Strategies for Controlling Filamentous Bulking in Activated Sludge Wastewater Treatment Plants: The Old and the New
Abstract
:1. Introduction
2. Filamentous Bacteria: Identification and Global Distribution
| Country | Ranking (in Order of Dominance) | Reference | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Bulking | ||||
| Australia | M. parvicella | Type 0041/0675 | Type 0092 | [43] |
| Denmark | Ca. Microthrix | Trichococcus | Chloroflexi | [44] |
| M. parvicella | Type 0041 | N. limicola | [22] | |
| Czech Republic | M. parvicella | N. limicola | Type 0092 | [45] |
| France | M. parvicella | Type 0675 | GALO | [46] |
| Germany | Type 0092 | M. parvicella | Type 0041 | [47] |
| Italy | M. parvicella | GALO | Type 0675 | [17] |
| Netherlands | M. parvicella | Type 0041 | Type 021N | [22] |
| South Africa | Type 0092 | M. parvicella | Type 0041 | [48] |
| Type 0092 | Type 1851 | GALO | [49] | |
| GALO | Type 0041 | Type 0675 | [50] | |
| Type 0092 | Type 0675 | Type 0041 | [21] | |
| Switzerland | S. natans | Type 021N | Type 0961 | [51] |
| Foaming | ||||
| Australia | M. parvicella | GALOs | Type 0092 | [52] |
| Czech Republic | M. parvicella | N. limicola | GALO | [53] |
| France | M. parvicella | Type 0675 | GALO | [46] |
| Italy | M. parvicella | GALO | Type 0675 | [17] |
| Netherlands | M. parvicella | GALO | N. limicola | [54] |
| South Africa | Type 0092 | M. parvicella | GALO | [21] |
| United Kingdom | M. parvicella | N. limicola | GALO | [55] |
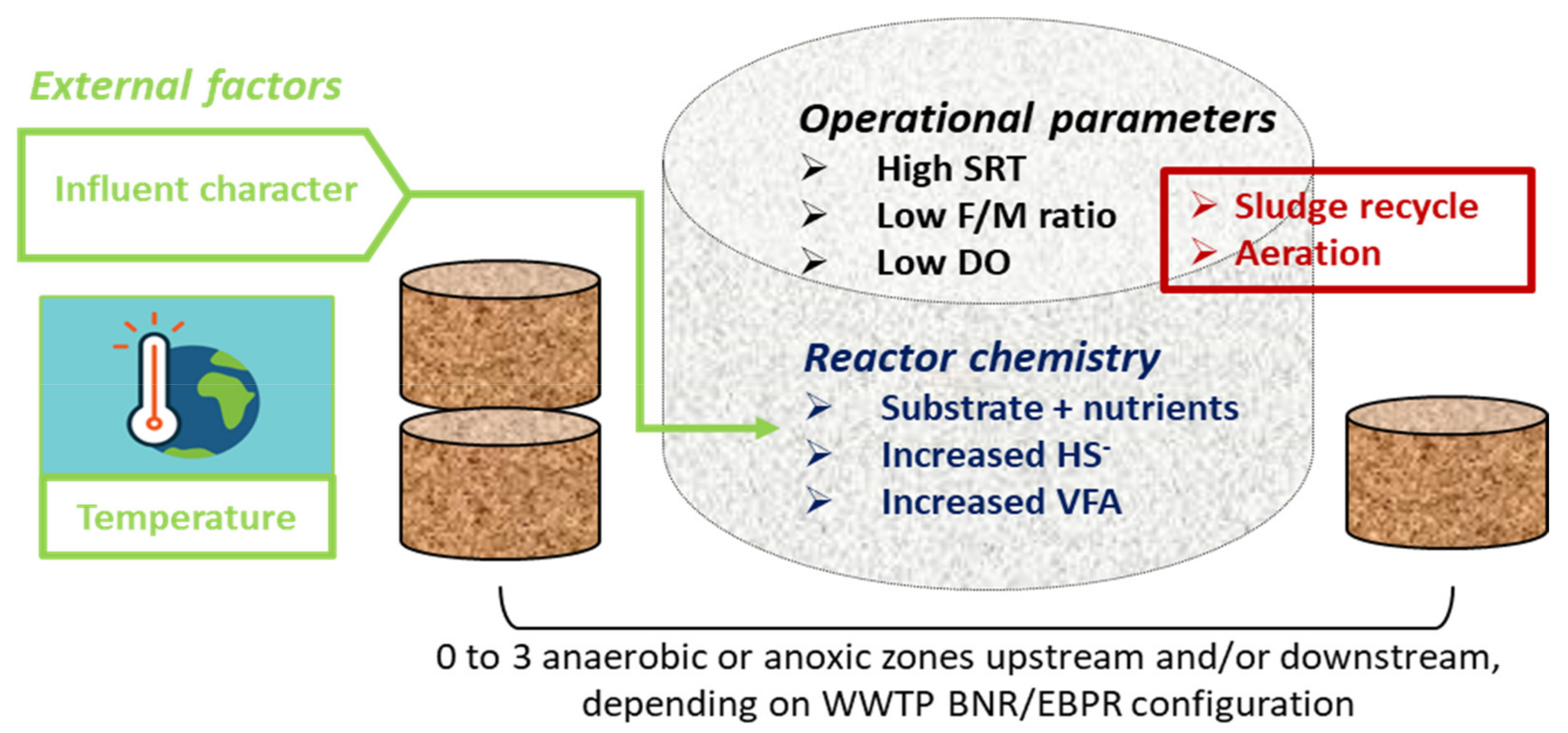
3. Factors Favouring the Growth of Filamentous Bacteria
3.1. Wastewater and Mixed Liquor Composition
3.1.1. Food-to-Microorganism Ratio/Substrate Availability
3.1.2. Inorganic Nutrients: Nitrogen and Phosphorus
3.1.3. Sulphides and Volatile Organic Acids
3.1.4. Toxic Metals
3.2. Other Operational Parameters
3.2.1. Dissolved Oxygen
3.2.2. Sludge Retention Time and Sludge Load
3.3. Climatic and Seasonal Factors
Temperature
4. Control Strategies for Filamentous Bulking Sludge
4.1. Non-Specific Methods
4.1.1. Chlorination-Based Control
4.1.2. Hydrogen Peroxide-Based Control
4.1.3. Ozone
4.1.4. Metal Application (Aluminium and Iron)
4.1.5. Synthetic Polymer-Based Control
4.1.6. Magnetic Field Application
4.1.7. Quorum Sensing
4.2. Specific Methods
4.2.1. Selector-Based Control
4.2.2. Condition-Based Control
4.3. Biological Control
4.3.1. Rotifer-Based Control
4.3.2. Bacteriophage-Mediated Control
5. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Juang, D.F. Effects of synthetic polymer on the filamentous bacteria in activated sludge. Bioresour. Technol. 2005, 96, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Mielczarek, A.T.; Nguyen, H.T.T.; Nielsen, J.L.; Nielsen, P.H. Population dynamics of bacteria involved in enhanced biological phosphorus removal in Danish wastewater treatment plants. Water Res. 2013, 47, 1529–1544. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, Q.; Wang, D.; Li, X.; Zhong, Y.; Li, X.; Deng, Y.; Wang, L.; Yi, K.; Zeng, G. Enhanced dewaterability of waste activated sludge by Fe (II)-activated peroxymonosulfate oxidation. Bioresour. Technol. 2016, 206, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Zhang, T. Profiling bulking and foaming bacteria in activated sludge by high throughput sequencing. Water Res. 2012, 46, 2772–2782. [Google Scholar] [CrossRef] [PubMed]
- Seka, M.; Hammes, F.; Verstraete, W. Predicting the effects of chlorine on the micro-organisms of filamentous bulking activated sludges. Appl. Microbiol. Biotechnol. 2003, 61, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Burger, W.; Krysiak-Baltyn, K.; Scales, P.J.; Martin, G.J.; Stickland, A.D.; Gras, S.L. The influence of protruding filamentous bacteria on floc stability and solid-liquid separation in the activated sludge process. Water Res. 2017, 123, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.; Richard, M.G.; Daigger, G.T. Manual on the Causes and Control of Activated Sludge Bulking, Foaming, and Other Solids Separation Problems, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2003; pp. 38–54. [Google Scholar]
- Mesquita, D.; Amaral, A.; Ferreira, E. Identifying different types of bulking in an activated sludge system through quantitative image analysis. Chemosphere 2011, 85, 643–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, J.; Lou, I.; de los Reyes, F.L. Relationship of species-specific filament levels to filamentous bulking in activated sludge. Appl. Environ. Microbiol. 2004, 70, 2420–2428. [Google Scholar] [CrossRef] [Green Version]
- Naidoo, D. Molecular Analyses of Pure Cultures of Filamentous Bacteria Isolated from Activated Sludge. Ph.D. Thesis, Durban Institute of Technology, Durban, South Africa, 2005. [Google Scholar]
- Schuler, A.J.; Jassby, D. Filament content threshold for activated sludge bulking: Artifact or reality. Water Res. 2007, 41, 4349–4356. [Google Scholar] [CrossRef]
- Li, W.M.; Liao, X.W.; Guo, J.S.; Zhang, Y.L.; Chen, Y.P.; Fang, F.; Yan, P. New insights into filamentous sludge bulking: The potential role of extracellular polymeric substances in sludge bulking in the activated sludge process. Chemosphere 2020, 248, 126012. [Google Scholar] [CrossRef]
- Shen, Y.; Huang, D.M.; Chen, Y.P.; Yan, P.; Gao, X. New insight into filamentous sludge bulking during wastewater treatment: Surface characteristics and thermodynamics. Sci. Total Environ. 2020, 712, 135–795. [Google Scholar] [CrossRef]
- Xu, P.; Xie, Z.; Shi, L.; Yan, X.; Fu, Z.; Ma, J.; Zhang, W.; Wang, H.; Xu, B.; He, Q. Distinct responses of aerobic granular sludge sequencing batch reactors to nitrogen and phosphorus deficient conditions. Sci. Total Environ. 2022, 834, 155–369. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, H.L.; Yang, S.F.; Tay, J.H. Mechanisms and models for anaerobic granulation in upflow anaerobic sludge blanket reactor. Water Res. 2003, 37, 661–673. [Google Scholar] [CrossRef]
- Khairnar, K.; Pal, P.; Chandekar, R.H.; Paunikar, W.N. Isolation and characterization of bacteriophages infecting nocardioforms in wastewater treatment plant. Biotechnol. Res. Int. 2014, 14, 224–625. [Google Scholar] [CrossRef] [Green Version]
- Madoni, P.; Davoli, D.; Gibin, G. Survey of filamentous microorganisms from bulking and foaming activated-sludge plants in Italy. Water Res. 2000, 34, 1767–1772. [Google Scholar] [CrossRef]
- Song, Y.; Liang, Z.L.; Zhu, H.Z.; Jiand, Y.; Yin, Y.; Qin, Y.L.; Juang, H.J.; Wang, B.J.; Wei, Z.Y.; Cheng, R.X.; et al. Candidatus Kaistella beijingensis sp. nov., Isolated from a Municipal Wastewater Treatment Plant, Is Involved in Sludge Foaming. Appl. Environ. Microbiol. 2021, 87, 15–34. [Google Scholar] [CrossRef]
- Mamais, D.; Andreadakis, A.; Noutsopoulos, C.; Kalergis, C. Causes of, and control strategies for, Microthrix parvicella bulking and foaming in nutrient removal activated sludge systems. Water Sci. Technol. 1998, 37, 9–17. [Google Scholar] [CrossRef]
- Liu, M.; Gill, J.J.; Young, R.; Summer, E.J. The diversity and host interactions of Propionibacterium acnes bacteriophages on human skin. ISME J. 2015, 9, 2078–2093. [Google Scholar] [CrossRef] [Green Version]
- Blackbeard, J.R.; Gabb, D.M.D.; Ekama, G.A.; Marais, G. Identification of filamentous organisms in nutrient removal activated sludge plants in South Africa. Water SA 1988, 14, 29–33. [Google Scholar] [CrossRef]
- Eikelboom, D.H.; Andreadakis, A.; Andreasen, K. Survey of filamentous populations in nutrient removal plants in four European countries. Water Sci. Technol. 1998, 37, 281–289. [Google Scholar] [CrossRef]
- Eikelboom, D.H.; Geurkink, B. Filamentous micro-organisms observed in industrial activated sludge plants. Water Sci. Technol. 2002, 46, 535–542. [Google Scholar] [CrossRef] [Green Version]
- Speirs, L.B.; Rice, D.T.; Petrovski, S.; Seviour, R.J. The phylogeny, biodiversity, and ecology of the Chloroflexi in activated sludge. Front. Microbiol. 2019, 10, 2015. [Google Scholar] [CrossRef] [Green Version]
- Kanagawa, T.; Kamagata, Y.; Aruga, S.; Kohno, T.; Horn, M.; Wagner, M. Phylogenetic analysis of and oligonucleotide probe development for Eikelboom type 021N filamentous bacteria isolated from bulking activated sludge. Appl. Environ. Microbiol. 2000, 66, 5043–5052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunkel, T.; Gallegos, E.L.; Bock, C.; Lange, A.; Hoffman, D.; Boenigk, J.; Denecke, M. Illumina sequencing for the identification of filamentous bulking and foaming bacteria in industrial activated sludge plants. Int. J. Environ. Sci. Technol. 2018, 15, 1139–1158. [Google Scholar] [CrossRef]
- Zhang, M.; Yao, J.; Wang, X.; Hong, Y.; Chen, Y. The microbial community in filamentous bulking sludge with the ultra-low sludge loading and long sludge retention time in oxidation ditch. Sci. Rep. 2019, 9, 13693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.; Yu, Z.; Qi, R.; Zhang, H. Detailed comparison of bacterial communities during seasonal sludge bulking in a municipal wastewater treatment plant. Water Res. 2016, 105, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Nittami, T.; Batinovic, S. Recent advances in understanding the ecology of the filamentous bacteria responsible for activated sludge bulking. Lett. Appl. Microbiol. 2021, 75, 759–775. [Google Scholar] [CrossRef] [PubMed]
- NIttami, T.; Spiers, L.B.M.; Yamada, T.; Suzuki, I.; Fukuda, J.; Kurisu, F.; Seviour, R.J. Quantification of Chloroflexi Eikelboom morphotype 1851 for prediction and control of bulking events in municipal activated sludge plants in Japan. Appl. Microbiol. Biotechnol. 2017, 101, 3861–3869. [Google Scholar] [CrossRef]
- Midas Field Guide. Available online: https://www.midasfieldguide.org (accessed on 27 June 2022).
- Eikelboom, D.H. Process Control of Activated Sludge Plants by Microscopic Investigation, 1st ed.; IWA Publishing: London, UK, 2000; pp. 159–160. [Google Scholar]
- Khan, M.; Faheem, S. Monitoring Bacterial Diversity in a full-scale Municipal Wastewater Treatment plant in Dubai by Fluorescence in situ hybridization Technique. Int. J. Environ. Res. 2013, 7, 479–484. [Google Scholar]
- Martins, A.M.; Pagilla, K.; Heijnen, J.J.; van Loosdrecht, M.C. Filamentous bulking sludge—A critical review. Water Res. 2004, 38, 793–817. [Google Scholar] [CrossRef]
- Wang, J.; Li, Q.; Qi, R.; Tandoi, V.; Yang, M. Sludge bulking impact on relevant bacterial populations in a full-scale municipal wastewater treatment plant. Process Biochem. 2014, 49, 2258–2265. [Google Scholar] [CrossRef]
- Guo, J.; Peng, Y.; Yang, X.; Wang, Z.; Zhu, A. Effects of feeding pattern and dissolved oxygen concentration on microbial morphology and community structure: The competition between floc-forming bacteria and filamentous bacteria. J. Water Process. Eng. 2014, 1, 108–114. [Google Scholar] [CrossRef]
- Aruga, S.; Kamagata, Y.; Kohno, T.; Hanada, S.; Nakamura, K.; Kanagawa, T. Characterization of filamentous Eikelboom type 021N bacteria and description of Thiothrix disciformis sp. nov. and Thiothrix flexilis sp. nov. Int. J. Syst. Evol. Microbiol. 2002, 52, 1309–1316. [Google Scholar] [CrossRef] [Green Version]
- Speirs, L.; Nittami, T.; McIlroy, S.; Schroeder, S.; Seviour, R.J. Filamentous bacterium Eikelboom type 0092 in activated sludge plants in Australia is a member of the phylum Chloroflexi. Appl. Environ. M.icrobiol. 2009, 75, 2446–2452. [Google Scholar] [CrossRef] [Green Version]
- Nittami, T.; Kasakura, R.; Kobayashi, T.; Suzuki, K.; Koshiba, Y.; Fukuda, J.; Takeda, M.; Tobino, T.; Kurisu, F.; Rice, D.; et al. Exploring the operating factors controlling Kouleothrix (type 1851), the dominant filamentous bacterial population, in a full-scale A2O plant. Sci. Rep. 2020, 10, 6809. [Google Scholar] [CrossRef] [Green Version]
- Rossetti, S.; Tomei, M.C.; Nielsen, P.H.; Tandoi, V. “Microthrix parvicella”, a filamentous bacterium causing bulking and foaming in activated sludge systems: A review of current knowledge. FEMS Microbiol. Rev. 2005, 1, 49–64. [Google Scholar] [CrossRef] [Green Version]
- Soddell, J.A.; Stainsby, F.M.; Eales, K.L.; Kroppenstedt, R.M.; Seviour, R.J.; Goodfellow, M. Millisia brevis gen. nov., sp. nov., an actinomycete isolated from activated sludge foam. Int. J. Syst. Evol. Microbiol. 2006, 56, 739–744. [Google Scholar] [CrossRef] [Green Version]
- Aonofriesei, F.; Petrosanu, M. Activated sludge bulking episodes and dominant filamentous bacteria at wastewater treatment plant. Proc. Rom. Acad. Series B 2007, 2, 83–87. [Google Scholar]
- Seviour, E.M.; Williams, C.; DeGrey, B.; Soddell, J.A.; Seviour, R.J.; Lindrea, K.C. Studies on filamentous bacteria from Australian activated sludge plants. Water Res. 1994, 28, 2335–2342. [Google Scholar] [CrossRef]
- Nierychlo, M.; McIlroy, S.J.; Kucheryavskiy, S.; Jiang, C.; Ziegler, A.S.; Kondrotaite, Z.; Stokholm-Bjerrgaard, M.; Nielsen, P.H. Candidatus Amarolinea and Candidatus Microthrix are mainly responsible for filamentous bulking in Danish municipal wastewater treatment plants. Front. Microbiol. 2020, 11, 1214. [Google Scholar] [CrossRef]
- Pujol, R.; Canler, J.P. Contact zone: French practice with low F/M bulking control. Water Sci. Technol. 1994, 29, 221–228. [Google Scholar] [CrossRef]
- Pujol, R.; Duchene, P.; Schetrite, S.; Canler, J.P. Biological foams in activated sludge plants: Characterization and situation. Water Res. 1991, 25, 1399–1404. [Google Scholar] [CrossRef]
- Wagner, F. Study of the causes and prevention of sludge bulking in Germany, Bulking of activated sludge. Water Sci. Technol. 1984, 16, 1–14. [Google Scholar] [CrossRef]
- Welz, P.J.; Kumari-Santosh, S.; Uys, C.; van Blerk, N.; Smith, A.; Bux, F.; Conco, T.; Thobejane, P.; Sonjica, N. National Survey of filamentous bacteria in activated sludge. WRC. Technical Report 2022, no 2471/1/22. Available online: https://search.wrc.org.za/ (accessed on 8 October 2022).
- Welz, P.J.; Esterhuysen, A.; Vulindlu, M.; Bezuidenhout, C. Filament identification and dominance of Eikelboom Type 0092 in activated sludge from wastewater treatment facilities in Cape Town, South Africa. Water SA 2014, 40, 649–658. [Google Scholar] [CrossRef] [Green Version]
- Lacko, N.; Bux, F.; Kasan, H.C. Survey of filamentous bacteria in activated sludge plants in KwaZulu-Natal. Water SA 1999, 25, 63–68. [Google Scholar] [CrossRef]
- Kappeler, J.; Gujer, W. Bulking in activated sludge systems: A qualitative simulation model for Sphaerotilus natans, Type 021N and Type 0961. Water Sci. Technol. 1992, 26, 473–482. [Google Scholar] [CrossRef]
- Seviour, E.M.; Williams, C.J.; Seviour, R.J.; Soddell, J.A.; Lindrea, K.C. A survey of filamentous bacterial populations from foaming activated sludge plants in eastern states of Australia. Water Res. 1990, 24, 493–498. [Google Scholar] [CrossRef]
- Wanner, J. Stable foams and sludge bulking: The largest remaining problems (Abridged). Water Environ. J. 1998, 12, 368–374. [Google Scholar] [CrossRef]
- Eikelboom, D.H. Scum formation in carrousel treatment plants. In Proceedings of the IAWPRC Specialized Seminar on Interactions of Wastewater, Biomass and Reactor configurations in Biological Treatment Plants, Copenhagen, Denmark, 21–23 August 1991. [Google Scholar]
- Foot, R.; Kocianova, E.; Forster, C. Variable morphology of Microthrix parvicella in activated sludge systems. Water Res. 1992, 26, 875–880. [Google Scholar] [CrossRef]
- Miłobędzka, A.; Witeska, A.; Muszyński, A. Factors affecting population of filamentous bacteria in wastewater treatment plants with nutrients removal. Water Sci. Technol. 2016, 73, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Beer, M.; Seviour, E.M.; Kong, Y.; Cunningham, M.; Blackall, L.L.; Seviour, R.J. Phylogeny of the filamentous bacterium Eikelboom Type 1851, and design and application of a 16S rRNA targeted oligonucleotide probe for its fluorescence in situ identification in activated sludge. FEMS Microbiol. Lett. 2002, 207, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.Q.; Nguyen, L.N.; Abu Hasan, A.B.; Ngo, H.H.; Nghiem, L.D. Linking endogenous decay and sludge bulking in the microbial community to membrane fouling at sub-critical flux. JMS Lett. 2022, 2, 100023. [Google Scholar] [CrossRef]
- Jenkins, D.; Richard, M.; Daigger, G. Manual on the Causes and Control of Activated Sludge Bulking and Foaming, 2nd ed.; Lewis Publishers: Boca Raton, FL, USA, 1993. [Google Scholar]
- Amin, L.; van der Steen, P.; Lopez-Vazquez, C.M. Expanding the activated sludge model no. 1 to describe filamentous bulking: The filamentous model. J. Water Process. Eng. 2022, 48, 102896. [Google Scholar] [CrossRef]
- Lou, I.; In Ieong, I. Modeling growth of filaments and floc formers in activated sludge flocs: Integrating the effects of kinetics and diffusion. Environ. Model. Assess. 2015, 20, 225–237. [Google Scholar] [CrossRef]
- Martins, A.M.P.; Karahan, Ö.; van Loosdrecht, M.C.M. Effect of polymeric substrate on sludge settleability. Water Res. 2011, 45, 263–273. [Google Scholar] [CrossRef]
- Chudoba, J.; Grau, P.; Ottová, V. Control of activated-sludge filamentous bulking–II. Selection of microorganisms by means of a selector. Water Res. 1973, 7, 1389–1406. [Google Scholar] [CrossRef]
- Noutsopoulos, C.; Andreadakis, A.; Mamais, D.; Gavalakis, E. Identification of type and causes of filamentous bulking under Mediterranean conditions. Environ. Technol. 2007, 28, 115–122. [Google Scholar] [CrossRef]
- Richard, M.; Brown, S.; Collins, F. Activated sludge microbiology problems and their control. In Proceedings of the 20th Annual USEPA National Operator Trainers Conference, Buffalo, NY, USA, 8 June 2003. [Google Scholar]
- Peng, Y.; Gao, C.; Wang, S.; Ozaki, M.; Takigawa, A. Non-filamentous sludge bulking caused by a deficiency of nitrogen in industrial wastewater treatment. Water Sci. Technol. 2003, 47, 289–295. [Google Scholar] [CrossRef]
- Kjellerup, B.V.; Keiding, K.; Nielsen, P.H. Monitoring and troubleshooting of non-filamentous settling and dewatering problems in an industrial activated sludge treatment plant. Water Sci. Technol. 2001, 44, 155–162. [Google Scholar] [CrossRef]
- Jobbágy, A.; Literáthy, B.; Tardy, G. Implementation of glycogen accumulating bacteria in treating nutrient-deficient wastewater. Water Sci. Technol. 2002, 46, 185–190. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, A.H.; Vollertsen, J. Model parameters for aerobic biological sulfide oxidation in sewer wastewater. Water 2021, 13, 981. [Google Scholar] [CrossRef]
- Nielsen, P.H.; de Muro, M.A.; Nielsen, J.L. Studies on the in-situ physiology of Thiothrix spp. present in activated sludge. Environ. Microbiol. 2000, 2, 89–398. [Google Scholar] [CrossRef]
- De Graff, D.R.; van Loosdrecht, M.C.M.; Pronk, M. Stable granulation of seawater-adapted aerobic granular sludge with filamentous Thiothrix bacteria. Water Res. 2017, 175, 115683. [Google Scholar] [CrossRef]
- Rubio-Rincón, F.J.; Welles, L.; Lopez-Vazquez, C.M.; Nierychlo, M.; Abbas, B.; Geleijnse, M.; Nielsen, P.H.; van Loosdrecht, M.C.M.; Brdjanovic, D. Long-term effects of sulphide on the enhanced biological removal of phosphorus: The symbiotic role of Thiothrix caldifontis. Water Res. 2017, 116, 53–64. [Google Scholar] [CrossRef]
- Pelletier, C.; Ann Fitzsimmons, M.; Deschênes, S.; Paice, M. Impact of septic compounds and operational conditions on the microbiology of an activated sludge system. Water Sci. Technol. 2007, 55, 135–142. [Google Scholar] [CrossRef]
- Gulez, G.; de los Reyes, F.L. Multiple approaches to assess filamentous bacterial growth in activated sludge under different carbon source conditions. J. Appl. Microbiol. 2009, 106, 682–691. [Google Scholar] [CrossRef]
- Miyazato, N.; Yamamoto-Ikemoto, R.; Takamatsu, S. Microbial community change of sulfate reduction and sulfur oxidation bacteria in the activated sludge cultivated with acetate and peptone. Water Sci. Technol. 2006, 54, 111–119. [Google Scholar] [CrossRef]
- Yamamoto-Ikemoto, R.; Matsui, S.; Komori, T.; Bosque-Hamilton, E.K. Interactions between filamentous sulfur bacteria, sulfate reducing bacteria and poly-P accumulating bacteria in anaerobic-oxic activated sludge from a municipal plant. Water Sci. Technol. 1998, 37, 599–603. [Google Scholar] [CrossRef]
- Yamamoto-Ikemoto, R.; Matsui, S.; Komori, T.; Bosque-Hamilton, E.K. Control of filamentous bulking and interactions among sulfur oxidation-reduction and iron oxidation-reduction in activated sludge using an iron coagulant. Water Sci. Technol. 1998, 38, 9–17. [Google Scholar] [CrossRef]
- Fan, N.; Yang, M.; Rossetti, S.; Levantesi, C.; Qi, R. Monitoring, isolation and characterization of Microthrix parvicella strains from a Chinese wastewater treatment plant. Water Sci. Technol. 2019, 79, 1406–1416. [Google Scholar] [CrossRef] [PubMed]
- Shuttleworth, K.L.; Unz, R.F. Influence of metals and metal speciation on the growth of filamentous bacteria. Water Res. 1991, 25, 1177–1186. [Google Scholar] [CrossRef]
- Anderson, J.; Kim, H.; McAvoy, T.; Hao, O. Control of an alternating aerobic–anoxic activated sludge system—Part 1: Development of a linearization-based modeling approach. Control Eng. Pract. 2000, 8, 271–278. [Google Scholar] [CrossRef]
- Wilén, B.M.; Balmer, P. The effect of dissolved oxygen concentration on the structure, size and size distribution of activated sludge flocs. Water Res. 1999, 33, 391–400. [Google Scholar] [CrossRef]
- Martins, A.M.P.; Pagilla, K.; Heijnen, J.J.; van Loosdrecht, M.C.M. Effect of dissolved oxygen concentration on sludge settleability. Appl. Microbiol. Biotechnol. 2003, 62, 586–593. [Google Scholar] [CrossRef]
- Vaiopoulou, E.; Melidis, P.; Aivasidis, A. An activated sludge treatment plant for integrated removal of carbon, nitrogen and phosphorus. Desalination 2007, 211, 192–199. [Google Scholar] [CrossRef]
- Campbell, K.; Wang, J.; Daigger, G.T. Filamentous organisms degrade oxygen transfer efficiency by increasing mixed liquor apparent viscosity: Mechanistic understanding and experimental verification. Water Res. 2020, 173, 115570. [Google Scholar] [CrossRef]
- Liu, G.; Wang, J.; Campbell, K. Formation of filamentous microorganisms impedes oxygen transfer and decreases aeration efficiency for wastewater treatment. J. Clean. Prod. 2018, 189, 502–509. [Google Scholar] [CrossRef]
- Adonadaga, M.G.; Ampadu, B.; Martienssen, M. Effect of sludge loading and dissolved oxygen concentration on proliferation of Thiothrix and Eikelboom Types 1851 and 0041 in activated sludge wastewater treatment plants. Int. J. Appl. Sci. 2016, 6, 8–13. [Google Scholar]
- Noutsopoulos, C.; Mamais, D.; Andreadakis, A. A hypothesis on Microthrix parvicella proliferation in biological nutrient removal activated sludge systems with selector tanks. FEMS Microbiol. Ecol. 2012, 80, 380–389. [Google Scholar] [CrossRef]
- Slijkhuis, H. Microthrix parvicella, a filamentous bacterium isolated from activated sludge: Cultivation in a chemically defined medium. Appl. Environ. Microbiol. 1983, 46, 832–839. [Google Scholar] [CrossRef] [Green Version]
- Fan, N.; Wang, R.; Qi, R.; Gao, Y.; Rossetti, S.; Tandoi, V.; Yang, M. Control strategy for filamentous sludge bulking: Bench-scale test and full-scale application. Chemosphere 2018, 210, 709–716. [Google Scholar] [CrossRef]
- Guo, P.; Wang, B.Z.; Hang, B.J.; Li, L.; Li, S.P.; He, J. Sphingobium faniae sp. nov., a pyrethroid-degrading bacterium isolated from activated sludge treating wastewater from pyrethroid manufacture. Int. J. Syst. Evol. Microbiol. 2010, 60, 408–412. [Google Scholar] [CrossRef] [Green Version]
- Friedrich, M.; Takács, I.; Tränckner, J. Physiological adaptation of growth kinetics in activated sludge. Water Res. 2015, 85, 22–30. [Google Scholar] [CrossRef]
- Li, D.; Cui, F.; Zhao, Z.; Liu, D.; Xu, Y.; Li, H.; Yang, X. The impact of titanium dioxide nanoparticles on biological nitrogen removal from wastewater and bacterial community shifts in activated sludge. Biodegradation 2014, 2, 167–177. [Google Scholar] [CrossRef]
- Campbell, K.; Wang, J.; Daniels, M. Assessing activated sludge morphology and oxygen transfer performance using image analysis. Chemosphere 2019, 223, 694–703. [Google Scholar] [CrossRef]
- Chen, Y.; Xiao, K.; Jiang, X.; Shen, N.; Zeng, R.J.; Zhou, Y. Long solid retention time (SRT) has minor role in promoting methane production in a 65 C single-stage anaerobic sludge digester. Bioresour. Technol. 2018, 247, 724–729. [Google Scholar] [CrossRef]
- Maza-Márquez, P.; Castellano-Hinojosa, A.; González-Martinez, A.; Juárez-Jiménez, B.; González-López, J.; Rodelas, B. Abundance of total and metabolically active Candidatus Microthrix and fungal populations in three full-scale wastewater treatment plants. Chemosphere 2019, 232, 26–34. [Google Scholar] [CrossRef]
- Sweetapple, C.; Fu, G.; Farmani, R.; Butler, D. Exploring wastewater system performance under future threats: Does enhancing resilience increase sustainability? Water Res. 2019, 149, 448–459. [Google Scholar] [CrossRef]
- Zlateva, P. Sliding mode control of wastewater treatment process with activated sludge under extreme weather events. In Proceedings of the 6th International Conference on Advances in Environmental Research, Sapporo, Japan, 26–28 August 2020. [Google Scholar]
- Johnston, J.; Behrens, S. Seasonal dynamics of the activated sludge microbiome in sequencing batch reactors, assessed using 16S rRNA transcript amplicon sequencing. Appl. Environ. Microbiol. 2020, 86, 597. [Google Scholar] [CrossRef]
- Wagner, D.S.; Peces, M.; Nierychlo, M.; Mielczarek, A.T.; Thornberg, D.; Nielsen, P.H. Seasonal microbial community dynamics complicates the evaluation of filamentous bulking mitigation strategies in full-scale WRRFs. Water Res. 2022, 216, 118. [Google Scholar] [CrossRef]
- Chen, X.; Yuan, L.; Lv, J.; Nie, K. Influence of anoxic to aerobic volume ratio on sludge settleability and bacterial community structure in a denitrifying–nitrifying activated sludge system. Desalination Water Treat. 2015, 56, 1863–1876. [Google Scholar] [CrossRef]
- Abusam, A.; Al-Salameen, F.; Mydlarczyk, A.; Ahmed, M.E. Seasonal variations of the growth of filamentous bacteria in Kuwait’s wastewater treatment plants. Desalination Water Treat. 2019, 176, 370–374. [Google Scholar] [CrossRef]
- Fan, N.; Qi, R.; Rossetti, S.; Tandoi, V.; Gao, Y.; Yang, M. Factors affecting the growth of Microthrix parvicella: Batch tests using bulking sludge as seed sludge. Sci. Total Environ. 2017, 609, 1192–1199. [Google Scholar] [CrossRef]
- Jiang, X.T.; Guo, F.; Zhang, T. Population dynamics of bulking and foaming bacteria in a full-scale wastewater treatment plant over five years. Sci. Rep. 2016, 6, 24180. [Google Scholar] [CrossRef] [Green Version]
- Janczukowicz, W.; Szewczyk, M.; Krzemieniewski, M.; Pesta, J. Settling properties of activated sludge from a sequencing batch reactor (SBR). Pol. J. Environ. Stud. 2001, 10, 15–20. [Google Scholar]
- Ramothokang, T.; Drysdale, G.; Bux, F. Isolation and cultivation of filamentous bacteria implicated in activated sludge bulking. Water SA 2003, 29, 405–410. [Google Scholar] [CrossRef] [Green Version]
- Abusam, A.; Al-Salameen, F.; Mydlarczyk, A.; Ahmed, M.E. Filamentous Bacteria Identification by VIT Method. Int. J. Environ. Sci. 2016, 7, 177. [Google Scholar] [CrossRef] [Green Version]
- Roels, T.; Dauwe, F.; Van Damme, S.; De Wilde, K.; Roelandt, F. The influence of PAX-14 on activated sludge systems and in particular on Microthrix parvicella. Water Sci. Technol. 2002, 46, 487–490. [Google Scholar] [CrossRef]
- Mamais, D.; Kalaitzi, E.; Andreadakis, A. Foaming control in activated sludge treatment plants by coagulants addition. Glob. Nest J. 2011, 13, 237–245. [Google Scholar] [CrossRef]
- Ayers, D.; Kelly, R.; Crane, T. Solutions to Mitigate Effects of Microthrix parvicella at the Meridian WWTP. In Proceedings of the PNCWA, 2012 Annual Conference, Boise, ID, USA, 21–24 October 2012. [Google Scholar]
- Lever, M. Operator’s guide to activated sludge bulking. In Proceedings of the 35th Annual Q Water Industry, CQ University, Rockhampton, QLD, Australia, 22–24 June 2010. [Google Scholar]
- Cubbage, L.E.; Pitt, P.A.; Stone, A.L.; He, X.L.; de los Reyes, F. A green alternative for dissolved nutrient recovery in wastewater sidestreams. In Proceedings of the Water Environment Federation Conference, WEF 2011, 4, Alexandria, Egypt, 25–28 January 2011. [Google Scholar] [CrossRef]
- D’Antoni, B.M.; Iracà, F.; Romero, M. Filamentous Foaming and Bulking in Activated Sludge Treatments: Causes and Mitigation Action; Panta Rei Water Solutions: Fiesso, Italy, 2017. [Google Scholar]
- Caravelli, A.; Giannuzzi, L.; Zaritzky, N. Effect of chlorine on filamentous microorganisms present in activated sludge as evaluated by respirometry and INT-dehydrogenase activity. Water Res. 2004, 38, 2395–2405. [Google Scholar] [CrossRef]
- Zerva, I.; Remmas, N.; Kagalou, I.; Melidis, P.; Ariantsi, M.; Sylaios, G.; Ntougias, S.I. Effect of chlorination on microbiological quality of effluent of a full-scale wastewater treatment plant. Life 2021, 11, 68. [Google Scholar] [CrossRef] [PubMed]
- Saayman, G.B.; Schutte, C.F.; Van Leeuwen, J. Chemical control of filamentous sludge bulking in a full-scale biological nutrient removal activated sludge plant. Water SA 1997, 20, 1–15. [Google Scholar] [CrossRef]
- Caravelli, A.; Giannuzzi, L.; Zaritzky, N. Effect of ozone on filamentous bulking in a laboratory scale activated sludge reactor using respirometry and INT-dehydrogenase activity. J. Environ. Eng. 2006, 132, 1001–1010. [Google Scholar] [CrossRef]
- Francy, D.S.; Stelzer, E.A.; Bushon, R.N.; Brady, A.M.G.; Williston, A.G.; Riddell, K.R.; Borchardt, M.A.; Spencer, S.K.; Gellner, T.M. Comparative effectiveness of membrane bioreactors, conventional secondary treatment, and chlorine and UV disinfection to remove microorganisms from municipal wastewaters. Water. Res. 2012, 46, 4164–4178. [Google Scholar] [CrossRef]
- Pang, Y.C.; Xi, J.Y.; Xu, Y.; Huo, Z.Y.; Hu, H.Y. Shifts of live bacterial community in secondary effluent by chlorine disinfection revealed by Miseq high-throughput sequencing combined with propidium monoazide treatment. Appl. Microbiol. Biotechnol. 2016, 100, 6435–6446. [Google Scholar] [CrossRef]
- Ramírez, G.W.; Alonso, J.L.; Villanueva, A.; Guardino, R.; Basiero, J.A.; Bernecer, I.; Morenilla, J.J. A rapid, direct method for assessing chlorine effect on filamentous bacteria in activated sludge. Water Res. 2000, 34, 3894–3898. [Google Scholar] [CrossRef]
- Wells, G.F. Microbial biogeography across a full-scale wastewater treatment plant transect: Evidence for immigration between coupled processes. Appl. Microbiol. Biotechnol. 2014, 98, 4723–4736. [Google Scholar] [CrossRef]
- Klorman Industries Pty Ltd Chlorine in Water Treatment. Available online: https://klorman-industries.com/water-treatment/chlorine-in-water-treatment/ (accessed on 16 August 2022).
- Nasr, A. The effect of using microorganisms on sludge reduction in wastewater treatment plant. In Proceedings of the 14th International Water Technology Conference, Cairo, Egypt, 21–23 March 2010. [Google Scholar]
- Wen, Q.; Ji, Y.; Chen, Z.; Lee, D.J. Use of sodium chloride to rapidly restore polydroxyalkanoates production from filamentous bulking without polyhydroxyalkanoates productivity impairment. Bioresour. Technol. 2020, 313, 123–516. [Google Scholar] [CrossRef]
- Saleh, M.Y.; El Enany, G.; Elzahar, M.H.; Elshikhipy, M.Z. Removal of lead in high rate activated sludge system. Int. J. Environ. Ecol. Eng. 2014, 8, 413–418. [Google Scholar]
- Van Leeuwen, J. Preliminary investigation into the use of hydrogen peroxide for bulking control in a nutrient removal activated sludge system. Water SA 1992, 18, 101–103. [Google Scholar]
- He, Q.; Zhang, J.; Gao, S.; Chen, L.; Lyu, W.; Zhang, W.; Song, J.; Hu, X.; Chen, R.; Wang, H.; et al. A comprehensive comparison between non-bulking and bulking aerobic granular sludge in microbial communities. Bioresour. Technol. 2019, 294, 12215. [Google Scholar] [CrossRef]
- Koopman, B.; Bitton, G. Effect of suspended solids concentration and filament type on the toxicity of chlorine and hydrogen peroxide to bulking activated sludge. Environ. Toxicol. 1987, 2, 49–62. [Google Scholar] [CrossRef]
- Hammadi, L.; Ponton, A.; Belhadri, M. Effects of heat treatment and hydrogen peroxide (H2O2) on the physicochemical and rheological behavior of an activated sludge from a water purification plant. Procedia Eng. 2012, 33, 293–302. [Google Scholar] [CrossRef] [Green Version]
- Govoreanu, R.; Saveyn, H.; Van der Meeren, P.; Vanrolleghem, P.A. Simultaneous determination of activated sludge floc size distribution by different techniques. Water Sci. Technol. 2004, 12, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Cole, C.A.; Stamberg, J.B.; Bishop, D.F. Hydrogen peroxide cures filamentous growth in activated sludge. J. Water Pollut. 1973, 45, 829. [Google Scholar]
- Nilsson, F.; Hagman, M.; Mielczarek, A.T.; Nielsen, P.H.; Jönsson, K. Application of ozone in full-scale to reduce filamentous bulking sludge at Öresundsverket WWTP. Ozone Sci. Eng. 2014, 36, 238–243. [Google Scholar] [CrossRef]
- Levén, L.; Wijnbladh, E.; Tuvesson, M.; Kragelund, C.; Hallin, S. Control of Microthrix parvicella and sludge bulking by ozone in a full-scale WWTP. Water Sci. Technol. 2016, 73, 866–872. [Google Scholar] [CrossRef]
- Nilsson, F.; Davidsson, Å.; Falås, P.; Bengtsson, S.; Bester, K.; Jönsson, K. Impact of activated sludge ozonation on filamentous bacteria viability and possible added benefits. Environ. Technol. 2018, 40, 2601–2607. [Google Scholar] [CrossRef]
- Barbarroja, P.; Zornoza, A.; Aguado, D.; Borrás, L.; Alonso, J.L. A multivariate approach of changes in filamentous, nitrifying and protist communities and nitrogen removal efficiencies during ozone dosage in a full-scale wastewater treatment plant. Environ. Pollut. 2019, 252, 1500–1508. [Google Scholar] [CrossRef]
- Ojo, P.; Ifelebuegu, A.O. The impact of aluminium salt dosing for chemical phosphorus removal on the settleability of activated sludge. Environments 2018, 5, 88. [Google Scholar] [CrossRef] [Green Version]
- De Gregorio, C.; Caravelli, A.H.; Zaritzky, N.E. Performance and biological indicators of a laboratory-scale activated sludge reactor with phosphate simultaneous precipitation as affected by ferric chloride addition. Chem. Eng. J. 2010, 165, 607–616. [Google Scholar] [CrossRef]
- Kida, M.; Masłoń, A.; Tomaszek, J.; Koszelnik, P. The possibilities of limitation and elimination of activated sludge bulking. In Progress in Environmental Engineering, 1st ed.; CRC Press: London, UK, 2015. [Google Scholar]
- Agridiotis, V.; Forster, C.F.; Carliell-Marquet, C. Addition of Al and Fe salts during treatment of paper mill effluents to improve activated sludge settlement characteristics. Bioresour. Technol. 2007, 98, 2926–2934. [Google Scholar] [CrossRef]
- Zaidi, N.S.; Syafiuddin, A.; Sillanpää, M.; Bahrodin, M.B.; Zhan, L.Z.; Ratnasari, A.; Kadier, A.; Mehmood, M.A.; Boopathy, R. Insights into the potential application of magnetic field in controlling sludge bulking and foaming: A review. Bioresour. Technol. 2022, 358, 127416. [Google Scholar] [CrossRef]
- Seka, A.M.; Verstraete, W. Feasibility of a multi-component additive for efficient control of activated sludge filamentous bulking. Water Res. 2001, 35, 2995–3003. [Google Scholar] [CrossRef]
- Juang, D.; Chiou, L. Microbial population structures in activated sludge before and after the application of synthetic polymer. Int. J. Environ. Sci. Technol. 2007, 4, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Omar, A.H.; Muda, K.; Toemen, S.; Sulaiman, S.F.; Zaidi, N.S.; Affam, A.C. Study on the effect of a static magnetic field in enhancing initial state of biogranulation. J. Water Supply. Res. Technol-Aqua 2018, 67, 484–489. [Google Scholar] [CrossRef]
- Zaidi, N.S. Application of Magnetic Field for Reduction of Sludge Bulking in Activated Slude System. Ph.D. Thesis, University Teknologi Malaysia, Johor, Malaysia, 2016. [Google Scholar]
- Miller, M.B.; Bassler, B.L. Quorum sensing in bacteria. Ann. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.X.; Wang, X.; Guo, J.S.; Fang, F.; Chen, Y.P.; Yan, P. New insight into filamentous sludge bulking: Potential role of AHL-mediated quorum sensing in deteriorating sludge floc stability and structure. Water Res. 2022, 212, 118096. [Google Scholar] [CrossRef]
- Dong, X.; Qin, J.; Zhang, Y.; Chen, X.; Ge, S. Mitigation of membrane biofouling using quorum-quenching bacteria in a continuously operated membrane bioreactor. Int. Biodeterior. Biodegrad. 2022, 166, 105339. [Google Scholar] [CrossRef]
- Tampus, M.V.; Martins, A.M.P.; van Loosdrecht, M.C.M. The effect of anoxic selectors on sludge bulking. Water Sci. Technol. 2004, 50, 261–268. [Google Scholar] [CrossRef]
- Eleren, S.C.; Alkan, U. Reducing effect of aerobic selector on the toxicity of synthetic organic compounds in activated sludge process. Bioresour. Technol. 2009, 100, 5714–5720. [Google Scholar] [CrossRef] [PubMed]
- Duine, A.; Kunst, S. Control of bulking sludge caused by Type 021 N and Type 0961 in an industrial wastewater treatment plant with an aerobic selector. Water Sci. Technol. 2002, 46, 29–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prendi, L.; Kroib, H. Bulking sludge prevention by an aerobic selector. Water Sci. Technol. 1998, 38, 19–27. [Google Scholar] [CrossRef]
- Cardete, M.A.; Mata-Alvarez, J.; Dosta, J.; Nieto-Sánchez, R. Effect of the mixed liquor parameters on sludge settling for a petrochemical activated sludge system including an aerobic selector. J. Environ. Chem. Eng. 2018, 6, 1062–1071. [Google Scholar] [CrossRef]
- Nakhla, G.; Lugowski, A. Control of filamentous organisms in food-processing wastewater treatment by intermittent aeration and selectors. J. Chem. Technol. Biotechnol. 2018, 78, 420–430. [Google Scholar] [CrossRef]
- Al-Mutairi, N.Z. Functional biodiversity of microbial communities in aerobic selector slaughterhouse wastewater. Water Environ. Res. 2007, 79, 660–666. [Google Scholar] [CrossRef]
- Xin, G.; Gough, H.L.; Stensel, D.H. Effect of anoxic selector configuration on sludge volume index control and bacterial population fingerprinting. Water Environ. Res. 2008, 80, 2228–2240. [Google Scholar] [CrossRef]
- Ferreira, V.; Martins, C.; Pereira, M.O.; Nicolau, A. Use of an aerobic selector to overcome filamentous bulking in an activated sludge wastewater treatment plant. Environ. Technol. 2014, 35, 1525–1531. [Google Scholar] [CrossRef]
- Cardete, M.A.; Mata-Alvarez, J.; Dosta, J.; Nieto-Sánchez, R. Influence of hydraulic retention time, food-to-microorganism ratio and influent biodegradability on the performance of an aerobic selector treating petrochemical wastewater. J. Environ. Chem. Eng. 2017, 5, 5033–5042. [Google Scholar] [CrossRef]
- Gray, D.M.D.; De Lange, V.P.; Chien, M.H.; Esque, M.A.; Shao, Y.J. Investigating the fundamental basis for selectors to improve activated sludge settling. Water Environ. Res. 2010, 82, 541–555. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.S.; Teo, K.H.; Yuen, W.A.; Long, W.Y.; Seah, B. Performance analysis of anoxic selector in upgrading activated sludge process in tropical climate. Water Sci. Technol. 2005, 52, 2005. [Google Scholar] [CrossRef]
- Lee, Y.; Olezzkiewicz, J.A. Bench-scale assessment of the effectiveness of an anaerobic selector in controlling filamentous bulking. Environ. Technol. 2004, 25, 751–755. [Google Scholar] [CrossRef]
- Fan, N.S.; Qi, R.; Huang, B.C.; Jin, R.C.; Yang, M. Factors influencing Candidatus Microthrix parvicella growth and specific filamentous bulking control: A review. Chemosphere 2020, 244, 125371. [Google Scholar] [CrossRef]
- Marten, W.L.; Daigger, G.T. Full-scale evaluation of factors affecting the performance of anoxic selectors. Water Environ. Res. 1997, 69, 1272–1281. [Google Scholar] [CrossRef]
- Cisterna-Osorio, P.; Arancibia-Avila, P. Comparison of biodegradation of fats and oils by activated sludge on experimental and real scales. Water 2019, 11, 1286. [Google Scholar] [CrossRef] [Green Version]
- Clément, P. The phylogeny of rotifers: Molecular, ultrastructural and behavioural data. In Proceedings of the 6th International Rotifer Symposium, Banyoles, Spain, 3–8 June 1991. [Google Scholar]
- Rehman, A.; Shakoori, F.R.; Shakoori, A.R. Heavy metals resistant rotifers from a chromium contaminated wastewater can help in environmental clean-up. Pak. J. Zool 2008, 40, 309–316. [Google Scholar]
- Fiałkowska, E.; Pajdak-Stós, A. The role of Lecane rotifers in activated sludge bulking control. Water Res. 2008, 42, 2483–2490. [Google Scholar] [CrossRef]
- Pajdak-Stós, A.; Fiałkowska, E. The influence of temperature on the effectiveness of filamentous bacteria removal from activated sludge by rotifers. Water Environ. Res. 2012, 84, 619–625. [Google Scholar] [CrossRef]
- Kocerba-Soroka, W.; Fiałkowska, E.; Pajdak-Stós, A.; Sobczyk, M.; Starzycka, J.; Fyda, J. The use of rotifers for limiting filamentous bacteria Type 021N, a bacterium causing activated sludge bulking. Water Sci. Technol. 2013, 67, 1557–1563. [Google Scholar] [CrossRef]
- Drzewicki, A.; Kowalska, E.; Pajdak-Stós, A.; Fiałkowska, E.; Kocerba-Soroka, W.; Sobczyk, Ł.; Fyda, J. Experimental attempt at using Lecane inermis rotifers to control filamentous bacteria Eikelboom type 0092 in activated sludge. Water Environ. Res. 2015, 87, 205–210. [Google Scholar] [CrossRef]
- Kowalska, E.; Paturej, E.; Zielińska, M. Use of Lecane rotifers for limiting Thiothrix filamentous bacteria in bulking activated sludge in a dairy wastewater treatment plant. Arch. Biol. Sci. 2014, 66, 1371–1378. [Google Scholar] [CrossRef]
- Kowalska, E.; Paturej, E.; Zielińska, M. Use of Lecane inermis for control of sludge bulking caused by the Haliscomenobacter genus. Desalination Water Treat. 2016, 57, 10916–10923. [Google Scholar] [CrossRef]
- Hagiwara, A.; Yamamiya, N.; Belem de Araujo, A. Effect of water viscosity on the population growth of the rotifer Brachionus plicatilis Müller. Hydrobiologia 1998, 387, 489–494. [Google Scholar] [CrossRef]
- Pérez-Legaspi, I.A.; Rico-Martínez, R. Effect of temperature and food concentration in two species of littoral rotifers. In Rotifera VIII: A Comparative Approach; Springer: Berlin/Heidelberg, Germany, 1998; pp. 341–348. [Google Scholar] [CrossRef]
- Montagnes, D.; Kimmance, S.; Tsounis, G.; Gumbs, J. Combined effect of temperature and food concentration on the grazing rate of the rotifer Brachionus plicatilis. Mar. Biol. 2001, 139, 975–979. [Google Scholar] [CrossRef] [Green Version]
- Kocerba-Soroka, W.; Fiałkowska, E.; Pajdak-Stós, A.; Sobczyk, M.; Starzycka, J.; Fyda, J. Lecane tenuiseta rotifers improves activated sludge settleability in laboratory scale SBR system at 13 °C and 20 °C. Water Environ. J. 2017, 31, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Klimek, B.; Fiałkowska, E.; Kocerba-Soroka, W.; Fyda, J.; Sobczyk, M.; Pajdak-Stós, A. The toxicity of selected trace metals to Lecane inermis rotifers isolated from activated sludge. Bull. Environ. Contam. Toxicol. 2013, 91, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Clokie, M.R.; Millard, A.D.; Letarov, A.V.; Heaphy, S. Phages in nature. Bacteriophage 2011, 1, 31–45. [Google Scholar] [CrossRef] [Green Version]
- Rattanachaikunsopon, P.; Phumkhachorn, P. Construction of a food-grade cloning vector for Lactobacillus plantarum and its utilization in a food model. J. Gen. Appl. Microbiol. 2012, 58, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Withey, S.; Cartmell, E.; Avery, L.M.; Stephenson, T. Bacteriophages—Potential for application in wastewater treatment processes. Sci. Total Environ. 2005, 339, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Koskella, B.; Brockhurst, M.A. Bacteria–phage coevolution as a driver of ecological and evolutionary processes in microbial communities. FEMS Microbiol. Rev. 2014, 38, 916–931. [Google Scholar] [CrossRef] [Green Version]
- Thomas, J.A.; Soddell, J.A.; Kurtböke, D.I. Fighting foam with phages. Water Sci. Technol. 2002, 46, 511–518. [Google Scholar] [CrossRef]
- Petrovski, S.; Seviour, R. Activated sludge and foaming: Can phage therapy provide a control strategy? Microbiol. Aust. 2018, 39, 162–164. [Google Scholar] [CrossRef]
- Kotay, S.M.; Datta, T.; Choi, J.; Goel, R. Biocontrol of biomass bulking caused by Haliscomenobacter hydrossis using a newly isolated lytic bacteriophage. Water Res. 2011, 45, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.; Amado, R.; Padrão, J.; Ferreira, V.; Dias, N.M.; Melo, L.D.; Santos, S.B.; Nicolau, A. The first sequenced Sphaerotilus natans bacteriophage–characterization and potential to control its filamentous bacterium host. FEMS Microbiol. Ecol. 2021, 97, 29. [Google Scholar] [CrossRef] [PubMed]
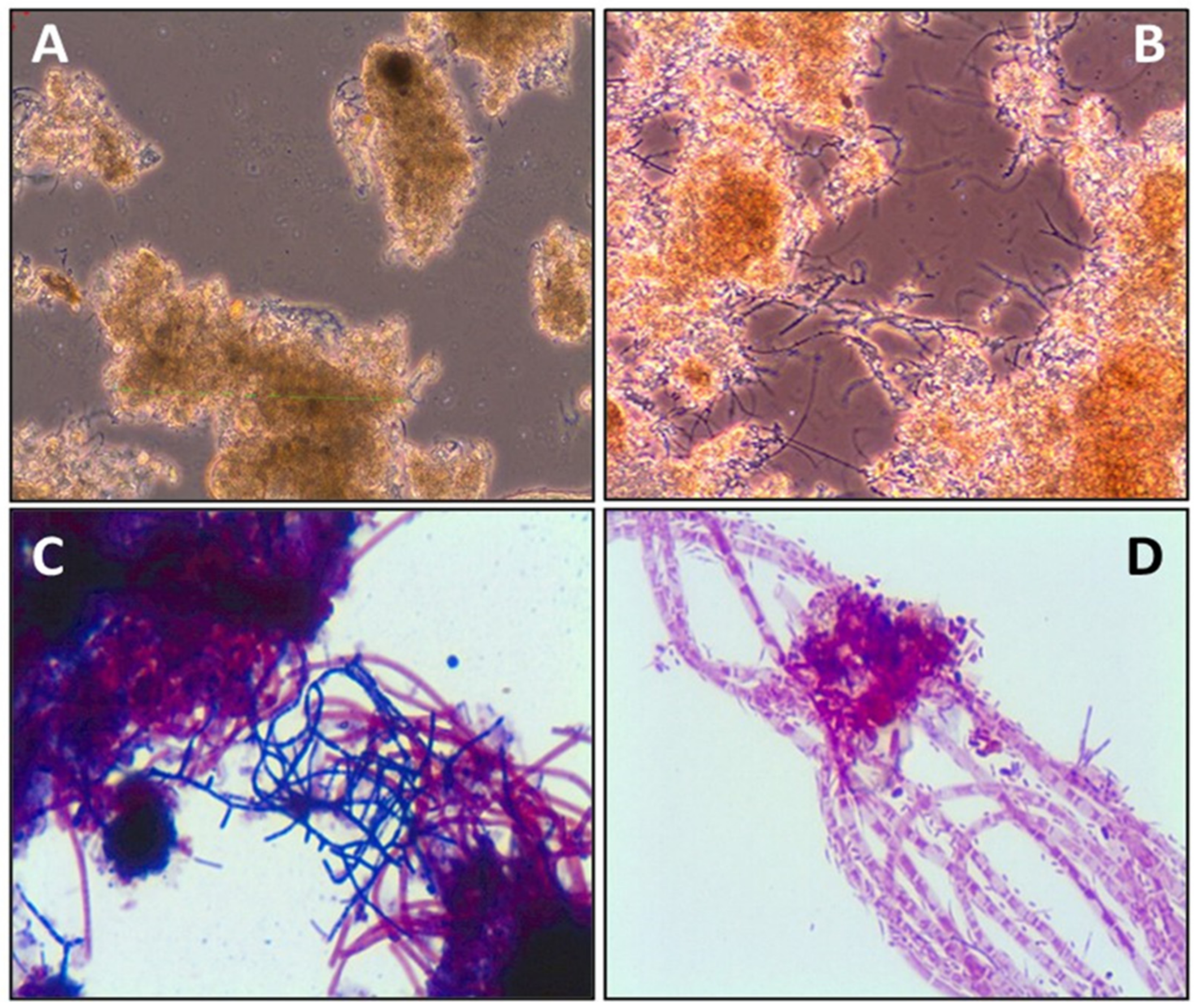
| Method | Advantages | Disadvantages |
|---|---|---|
| Chlorination |
|
|
| Hydrogen peroxide |
|
|
| Ozonation |
|
|
| Metal addition |
|
|
| Selectors |
|
|
| Rotifers |
|
|
| Bacterio-phages |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sam, T.; Le Roes-Hill, M.; Hoosain, N.; Welz, P.J. Strategies for Controlling Filamentous Bulking in Activated Sludge Wastewater Treatment Plants: The Old and the New. Water 2022, 14, 3223. https://doi.org/10.3390/w14203223
Sam T, Le Roes-Hill M, Hoosain N, Welz PJ. Strategies for Controlling Filamentous Bulking in Activated Sludge Wastewater Treatment Plants: The Old and the New. Water. 2022; 14(20):3223. https://doi.org/10.3390/w14203223
Chicago/Turabian StyleSam, Thandokazi, Marilize Le Roes-Hill, Nisreen Hoosain, and Pamela J. Welz. 2022. "Strategies for Controlling Filamentous Bulking in Activated Sludge Wastewater Treatment Plants: The Old and the New" Water 14, no. 20: 3223. https://doi.org/10.3390/w14203223







