Vertical Distribution of Phytoplankton Community and Pigment Production in the Yellow Sea and the East China Sea during the Late Summer Season
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Environmental Data
2.2. Size-Fractionated Chlorophyll-a Measurement
2.3. In Situ Culture Experiment for Photosynthetic Pigment Production
2.4. Extraction and Analysis for Photosynthetic Pigment Production
2.5. Calculation of the Pigment Concentration and Production Rate
2.6. Chemotaxonomic Analysis
3. Results
3.1. Environmental Conditions
3.2. Total and Size-Fractionated Chlorophyll-a Concentration in the YS and the ECS
3.3. Phytoplankton Community Structure
3.4. Relationships between Phytoplankton Groups and Different Environmental Factors
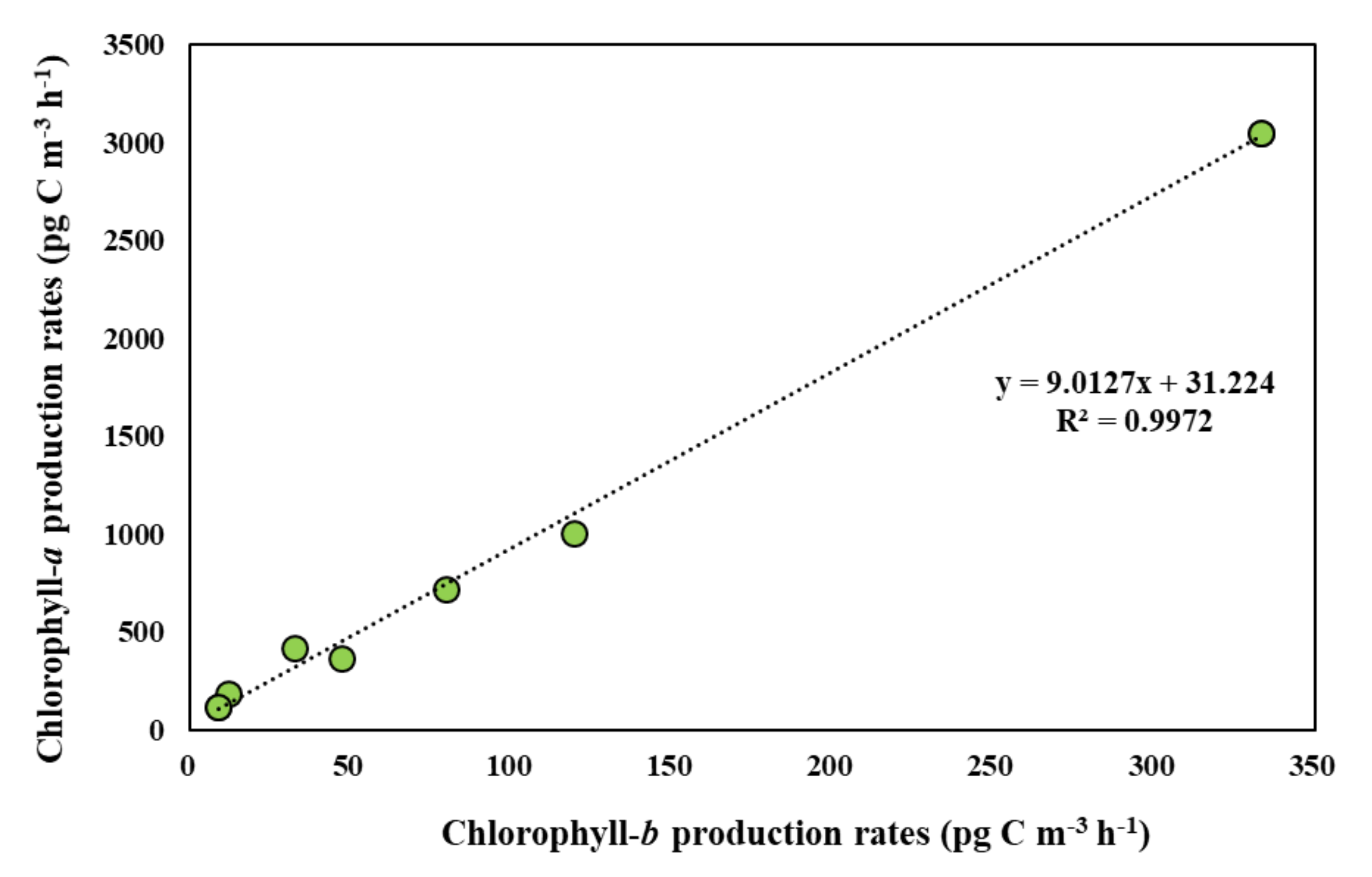
3.5. Photosynthetic Pigment Production Rates
4. Discussion
5. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elser, J.J.; Elser, M.M.; Carpenter, S.R. Size fraction of algal chlorophyll, carbon fixation and phosphatase activity: Relationships with species-specific size distributions and zooplankton community structure. J. Plankton Res. 1986, 8, 365–383. [Google Scholar] [CrossRef]
- Iriarte, J.L.; Pizarro, G.; Troncoso, V.A.; Sobarzo, M. Primary production and biomass of size-fractionated phytoplankton off Antofagasta, Chile (23–24°S) during pre-El Niño and El Niño 1997. J. Mar. Syst. 2000, 26, 37–51. [Google Scholar] [CrossRef]
- Boyce, D.G.; Lewis, M.R.; Worm, B. Global phytoplankton decline over the past century. Nature 2010, 466, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M.; Cembella, A.D.; Hallegraeff, G.M. Progress in understanding harmful algal blooms: Paradigm shifts and new technologies for research, monitoring, and management. Annu. Rev. Mar. Sci. 2012, 4, 143–176. [Google Scholar] [CrossRef]
- Doney, S.C.; Ruckelshaus, M.; Duffy, J.E.; Barry, J.P.; Chan, F.; English, C.A.; Galindo, H.M.; Grebmeier, J.M.; Hollowed, A.B.; Knowlton, N.; et al. Climate change impacts on marine ecosystems. Annu. Rev. Mar. Sci. 2012, 4, 11–37. [Google Scholar] [CrossRef] [PubMed]
- Morán, X.A.G.; López-Urrutia, Á.; Calvo-díaz, A.; Li, W.K. Increasing importance of small phytoplankton in a warmer ocean. Glob. Chang. Biol. 2010, 16, 1137–1144. [Google Scholar] [CrossRef]
- Flombaum, P.; Gallegos, J.L.; Gordillo, R.A.; Rincon, J.; Zabala, L.L.; Jiao, N.; Karl, D.M.; Li, W.K.; Lomas, M.W.; Veneziano, D.; et al. Present and future global distributions of the marine Cyanobacteria Prochlorococcus and Synechococcus. Proc. Natl. Acad. Sci. USA 2013, 110, 9824–9829. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.J.; Jang, H.K.; Lim, J.H.; Lee, D.; Lee, J.H.; Bae, H.; Lee, C.H.; Kang, C.-K.; Lee, S.H. Characteristics of Different Size Phytoplankton for Primary Production and Biochemical Compositions in the Western East/Japan Sea. Front. Microbiol. 2020, 11, 3306. [Google Scholar] [CrossRef]
- Litchman, E.; Klausmeier, C.A.; Schofield, O.M.; Falkowski, P.G. The role of functional traits and trade-offs in structuring phytoplankton communities: Scaling from cellular to ecosystem level. Ecol. Lett. 2007, 10, 1170–1181. [Google Scholar] [CrossRef]
- Barton, A.D.; Pershing, A.J.; Litchman, E.; Record, N.R.; Edwards, K.F.; Finkel, Z.V.; Kiørboe, T.; Ward, B.A. The biogeography of marine plankton traits. Ecol. Lett. 2013, 16, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Finkel, Z.V.; Irwin, A.J.; Schofield, O. Resource limitation alters the 3/4 size scaling of metabolic rates in phytoplankton. Mar. Ecol. Prog. Ser. 2004, 273, 269–279. [Google Scholar] [CrossRef]
- Roy, S.; Llewellyn, C.A.; Egeland, E.S.; Johnsen, G. (Eds.) Phytoplankton Pigments: Characterization, Chemotaxonomy and Applications in Oceanography; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Ha, S.Y.; La, H.S.; Min, J.O.; Chung, K.H.; Kang, S.H.; Shin, K.H. Photoprotective function of mycosporine-like amino acids in a bipolar diatom (Porosira glacialis): Evidence from ultraviolet radiation and stable isotope probing. Diatom Res. 2014, 29, 399–409. [Google Scholar] [CrossRef]
- Dai, D.J.; Qiao, F.L.; Xia, C.S.; Jung, K.T. A numerical study on dynamic mechanisms of seasonal temperature variability in the Yellow Sea. J. Geophys. Res. 2006, 111, C11S05. [Google Scholar] [CrossRef]
- Huang, B.Q.; Liu, Y.; Chen, J.X.; Wang, D.Z.; Hong, H.S.; Lv, R.H.; Huang, L.F.; Lin, Y.A.; Wei, H. Temporal and spatial distribution of size-fractioated phytoplankton biomass in East China Sea and Huanghai Sea. Acta Oceanol. Sin. 2006, 28, 156–164, (In Chinese with English Abstract). [Google Scholar]
- Fu, M.Z.; Wang, Z.L.; Li, Y.; Li, R.; Sun, P.; Wei, X.; Lin, X.; Guo, J. Phytoplankton biomass size structure and its regulation in the southern Yellow Sea (China): Seasonal variability. Cont. Shelf Res. 2009, 29, 2178–2194. [Google Scholar] [CrossRef]
- Gong, G.C.; Chen, Y.L.L.; Liu, K.K. Chemical hydrography and chlorophyll a distribution in the East China Sea in summer: Implications in nutrient dynamics. Cont. Shelf. Res. 1996, 16, 1561–1590. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, W.; Landry, M.R.; Chiang, K.-P.; Wang, L.; Huang, B. Responses of phytoplankton communities to environmental variability in the East China Sea. Ecosystems 2016, 19, 832–849. [Google Scholar] [CrossRef]
- Gardner, W.D.; Chung, S.P.; Richardson, M.J.; Walsh, I.D. The oceanic mixed-layer pump. Deep Sea Res. Part II Top. Stud. Oceanogr. 1995, 42, 757–775. [Google Scholar] [CrossRef]
- Kwak, J.H.; Lee, S.H.; Hwang, J.; Suh, Y.S.; Park, H.; Chang, K.I.; Kim, K.-R.; Kang, C.-K. Summer primary productivity and phytoplankton community composition driven by different hydrographic structures in the East/Japan Sea and the Western Subarctic Pacific. J. Geophys. Res. Oceans 2014, 119, 4505–4519. [Google Scholar] [CrossRef]
- Cho, B.C.; Park, M.G.; Shim, J.H.; Choi, D.H. Sea-surface temperature and f-ratio explain large variability in the ratio of bacterial production to primary production in the Yellow Sea. Mar. Ecol. Prog. Ser. 2001, 216, 31–41. [Google Scholar] [CrossRef]
- Parsons, T.R.; Maita, Y.; Lalli, C.M. A Manual of Chemical and Biological Methods for Seawater Analysis; Pergamon Press: Oxford, UK, 1984. [Google Scholar]
- Kang, J.J.; Joo, H.; Lee, J.H.; Lee, J.H.; Lee, H.W.; Lee, D.; Kang, C.K.; Yun, M.S.; Lee, S.H. Comparison of biochemical compositions of phytoplankton during spring and fall seasons in the northern East/Japan Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2017, 143, 73–81. [Google Scholar] [CrossRef]
- Lee, S.H.; Joo, H.; Lee, J.H.; Lee, J.H.; Kang, J.J.; Lee, H.W.; Lee, D.; Kang, C.K. Seasonal carbon uptake rates of phytoplankton in the northern East/Japan Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2017, 143, 45–53. [Google Scholar] [CrossRef]
- Kim, B.K.; Joo, H.; Song, H.J.; Yang, E.J.; Lee, S.H.; Hahm, D.; Rhee, T.S.; Lee, S.H. Large seasonal variation in phytoplankton production in the Amundsen Sea. Polar Biol. 2015, 38, 319–331. [Google Scholar] [CrossRef]
- Hama, T.; Miyazaki, T.; Ogawa, Y.; Iwakuma, T.; Takahashi, M.; Otsuki, A.; Ichimura, S. Measurement of photosynthetic production of a marine phytoplankton population using a stable 13C isotope. Mar. Biol. 1983, 73, 31–36. [Google Scholar] [CrossRef]
- Ha, S.Y.; Lee, Y.; Kim, M.S.; Kumar, K.S.; Shin, K.H. Seasonal changes in mycosporine-like amino acid production rate with respect to natural phytoplankton species composition. Mar. Drugs 2015, 13, 6740–6758. [Google Scholar] [CrossRef]
- Jang, S.J.; Park, M.O. Evaluation of grinding effects on the extraction of photosynthetic pigments for HPLC analysis. Sea 2015, 20, 71–77. [Google Scholar] [CrossRef][Green Version]
- Kwak, J.H.; Han, E.; Lee, S.H.; Park, H.J.; Kim, K.-R.; Kang, C.-K. A consistent structure of phytoplankton communities across the warm–cold regions of the water mass on a meridional transect in the East/Japan Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2017, 143, 36–44. [Google Scholar] [CrossRef]
- Zapata, M.; Rodríguez, F.; Garrido, J.L. Separation of chlorophylls and carotenoids from marine phytoplankton: A new HPLC method using a reversed phase C8 column and pyridine-containing mobile phases. Mar. Ecol. Prog. Ser. 2000, 195, 29–45. [Google Scholar] [CrossRef]
- Park, M.O. Composition and distribution of phytoplankton with size fraction results at southwestern East/Japan Sea. Ocean. Sci. J. 2006, 41, 301–313. [Google Scholar] [CrossRef]
- Mackey, M.D.; Mackey, D.J.; Higgins, H.W.; Wright, S.W. CHEMTAX—A program for estimating class abundances from chemical markers: Application to HPLC measurements of phytoplankton. Mar. Ecol. Prog. Ser. 1996, 144, 256–283. [Google Scholar] [CrossRef]
- Wright, S.W.; Thomas, D.P.; Marchant, H.J.; Higgins, H.W.; Mackey, M.D.; Mackey, M.D. Analysis of phytoplankton of the Australian sector of the Southern Ocean: Comparisons of microscopy and size frequency data with interpretations of pigment HPLC data using the ‘CHEMTAX’ matrix factorisation program. Mar. Ecol. Prog. Ser. 1996, 144, 285–298. [Google Scholar] [CrossRef]
- Wright, S.W.; van den Enden, R.L. Phytoplankton community structure and stocks in the Eastern Antarctic marginal ice zone (BROKE survey, January e March 1996) determined by CHEMTAX analysis of HPLC pigment signatures. Deep Sea Res. Part II Top. Stud. Oceanogr. 2000, 47, 2363–2400. [Google Scholar] [CrossRef]
- Liu, X.; Huang, B.Q.; Huang, Q.; Wang, L.; Ni, X.B.; Tang, Q.S.; Sun, S.; Wei, H.; Liu, S.M.; Li, C.L.; et al. Seasonal phytoplankton response to physical processes in the southern Yellow Sea. J. Sea Res. 2015, 95, 45–55. [Google Scholar] [CrossRef]
- Dugdale, R.C.; Goering, J.J. Uptake of new and regenerated forms of nitrogen in primary productivity. Limnol. Oceanogr. 1967, 12, 196–206. [Google Scholar] [CrossRef]
- Venrick, E.L.; McGowan, J.A.; Mantyla, A.W. Deep maxima of photosynthetic chlorophyll in the Pacific Ocean. Fish. Bull. 1973, 71, 41–52. [Google Scholar]
- Domingues, R.B.; Barbosa, A.B.; Sommer, U.; Galvão, H.M. Ammonium, nitrate and phytoplankton interactions in a freshwater tidal estuarine zone: Potential effects of cultural eutrophication. Aquat. Sci. 2011, 73, 331–343. [Google Scholar] [CrossRef]
- Jang, H.K.; Youn, S.H.; Joo, H.; Kim, Y.; Kang, J.J.; Lee, D.; Jo, N.; Kim, K.; Kim, M.-J.; Kim, S.; et al. First Concurrent Measurement of Primary Production in the Yellow Sea, the South Sea of Korea, and the East/Japan Sea, 2018. J. Mar. Sci. Eng. 2021, 9, 1237. [Google Scholar] [CrossRef]
- Furuya, K.; Hayashi, M.; Yabushita, T.; Ishikawa, A. Phytoplankton dynamics in the East China Sea in spring and summer as revealed by HPLC-derived pigment signatures. Deep Sea Res. Part II Top. Stud. Oceanogr. 2003, 50, 367–387. [Google Scholar] [CrossRef]
- Liu, X.; Huang, B.; Liu, Z.; Wang, L.; Wei, H.; Li, C.; Huang, Q. High-resolution phytoplankton diel variations in the summer stratified central Yellow Sea. J. Oceanogr. 2012, 68, 913–927. [Google Scholar] [CrossRef]
- Kwak, J.H.; Lee, S.H.; Park, H.J.; Choy, E.J.; Jeong, H.D.; Kim, K.R.; Kang, C.K. Monthly measured primary and new productivities in the Ulleung Basin as a biological” hot spot” in the East/Japan Sea. Biogeosciences 2013, 10, 4405–4417. [Google Scholar] [CrossRef]
- Kim, Y.; Youn, S.-H.; Oh, H.J.; Kang, J.J.; Lee, J.H.; Lee, D.; Kim, K.; Jang, H.K.; Lee, J.; Lee, S.H. Spatiotemporal Variation in Phytoplankton Community Driven by Environmental Factors in the Northern East China Sea. Water 2020, 12, 2695. [Google Scholar] [CrossRef]
- Mur, R.; Skulberg, O.; Utkilen, H. Cyanobacteria in the environment. In Toxic Cyanobacteria in Water; Chorus, I., Batram, J., Eds.; E&FN Spon: London, UK, 1999; pp. 15–40. [Google Scholar]
- Peperzak, L. Climate change and harmful algal blooms in the North Sea. Acta Oecol. 2003, 24, S139–S144. [Google Scholar] [CrossRef]
- Paerl, H.W.; Huisman, J. Climate change: A catalyst for global expansion of harmful cyanobacterial blooms. Environ. Microbiol. Rep. 2009, 1, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.E.; Kal, J. Size dependence of growth rate, respiratory electron transport system activity and chemical composition in marine diatoms in the laboratory. J. Phycol. 1982, 18, 275–284. [Google Scholar] [CrossRef]
- Fogg, G.E. Review Lecture-Picoplankton. Proc. R. Soc. Lond. B Biol. Sci. 1986, 228, 1–30. [Google Scholar]
- Raven, J. The twelfth Tansley Lecture. Small is beautiful: The picophytoplankton. Funct. Ecol. 1998, 12, 503–513. [Google Scholar] [CrossRef]
- Reed, M.L.; Pinckney, J.L.; Keppler, C.J.; Brock, L.M.; Hogan, S.B.; Greenfield, D.I. The influence of nitrogen and phosphorus on phytoplankton growth and assemblage composition in four coastal, southeastern USA systems. Estuar. Coast. Shelf Sci. 2016, 177, 71–82. [Google Scholar] [CrossRef]
- Nwankwegu, A.S.; Li, Y.; Huang, Y.; Wei, J.; Norgbey, E.; Ji, D.; Pu, Y.; Nuamah, L.A.; Yang, Z.; Paerl, H.W. Nitrate repletion during spring bloom intensifies phytoplankton iron demand in Yangtze River tributary, China. Environ. Pollut. 2020, 264, 114626. [Google Scholar] [CrossRef]
- Bronk, D.A.; Glibert, P.M.; Ward, B.B. Nitrogen uptake, dissolved organic nitrogen release, and new production. Science 1994, 265, 1843–1846. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Qi, M.; Tang, H.; Han, X. Spatial and temporal nutrient variations in the Yellow Sea and their effects on Ulva prolifera blooms. Estuar. Coast. Shelf Sci. 2015, 163, 36–43. [Google Scholar] [CrossRef]
- Goericke, R.; Welschmeyer, N.A. Pigment turnover in the marine diatom Thalassiosira weissflogii. II. The 14CO2-Labeling kinetics of carotenoids. J. Phycol. 1992, 28, 507–517. [Google Scholar] [CrossRef]
- Johnsen, G.; Prézelin, B.B.; Jovine, R.V.M. Fluorescence excitation spectra and light utilization in two red tide dinoflagellates. Limnol. Oceanogr. 1997, 42, 1166–1177. [Google Scholar] [CrossRef]
- Stolte, W.; Kraay, G.W.; Noordeloos, A.A.M.; Riegman, R. Genetic and physiological variation in pigment composition of Emiliania huxleyi (Prymnesiophyceae) and the potential use of its pigment rations as a quantitative physiological marker. J. Phycol. 2000, 36, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Falkowski, P.G.; Chen, Y.-B. Photoacclimation of light harvesting systems in eukaryotic algae. In Light-Harvesting Antennas in Photosynthesis; Green, B.R., Parson, W.W., Eds.; Kluwer Academic: Dordrecht, The Netherlands, 2003; pp. 423–447. [Google Scholar] [CrossRef]
- Rodríguez, F.; Chauton, M.; Johnsen, G.; Andresen, K.; Olsen, L.M.; Zapata, M. Photoacclimation in phytoplankton: Implications for biomass estimates, pigment functionality and chemotaxonomy. Mar. Biol. 2006, 148, 963–971. [Google Scholar] [CrossRef]
- Wright, S.W.; Jeffrey, S.W.; Mantoura, R.F.C. (Eds.) Phytoplankton Pigments in Oceanography: Guidelines to Modern Methods; Unesco: Paris, France, 2005. [Google Scholar]
- Young, A.J.; Frank, H.A. Energy transfer reactions involving carotenoids: Quenching of chlorophyll fluorescence. J. Photochem. Photobiol. B Biol. 1996, 36, 3–15. [Google Scholar] [CrossRef]
- Agustí, S. Light environment within dense algal populations: Cell size influences on self-shading. J. Plankton Res. 1991, 13, 863–871. [Google Scholar] [CrossRef]
- Agawin, N.S.; Duarte, C.M.; Agustí, S. Nutrient and temperature control of the contribution of picoplankton to phytoplankton biomass and production. Limnol. Oceanogr. 2000, 45, 591–600. [Google Scholar] [CrossRef]
- Mousing, E.A.; Ellegaard, M.; Richardson, K. Global patterns in phytoplankton community size structure—Evidence for a direct temperature effect. Mar. Ecol. Prog. Ser. 2014, 497, 25–38. [Google Scholar] [CrossRef]
- Kehoe, M.; O’Brien, K.R.; Grinham, A.; Burford, M.A. Primary production of lake phytoplankton, dominated by the cyanobacterium Cylindrospermopsis raciborskii, in response to irradiance and temperature. Inland Waters 2015, 5, 93–100. [Google Scholar] [CrossRef]
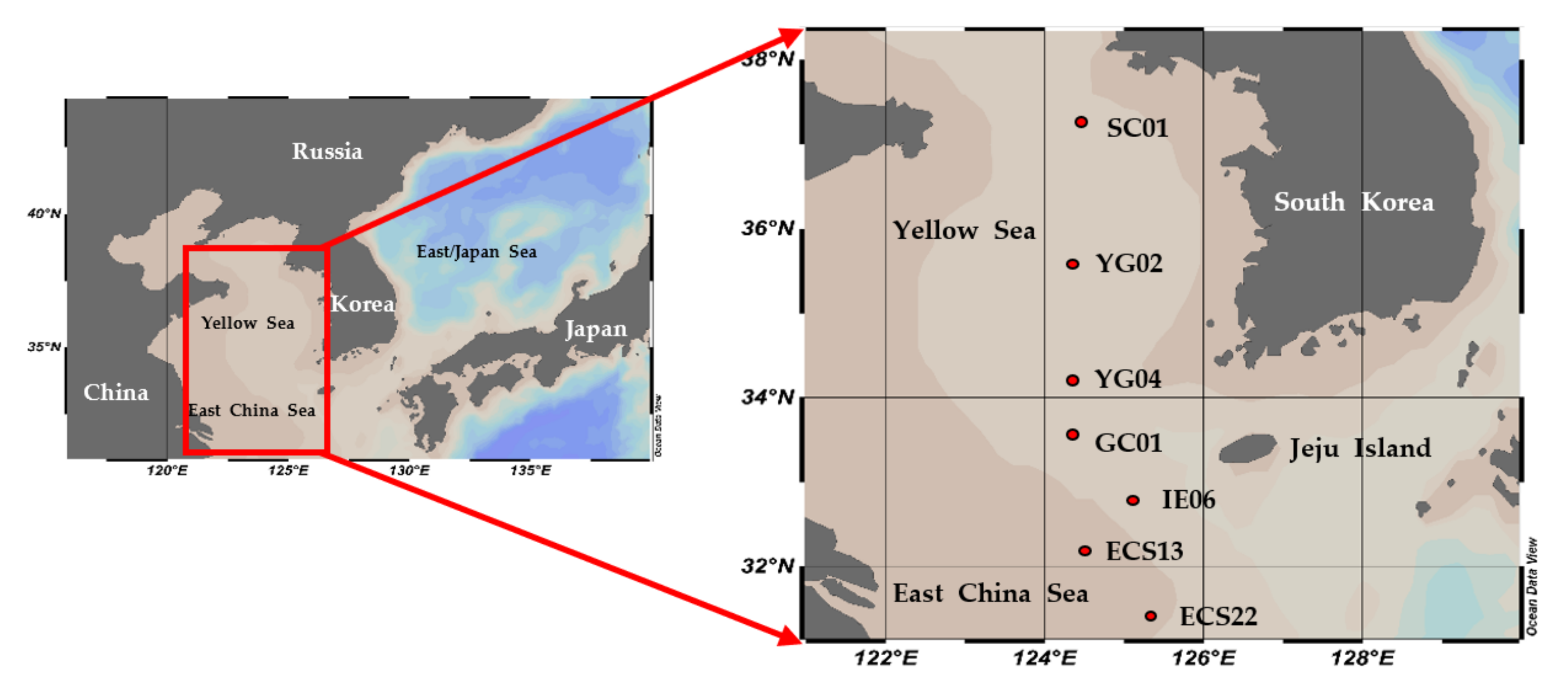

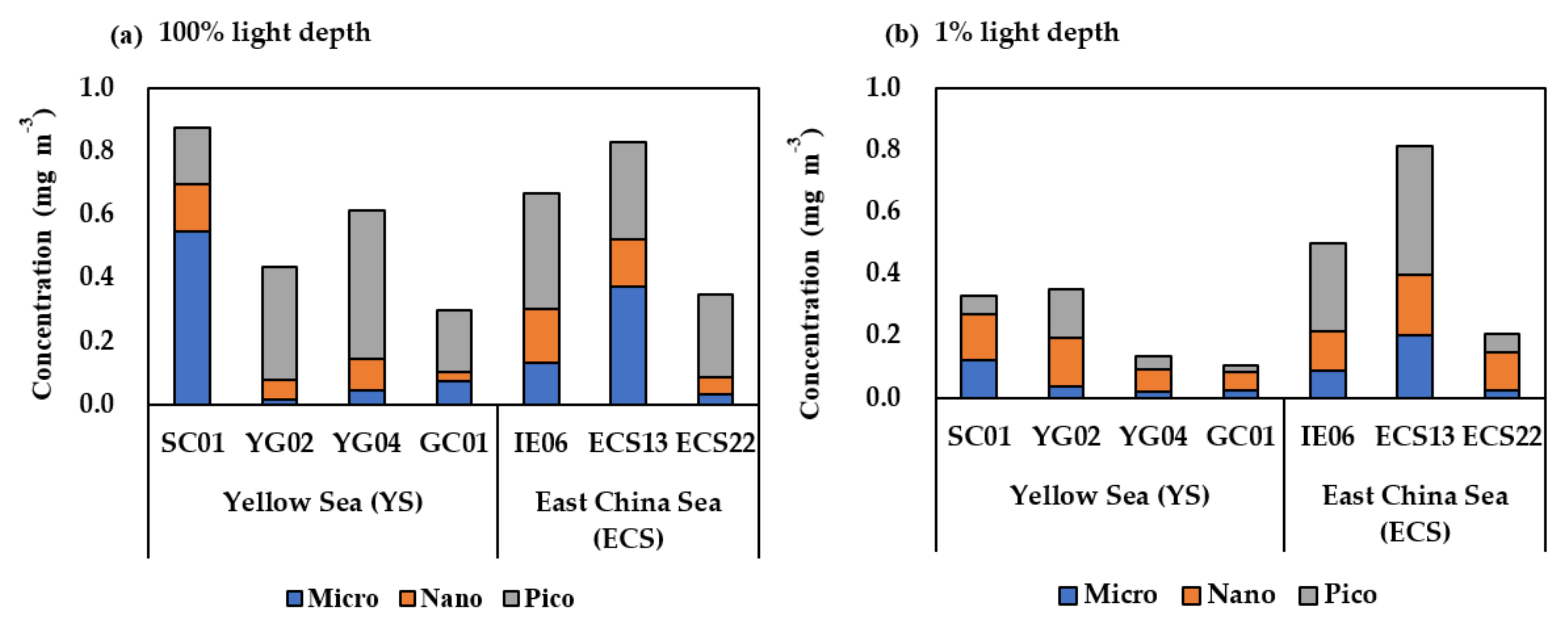
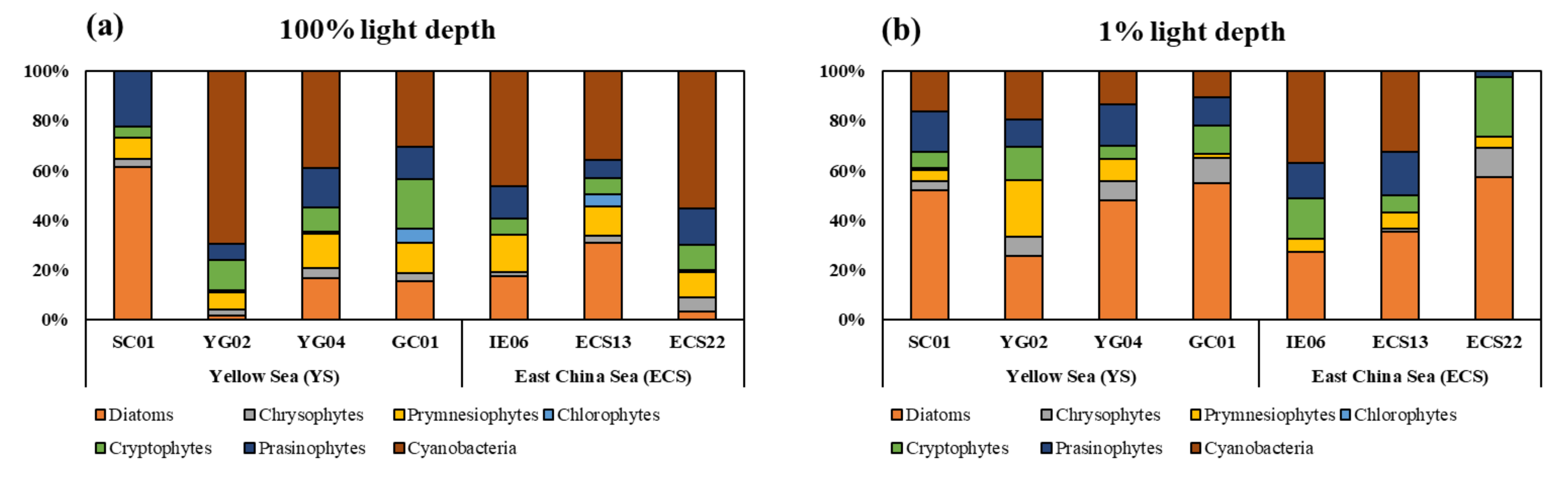
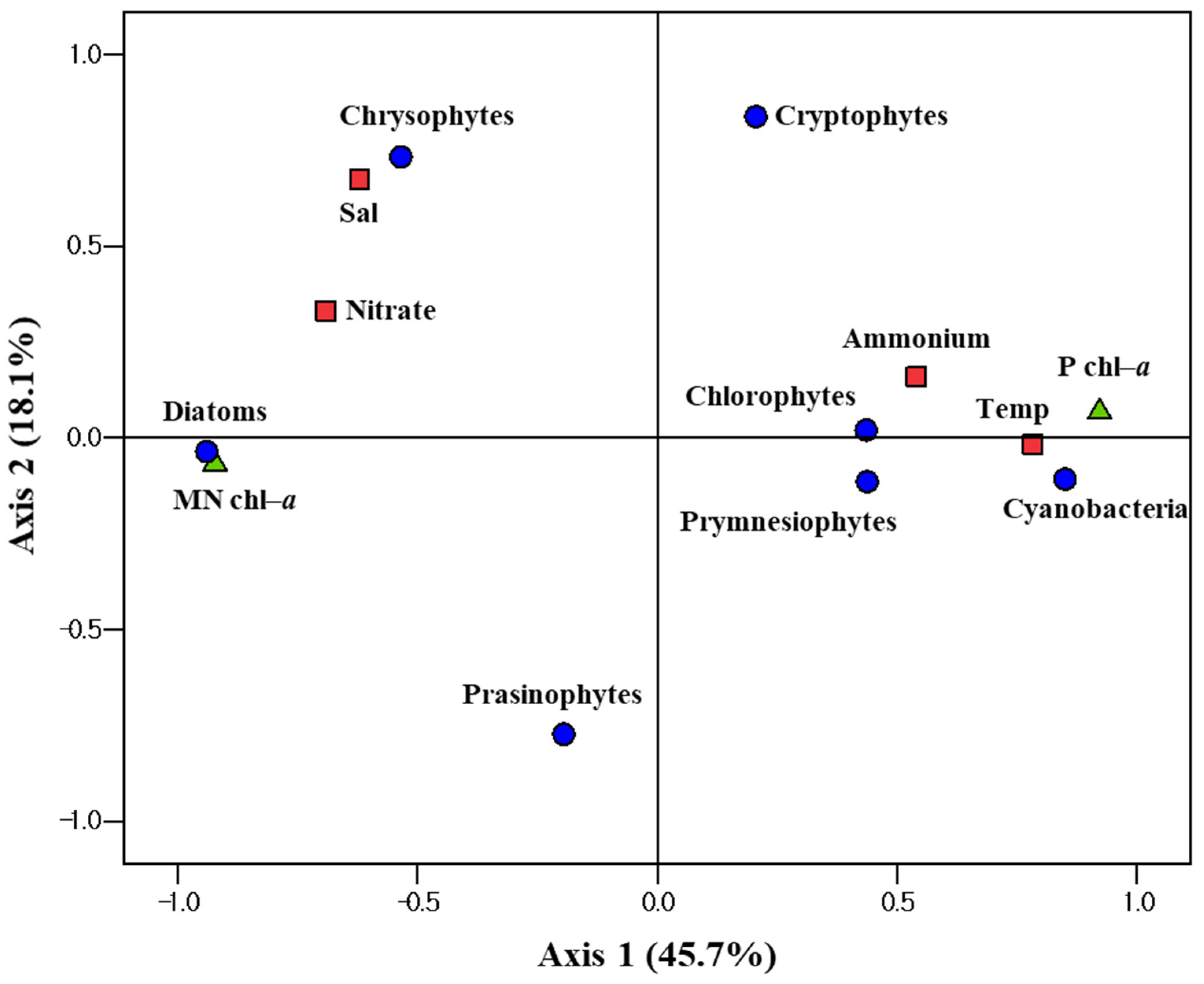
| Region | Station | Date (2020) | Latitude (°N) | Longitude (°E) | Mixed Layer Depth (m) | Stability Index | Light Depth (%) | Depth (m) | T (°C) | S (psu) | NO3 (µM) | NH4 (µM) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yellow Sea (YS) | SC01 | 13-Sep | 31.400 | 125.330 | 21 | 0.068 | 100 | 0 | 21.7 | 31.2 | 0.18 | 1.00 |
| 1 | 30 | 16.0 | 31.9 | 5.12 | 0.96 | |||||||
| YG02 | 12-Sep | 32.175 | 124.510 | 10 | 0.055 | 100 | 0 | 24.2 | 31.5 | 1.05 | 0.83 | |
| 1 | 27 | 21.0 | 32.2 | 5.53 | 1.00 | |||||||
| YG04 | 12-Sep | 32.771 | 125.108 | 15 | 0.096 | 100 | 0 | 23.2 | 30.8 | 3.76 | 1.45 | |
| 1 | 33 | 16.6 | 32.5 | 11.35 | 0.71 | |||||||
| GC01 | 12-Sep | 33.565 | 124.353 | 13 | 0.094 | 100 | 0 | 23.4 | 30.9 | 2.06 | 1.54 | |
| 1 | 51 | 11.8 | 33.3 | 13.42 | 1.00 | |||||||
| East China Sea (ECS) | IE06 | 11-Sep | 34.195 | 124.352 | 12 | 0.025 | 100 | 0 | 24.8 | 30.5 | 2.33 | 1.29 |
| 1 | 19 | 23.8 | 30.6 | 5.90 | 1.08 | |||||||
| ECS13 | 10-Sep | 35.556 | 124.349 | 12 | 0.065 | 100 | 0 | 25.9 | 30.2 | 0.80 | 0.79 | |
| 1 | 19 | 24.0 | 31.0 | 5.31 | 0.87 | |||||||
| ECS22 | 09-Sep | 37.254 | 124.449 | 12 | 0.044 | 100 | 0 | 26.2 | 32.0 | 1.51 | 1.37 | |
| 1 | 41 | 24.6 | 33.5 | 6.33 | 1.04 |
| * Unit: pg C m−3 h−1 | Carotenoid | Chlorophyll | Carotenoid Xanthophyll (Photoprotective Pigment) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | Station | But-fuco | Fuco | Hex-fuco | Neo | Pras | Allo | Chl-b | Chl-a | Diadino | Diato | Viola | Zea | |
| 100% light depth | Yellow Sea (YS) | SC01 | 0.00 | 48.35 | 2.34 | 1.42 | 1.81 | 28.86 | 79.38 | 719.93 | 165.75 | 0.00 | 6.03 | 14.62 |
| YG02 | 0.00 | 0.03 | 0.16 | 0.00 | 0.00 | 7.25 | 8.71 | 119.14 | 0.40 | 0.53 | 0.00 | 52.90 | ||
| YG04 | 0.22 | 15.56 | 2.70 | 4.52 | 1.44 | 17.61 | 332.90 | 3052.47 | 52.82 | 15.70 | 0.74 | 336.39 | ||
| GC01 | 0.10 | 2.03 | 0.00 | 0.34 | 0.03 | 8.32 | 32.32 | 423.36 | 10.10 | 4.97 | 0.00 | 44.27 | ||
| East China Sea (ECS) | IE06 | 0.00 | 1.52 | 0.64 | 0.27 | 0.00 | 1.50 | 11.54 | 187.42 | 1.38 | 1.78 | 0.00 | 27.37 | |
| ECS13 | 0.16 | 20.35 | 1.32 | 0.16 | 0.19 | 3.82 | 119.26 | 1008.49 | 34.34 | 8.12 | 0.56 | 109.18 | ||
| ECS22 | 0.36 | 1.26 | 0.00 | 0.23 | 0.00 | 0.53 | 47.19 | 367.08 | 0.00 | 1.10 | 0.35 | 63.84 | ||
| 1% Light depth | Yellow Sea (YS) | SC01 | 0.00 | 1.72 | 0.00 | 0.02 | 0.00 | 0.11 | 4.16 | 103.83 | 0.00 | 0.00 | 0.00 | 0.42 |
| YG02 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | 0.19 | 3.03 | 39.46 | 0.00 | 0.01 | 0.00 | 0.94 | ||
| YG04 | 0.00 | 0.71 | 0.00 | 0.00 | 0.00 | 0.16 | 3.75 | 24.64 | 0.00 | 0.06 | 0.00 | 1.52 | ||
| GC01 | 0.00 | 1.89 | 1.02 | 0.00 | 0.15 | 0.06 | 0.00 | 0.00 | 0.03 | 0.00 | 0.00 | 0.34 | ||
| East China Sea (ECS) | IE06 | 0.00 | 1.89 | 0.02 | 0.03 | 0.00 | 0.00 | 2.11 | 6.21 | 0.00 | 0.00 | 0.00 | 0.38 | |
| ECS13 | 0.00 | 5.38 | 0.12 | 0.06 | 0.55 | 0.20 | 4.51 | 107.54 | 0.06 | 0.04 | 0.06 | 0.22 | ||
| ECS22 | 0.00 | 3.57 | 0.63 | 0.00 | 0.00 | 0.65 | 0.00 | 0.00 | 0.12 | 0.07 | 0.00 | 0.00 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.-J.; Min, J.-O.; Kim, Y.; Lee, C.-H.; Yoo, H.; Jang, H.-K.; Kim, M.-J.; Oh, H.-J.; Lee, S.-H. Vertical Distribution of Phytoplankton Community and Pigment Production in the Yellow Sea and the East China Sea during the Late Summer Season. Water 2021, 13, 3321. https://doi.org/10.3390/w13233321
Kang J-J, Min J-O, Kim Y, Lee C-H, Yoo H, Jang H-K, Kim M-J, Oh H-J, Lee S-H. Vertical Distribution of Phytoplankton Community and Pigment Production in the Yellow Sea and the East China Sea during the Late Summer Season. Water. 2021; 13(23):3321. https://doi.org/10.3390/w13233321
Chicago/Turabian StyleKang, Jae-Joong, Jun-Oh Min, Yejin Kim, Chang-Hwa Lee, Hyeju Yoo, Hyo-Keun Jang, Myung-Joon Kim, Hyun-Ju Oh, and Sang-Heon Lee. 2021. "Vertical Distribution of Phytoplankton Community and Pigment Production in the Yellow Sea and the East China Sea during the Late Summer Season" Water 13, no. 23: 3321. https://doi.org/10.3390/w13233321
APA StyleKang, J.-J., Min, J.-O., Kim, Y., Lee, C.-H., Yoo, H., Jang, H.-K., Kim, M.-J., Oh, H.-J., & Lee, S.-H. (2021). Vertical Distribution of Phytoplankton Community and Pigment Production in the Yellow Sea and the East China Sea during the Late Summer Season. Water, 13(23), 3321. https://doi.org/10.3390/w13233321








