Horizontal Distribution and Carbon Biomass of Planktonic Foraminifera in the Eastern Indian Ocean
Abstract
:1. Introduction
2. Materials and Methods
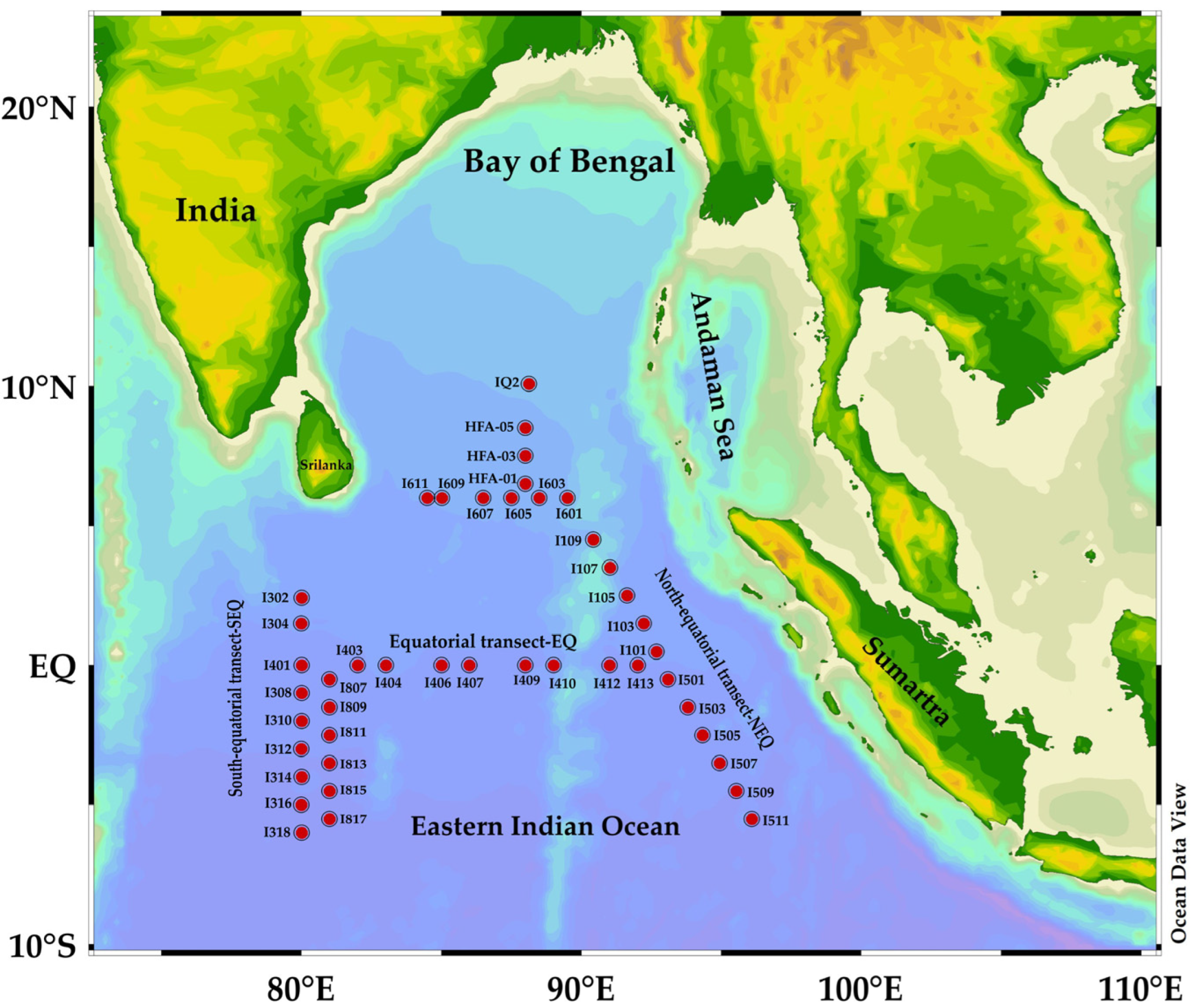
2.1. Study Area
2.2. CTD Data Collection
2.3. Plankton Tow Sampling and Cell Counting Method
2.4. Analysis of Diversity Measurements and Calculation Method
2.5. Carbon Biomass Conversion Factor
2.6. Graphics and Statistical Analysis
3. Results
3.1. Vertical Distribution of Temperature, Salinity, and Chlorophyll-a in the Eastern Indian Ocean
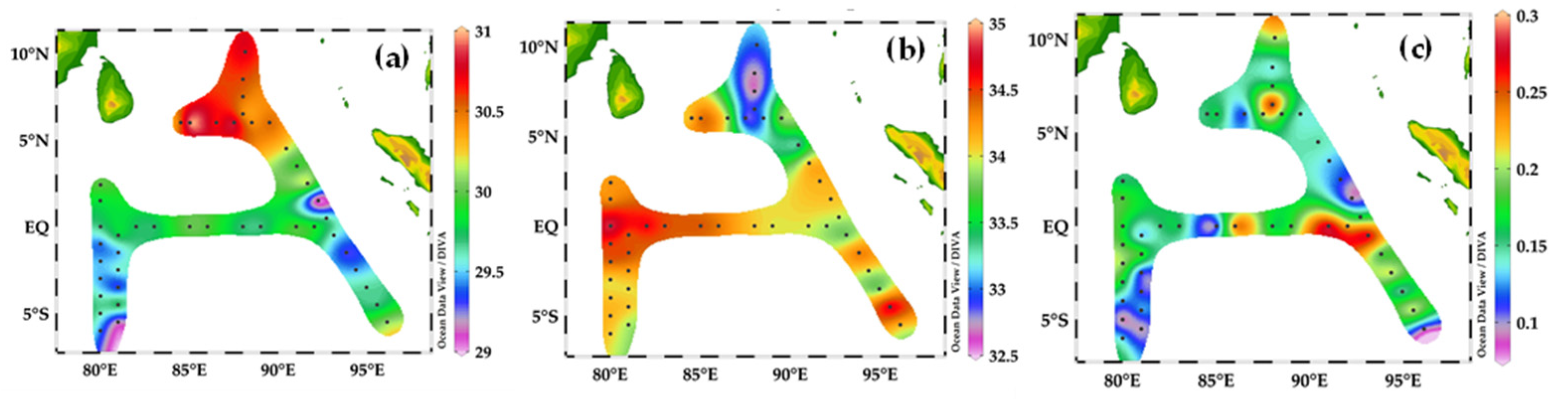
3.2. Horizontal Distribution of Temperature, Salinity, and Chlorophyll a
3.3. Community Structure and Diversity Pattern
3.4. Horizontal Distribution of Foraminifera and Their Contributions to Carbon Biomass
3.5. Carbon Biomass of Planktonic Foraminifera Species in the Eastern Indian Ocean
3.6. Correlation Analysis
4. Discussion
- (a)
- Foraminifera assemblages, horizontal distribution, and water masses
- (b)
- Contribution of foraminifera to the carbon biomass in the Eastern Indian Ocean
- (c)
- Environmental Factors affecting foraminifera assemblages
5. Conclusions
- (a)
- High abundance and carbon biomass of total foraminifera were recorded in the equatorial region.
- (b)
- Temperature and Chlorophyll-a are two main factors that trigger the dominant foraminifera species at a significant p > 0.05 level.
- (c)
- The foraminifera species with a size of approximately 150–188 μm is considered to be a major exporter of carbon from the Eastern Indian Ocean.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, Y.; Zhang, G.; Chen, J.; Wang, J.; Ding, C.; Zhang, X.; Sun, J. Dynamic responses of picophytoplankton to physicochemical variation in the eastern Indian Ocean. Ecol. Evol. 2019, 9, 5003–5017. [Google Scholar] [CrossRef]
- Adl, S.M.; Simpson, G.B.; Farmer, M.A.; Andersen, R.A.; Anderson, O.R.; Barta, J.R.; Bowser, S.S.; Brugerolle, G.; Fensome, R.A.; Fredericq, S.; et al. The new higher-level classification of Eukaryotes with Emphasis on the taxonomy of Protists. J. Eukaryot. Microbiol. 2005, 5, 399–451. [Google Scholar] [CrossRef]
- Hayward, B.W.; Le Coze, F.; Gross, D. World Foraminifera Database. Globigerina World Register of Marine Species. 2018. Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=112197 (accessed on 22 April 2022).
- Munir, S.; Sun, J. Modern planktonic foraminifera from the eastern Indian Ocean. Acta Oceanol. Sin. 2018, 37, 46–63. [Google Scholar] [CrossRef]
- Fenton, I.S.; Pearson, P.N.; Dunkley Jones, T.; Purvis, A. Environmental predictors of diversity in recent planktonic foraminifera as recorded in marine sediments. PLoS ONE 2016, 11, e0165522. [Google Scholar] [CrossRef] [Green Version]
- Schiebel, R.; Hemleben, C. Planktic Foraminifers in the Modern Ocean; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–358. [Google Scholar]
- Giamali, C.; Koskeridou, E.; Antonarakou, A.; Ioakim, C.; Kontakiotis, G.; Karageorgis, A.P.; Roussakis, G.; Karakitsios, V. Multiproxy ecosystem response of abrupt Holocene climatic changes in the northeastern Mediterranean sedimentary archive and hydrologic regime. Quat. Res. 2019, 92, 665–685. [Google Scholar] [CrossRef]
- Martinez, J.I.; Taylor, L.; De Deckker, P.; Barrows, T. Planktonic foraminifera from the Eastern Indian Ocean: Distribution and ecology in relation to the Western Pacific Warm Pool (WPWP). Mar. Micropaleontol. 1998, 34, 121–151. [Google Scholar] [CrossRef]
- Malmgren, B.A.; Kucera, M.; Nyberg, J.; Waelbroeck, C. Comparison of statistical and artificial neural network techniques for estimating past sea surface temperatures from planktonic foraminifer census data. Paleoceanography 2001, 16, 520–530. [Google Scholar] [CrossRef]
- Feldberg, M.J.; Mix, A.C. Planktonic foraminifera, sea surface temperatures, and mechanisms of oceanic change in the Peru and south equatorial currents, 0–150 ka BP. Paleoceanography 2003, 18, 1016. [Google Scholar] [CrossRef]
- Fraile, I.; Schulz, M.; Mulitza, S.; Kucera, M. Predicting the global distribution of planktonic foraminifera using a dynamic ecosystem model. Biogeosciences 2008, 5, 891–911. [Google Scholar] [CrossRef] [Green Version]
- Kroeker, K.J. Impacts of ocean acidification on marine organisms: Quantifying sensitivities and interaction with warming. Global Chang. Biol. 2013, 19, 1884–1896. [Google Scholar] [CrossRef] [Green Version]
- Barker, S.; Elderfield, H. Foraminiferal calcification response to glacial-interglacial changes in atmospheric CO2. Science 2002, 297, 833–836. [Google Scholar] [CrossRef]
- Zarkogiannis, S.D.; Antonarakou, A.; Tripati, A.; Kontakiotis, G.; Mortyn, P.G.; Drinia, H.; Greaves, M. Influence of surface ocean density on planktonic foraminifera calcification. Sci. Rep. 2019, 9, 533. [Google Scholar] [CrossRef] [PubMed]
- Kontakiotis, G.; Efstathiou, E.; Zarkogiannis, S.D.; Besiou, E.; Antonarakou, A. Latitudinal Differentiation among Modern Planktonic Foraminiferal Populations of Central Mediterranean: Species–Specific Distribution Patterns and Size Variability. J. Mar. Sci. Eng. 2021, 9, 551. [Google Scholar] [CrossRef]
- Morey, A.E.; Mix, A.C.; Pisias, N.G. Planktonic foraminiferal assemblages preserved in surface sediments correspond to multiple environment variables. Quat. Sci. Rev. 2005, 24, 925–950. [Google Scholar] [CrossRef]
- Seears, H. Biogeography, and Phylogenetics of the Planktonic Foraminifera. Ph.D. Thesis, University of Nottingham, Nottingham, UK, 2011. [Google Scholar]
- BouDagher-Fadel, M.K. Biostratigraphic and Geological Significance of Planktonic Foraminifera, 2nd ed.; UCL Press: London, UK, 2015. [Google Scholar] [CrossRef] [Green Version]
- Kontakiotis, G.; Butiseacă, G.A.; Antonarakou, A.; Agiadi, K.; Zarkogiannis, S.D.; Krsnik, E.; Besiou, E.; Zachariasse, W.J.; Lourens, L.; Thivaiou, D.; et al. Hypersalinity accompanies tectonic restriction in the eastern Mediterranean prior to the Messinian Salinity Crisis. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2022, 592, 110903. [Google Scholar] [CrossRef]
- Frerichs, W.E.; Heiman, M.E.; Borgman, L.E.; Be, A.W. Latitudal variations in planktonic foraminiferal test porosity. J. Foraminifer. Res. Part 1 Opt. Stud. 1972, 2, 6–13. [Google Scholar] [CrossRef]
- Marshall, B.J.; Thunell, R.C.; Spero, H.J.; Henehan, M.J.; Lorenzoni, L.; Astor, Y. Morphometric and stable isotopic differentiation of Orbulina universa morphotypes from the Cariaco Basin, Venezuela. Mar. Micropaleontol. 2015, 120, 46–64. [Google Scholar] [CrossRef] [Green Version]
- Erez, J. The source of ions for biomineralization in foraminifera and their implications for paleoceanographic proxies. Rev. Min. Geochem. 2003, 54, 115–149. [Google Scholar] [CrossRef] [Green Version]
- Schiebel, R.; Movellan, A. First-order estimate of the planktic foraminifer biomass in the modern ocean. Earth Syst. Sci. Data 2012, 4, 75–89. [Google Scholar] [CrossRef] [Green Version]
- Schiebel, R. Planktic foraminiferal sedimentation and the marine calcite budget. Glob. Biogeochem. Cycles 2002, 16, 1065. [Google Scholar] [CrossRef]
- Schiebel, R.; Barker, S.; Lendt, R.; Thomas, H.; Bollmann, J. Planktic foraminiferal dissolution in the twilight zone. J. Deep-Sea Res. II 2007, 54, 676–686. [Google Scholar] [CrossRef] [Green Version]
- Fabry, V.J.; Seibel, B.A.; Feely, R.A.; Orr, J.C. Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J. Mar. Sci. 2008, 65, 414–432. [Google Scholar] [CrossRef]
- Ramaswamy, V.; Gaye, B. Regional variations in the fluxes of foraminifera carbonate, coccolithophorid carbonate and biogenic opal in the northern Indian Ocean. Deep. -Sea Res. Part I Oceanogr. Res. Pap. 2006, 53, 271–293. [Google Scholar] [CrossRef]
- Langer, M.R. Assessing the contribution of foraminiferan protists to global ocean carbonate production. J. Eukaryot. Microbiol. 2008, 55, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Kleijne, A.; Kroon, D.; Zevenboom, W. Phytoplankton and foraminiferal frequencies in the northern Indian Ocean and Red Sea surface waters. Neth. J. Sea Res. 1989, 24, 531–539. [Google Scholar] [CrossRef]
- Stainbank, S.; Kroon, D.; Ru¨ggeberg, A.; Raddatz, J.; de Leau, E.S.; Zhang, M.; Spezzaferri, S. Controls on planktonic foraminifera apparent calcification depths for the northern equatorial Indian Ocean. PLoS ONE 2019, 14, e0222299. [Google Scholar] [CrossRef] [Green Version]
- Ujiie, H.; Nagase, K. Cluster analysis of living planktonic foraminifera from the south-eastern Indian Ocean. In Proceedings of the 2nd International Planktonic Conference, Rome, Italy; 1971; pp. 1251–1258. [Google Scholar]
- Strickland, J.D.H.; Parsons, T.R. A Practical Handbook of Seawater Analysis; The Alger Press Ltd.: Ottawa, ON, Canada, 1972. [Google Scholar]
- Martin, J.L.; Wildish, D.J. Integrated Water Column versus Niskin Bottle Sampling in the Southwest Bay of Fundy; Canadian Technical Report of Fisheries and Aquatic Sciences 1992(1893); Fisheries and Oceans Canada: Ottawa, ON, Canada, 1992; pp. 18–19.
- Sun, J.; Liu, D.; Qian, S. A quantitative research and analysis method for marine phytoplankton: An introduction to Utermöhl method and its modification. J. Oceanogr. Huanghai Bohai Seas 2002, 20, 105–112. (In Chinese) [Google Scholar]
- Shannon, C.E. A mathematical theory of communications. Bell Syst. Techical. J. 1948, 27, 379–423. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.L.; Chen, Y.Q. Aggregated intensity of dominant species of zooplankton in autumn in the East China Sea and the Yellow Sea. J. Ecol. 1989, 8, 13–15. (In Chinese) [Google Scholar]
- Michaels, A.F.; Caron, D.A.; Swanberg, N.R.; Howse, F.A.; Michaels, C.M. Planktonic sarcodines (Acantharia, Radiolaria, Foraminifera) in surface waters near Bermuda: Abundance, biomass, and vertical flux. J. Plankton Res. 1995, 17, 131–163. [Google Scholar] [CrossRef]
- Ly, A.; Verhagen, J.; Wagenmakers, E.-J. Harold Jeffreys’s default Bayes factor hypothesis tests: Explanation, extension, and application in psychology. J. Math. Psychol. 2016, 72, 19–32. [Google Scholar] [CrossRef]
- Xue, B.; Sun, J.; Ding, C.; Wang, D. Diatom communities in the equatorial region and its adjacent areas of Eastern Indian Ocean during spring intermonsoon 2014. Haiyang Xuebao 2016, 38, 112–120. [Google Scholar]
- Zhang, C.; Sun, J.; Wang, D.; Song, D.; Zhang, X.; Munir, S. Tintinnid community structure in the eastern equatorial Indian Ocean during the spring inter-monsoon period. Aquat. Biol. 2017, 26, 87–100. [Google Scholar] [CrossRef] [Green Version]
- Munir, S.; Rogers, J.; Zhang, X.; Ding, C.; Sun, J. The Horizontal Distribution of Siliceous Planktonic Radiolarian Community in the Eastern Indian Ocean. Water 2020, 12, 3502. [Google Scholar] [CrossRef]
- Evans, M.S.; Sell, D.W. Mesh size and collection characteristics of 50-cm diameter conical plankton nets. Hydrobiologia 1985, 122, 97–104. [Google Scholar] [CrossRef]
- Schiebel, R.; Hemleben, C. Modern planktic foraminifera. Palaeont Zool. 2005, 79, 135–148. [Google Scholar] [CrossRef] [Green Version]
- Carstens, J.; Hebbeln, D.; Wefer, G. Distribution of planktic foraminifera at the ice margin in the Arctic (Fram Strait). Mar. Micropaleontol 1997, 29, 257–269. [Google Scholar] [CrossRef]
- Taylor, B.J.; Rae, J.W.; Gray, W.R.; Darling, K.F.; Burke, A.; Gersonde, R.; Ziveri, P. Distribution and ecology of planktic foraminifera in the North Pacific: Implications for paleo-reconstructions. Quat. Sci. Rev. 2018, 191, 256–274. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Sun, J.; Wang, D.; Zhang, X.; Zhang, C.; Song, S.; Thangaraj, S. Distribution of living Coccolithophores in the eastern Indian Ocean during spring Inter-monsoon. Sci. Rep. 2018, 8, 12488. [Google Scholar] [CrossRef] [PubMed]
- Hemleben, C.; Spindler, M.; Anderson, O.R. Modern Planktonic Foraminifera; Springer Science & Business Media: Berlin, Germany, 1989. [Google Scholar]
- Chernihovsky, N.; Almogi Labin, A.; Kienast, S.S.; Torfstein, A. The daily resolved temperature dependence and structure of planktonic foraminifera blooms. Sci. Rep. 2020, 10, 17456. [Google Scholar] [CrossRef]
- Anbuselvan, N.; Senthil Nathan, D. Distribution and environmental implications of planktonic foraminifera in the surface sediments of the southwestern part of Bay of Bengal, India. J. Sediment. Environ. 2021, 6, 213–235. [Google Scholar] [CrossRef]
- Bradbury, M.G.; Abbott, D.P.; Bovbjerg, R.V.; Mariscal, R.N.; Fielding, W.C.; Barber, R.T.; Pearse, V.B.; Proctor, S.J.; Ogden, J.C.; Wourms, L.R.; et al. Studies in the fauna associated with the deep scattering layers in the equatorial Indian Ocean, conducted on R/V Te Vega during October and December 1964. In Proceedings of the an International Symposium on Biological Sound Scattering in the Ocean, Warrenton, WV, USA, 31 March–2 April 1970; pp. 409–452. [Google Scholar]
- Bé, A.W.H.; Hutson, W.H. Ecology of planktonic foraminifera and biogeographic patterns of life and fossil assemblages in the Indian Ocean. Micropaleontology 1977, 23, 369–414. [Google Scholar] [CrossRef]
- Peeters, F.; Ivanova, E.; Conan, S.; Brummer, G.J.; Ganssen, G.; Troelstra, S.; van Hinte, J. A size analysis of planktic foraminifera from the Arabian Sea. Mar. Micropaleontol. 1999, 36, 31–63. [Google Scholar] [CrossRef]
- Wei, Y.; Sun, J.; Zhang, X.; Wang, J.; Huang, K. Picophytoplankton size and biomass around equatorial eastern Indian Ocean. Microbiol. Open 2019, 8, e00629. [Google Scholar] [CrossRef] [PubMed]
- Tittensor, D.; Mora, C.; Jetz, W.; Lotze, H.; Ricard, D.; Berghe, E.; Worm, B. Global patterns and predictors of marine biodiversity across taxa. Nature 2010, 466, 1098–1101. [Google Scholar] [CrossRef]
- Longhurst, A.R. Ecological Geography of the Sea, 2nd ed.; Academic Press: Cambridge, MA, USA, 2007; p. 542. [Google Scholar]
- Rutherford, S.; D’Hondt, S.; Prell, W. Environmental controls on the geographic distribution of zooplankton diversity. Nature 1999, 400, 749–753. [Google Scholar] [CrossRef]






| Species | (Pi)% | (fi)% | (Y) |
|---|---|---|---|
| Dentigloborotalia anfracta | 0.091 | 4 | 0.001 |
| Globigerina bulloides | 0.864 | 38 | 0.406 |
| Globigerinoides ruber (white) | 0.727 | 32 | 0.14 |
| Globorotalia scitula | 0.045 | 2 | 0 |
| Globigerinella siphonifera | 0.409 | 18 | 0.039 |
| Globorotalia menardii | 0.114 | 5 | 0.001 |
| Globigerinella calida | 0.273 | 12 | 0.008 |
| Globigerinella glutinata | 0.159 | 7 | 0.004 |
| Globorotalia tumida | 0.045 | 2 | 0 |
| Globorotalia ungulata | 0.023 | 1 | 0 |
| Hastigerina pelagica | 0.159 | 7 | 0.002 |
| Orcadia riedeli | 0.114 | 20 | 0.001 |
| Orbulina universa | 0.227 | 10 | 0.009 |
| Turborotalita quinqueloba | 0.386 | 17 | 0.019 |
| Trilobatus sacculifer | 0.159 | 7 | 0.002 |
| Tenuitella parkerae | 0.023 | 1 | 0 |
| Tenuitella fleisheri | 0.023 | 1 | 0 |
| Turborotalita humilis | 0.023 | 1 | 0 |
| Species | Shell Size µm | Biovolume | Carbon Biomass/Cell (0.05 pg C µm−3) | Contribution to Total Carbon Biomass |
|---|---|---|---|---|
| (µm3) | μg C m−3 | |||
| Dentigloborotalia anfracta | 57.91–82.32 | 23,255 | 0.117 | 0.0021 |
| Globigerina bulloides | 79.64–281.5 | 77,191 | 0.108 | 0.0069 |
| Globigerinoides ruber (white) | 116–287 | 2,548,920 | 0.087 | 0.2269 |
| Globorotalia scitula | 78–106 | 452,002 | 0.097 | 0.0402 |
| Globigerinella siphonifera | 94–150 | 634,929 | 0.095 | 0.0565 |
| Globorotalia menardii | 188 | 44,897,792 | 0.073 | 3.9959 |
| Globorotalia tumida | 299–508 | 1,775,708 | 0.089 | 0.1580 |
| Globorotalia ungulate | 299 | 67,759 | 0.109 | 0.0054 |
| Tenuitella parkerae | 103 | 113,353 | 0.106 | 0.0101 |
| Turborotalita humilis | 57–63 | 67,800 | 0.109 | 0.0060 |
| Turborotalita quinqueloba | 17–88 | 83,635 | 0.108 | 0.0074 |
| Trilobatus sacculifer | 175–189 | 4,371,861 | 0.085 | 0.3891 |
| Tenuitella fleisheri | 51.57–63.23 | 30,116 | 0.115 | 0.0027 |
| Orcadia riedeli | 65–72.78 | 83,669 | 0.108 | 0.0074 |
| Orbulina universa | 100–290 | 6,386,181 | 0.098 | 0.5684 |
| Hastigerina pelagica | 83–195 | 2,119,619 | 0.104 | 0.1186 |
| Globigerinella calida | 118–303 | 7,712,913 | 0.097 | 0.6864 |
| Globigerinella glutinata | 106–167 | 1,531,128 | 0.106 | 0.1365 |
| Species | Temperature °C | Salinity | Chlorophyll a |
|---|---|---|---|
| Globigerina bulloides | 0.334 ** | 0.307 ** | 0.372 ** |
| Globigerinoides ruber (white) | 0.196 | 0.265 | 0.189 |
| Orcadia riedeli | –0.31 | 0.184 | –0.059 |
| Globigerinella calida | 0.206 | 0.251 | 0.153 |
| Globigerinella siphonifera | 0.256 | 0.315 ** | 0.195 |
| Trilobatus sacculifer | –0.31 | 0.204 | –0.02 |
| Globigerinella glutinata | 0.086 | –0.04 | 0.255 |
| Turborotalita quinqueloba | 0.027 | 0 | 0.238 |
| Globorotalia scitula | –0.006 | –0.103 | –0.183 |
| Hastigerina pelagica | –0.121 | 0.241 | –0.083 |
| Orbulina universa | 0.235 | –0.092 | 0.013 |
| Globorotalia tumida | –0.174 | 0.044 | –0.099 |
| Globorotalia menardii | 0.027 | –0.086 | –0.137 |
| Dentigloborotalia anfracta | –0.05 | –0.024 | 0.448 ** |
| Globorotalia inflanta | 0.086 | 0.149 | –0.014 |
| Globorotalia ungulanta | 0.027 | –0.086 | –0.137 |
| Tenuitella fleisheri | 0.027 | –0.086 | –0.137 |
| Tenuitella parakera | –0.069 | 0.015 | 0.171 |
| Streptochilius globigerum | 0.136 | 0.109 | 0.053 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munir, S.; Sun, J.; Morton, S.L.; Zhang, X.; Ding, C. Horizontal Distribution and Carbon Biomass of Planktonic Foraminifera in the Eastern Indian Ocean. Water 2022, 14, 2048. https://doi.org/10.3390/w14132048
Munir S, Sun J, Morton SL, Zhang X, Ding C. Horizontal Distribution and Carbon Biomass of Planktonic Foraminifera in the Eastern Indian Ocean. Water. 2022; 14(13):2048. https://doi.org/10.3390/w14132048
Chicago/Turabian StyleMunir, Sonia, Jun Sun, Steve L. Morton, Xiaodong Zhang, and Changling Ding. 2022. "Horizontal Distribution and Carbon Biomass of Planktonic Foraminifera in the Eastern Indian Ocean" Water 14, no. 13: 2048. https://doi.org/10.3390/w14132048
APA StyleMunir, S., Sun, J., Morton, S. L., Zhang, X., & Ding, C. (2022). Horizontal Distribution and Carbon Biomass of Planktonic Foraminifera in the Eastern Indian Ocean. Water, 14(13), 2048. https://doi.org/10.3390/w14132048








