; Papelis, C.; Leckie, J.O. Modeling ionic strength effects on anion adsorption at hydrous oxide/solution interfaces. J. Colloid Interface Sci. 1988, 125, 717–726. [Google Scholar] [CrossRef]
- Dzombak, D.A.; Morel, F.M. Surface Complexation Modeling: Hydrous Ferric Oxide; John Wiley & Sons: Hoboken, NJ, USA, 1990. [Google Scholar]
- Xie, J.; Gu, X.; Tong, F.; Zhao, Y.; Tan, Y. Surface complexation modeling of Cr(VI) adsorption at the goethite–water interface. J. Colloid Interface Sci. 2015, 455, 55–62. [Google Scholar] [CrossRef]
- Cui, J.; Du, J.; Yu, S.; Jing, C.; Chan, T. Groundwater arsenic removal using granular TiO2: Integrated laboratory and field study. Environ. Sci. Pollut. Res. 2015, 22, 8224–8234. [Google Scholar] [CrossRef]






| As(V) Aqueous-Phase-Dissociation Reaction | logK |
|---|---|
| H3AsO4 = AsO43− + 3H+ | −20.7 |
| H+ + AsO43− = HAsO42− | 11.5 |
| 2 H+ + AsO43− = H2AsO4− | 18.46 |
| Surface Reaction of Adsorption of AsO43− by PC-Fe/C-B | logK |
|---|---|
| ≡FeOH = ≡FeO− + H+ | −8.93 |
| ≡FeOH + H+ = ≡FeOH2+ | 7.29 |
| 2 ≡FeOH + 2 H+ + AsO43− = ≡Fe2O2AsO2− + 2 H2O | 29.29 |
| 2 ≡FeOH + 3 H+ + AsO43− = ≡Fe2O2AsOOH + 2 H2O | 32.69 |
| ≡FeOH + 2 H+ + AsO43− = ≡FeOAsO2OH− + H2O | 26.62 |
| The surface site density of PC-Fe/C-B/(sites/nm2): 2.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Li, Y.; Tang, S.; Zhang, L.; Zhang, J.; Zhao, Y.; Zhang, X.; Zhu, Y. Dynamic Adsorption of As(V) onto the Porous α-Fe2O3/Fe3O4/C Composite Prepared with Bamboo Bio-Template. Water 2022, 14, 1848. https://doi.org/10.3390/w14121848
Peng Y, Li Y, Tang S, Zhang L, Zhang J, Zhao Y, Zhang X, Zhu Y. Dynamic Adsorption of As(V) onto the Porous α-Fe2O3/Fe3O4/C Composite Prepared with Bamboo Bio-Template. Water. 2022; 14(12):1848. https://doi.org/10.3390/w14121848
Chicago/Turabian StylePeng, Yuqing, Yanhong Li, Shen Tang, Lihao Zhang, Jing Zhang, Yao Zhao, Xuehong Zhang, and Yinian Zhu. 2022. "Dynamic Adsorption of As(V) onto the Porous α-Fe2O3/Fe3O4/C Composite Prepared with Bamboo Bio-Template" Water 14, no. 12: 1848. https://doi.org/10.3390/w14121848
APA StylePeng, Y., Li, Y., Tang, S., Zhang, L., Zhang, J., Zhao, Y., Zhang, X., & Zhu, Y. (2022). Dynamic Adsorption of As(V) onto the Porous α-Fe2O3/Fe3O4/C Composite Prepared with Bamboo Bio-Template. Water, 14(12), 1848. https://doi.org/10.3390/w14121848






