Abstract
Water is a precious natural resource. Unfortunately, bodies of water become polluted by waste, such as untreated wastewater and detritus, along with oil spills, with minimum or no consideration for their limited capacity to renew themselves. Among these pollutants, dyes are harmful as they are persistent and not biodegradable in nature. The present study demonstrates the removal of crystal violet (CV), a toxic cationic dye, by using three systems: Peroxymonosulfate (PMS), UV-254 nm radiation and UV/P5MS. The effects of various parameters, such as the effects of the initial dose of crystal violet, initial concentration of PMS, pH, typical inorganic ions, etc., were also investigated. The effect of pH was investigated in the range of 1.92–12.07. Similarly, the effect of various anions such as NO2•−, HCO3•−, CO3•2−, SO4•2− and CH3COO•− was investigated for the degradation of target pollutants. The order of degradation of crystal violet was UV/PMS > PMS > UV with removal efficiencies of 97%, 76% and 42%, respectively, at reaction times of 60 min. The degradation of crystal violet was enhanced significantly at a pH range of 10.52–12.07. Electrical energy per order (EE/O) values for UV/PMS, PMS and UV were calculated to be 1.68, 3.62 and 48.96 KWh/m3/order, respectively. The addition of inorganic ions inhibited the removal of CV in the order of SO4•2− > NO2•− > HCO3•− > CO3•2− > CH3COO•−. Moreover, the kinetic studies on the degradation of CV by the UV-254 nm, PMS, and UV/PMS systems, were also carried out and found to follow pseudo-first-order kinetics. The study revealed that oxidation processes are most efficacious for the removal of organic dyes from wastewater.
1. Introduction
The textile industry is a major source of dyes and about 15% of the dyes are wasted during synthetic processes and the treatment of industrial effluents, which pose a serious threat to marine life and are also harmful to human beings [1,2,3]. The residues of textile industries have aroused much concern due to their harmful effect on aquatic life as well as human beings and is usually considered one of the major coloring contaminants in water bodies [4]. The release of these textile effluents into water bodies has been reported to be carcinogenic and hazardous as they cause major health issues [5,6,7]. Among the broad range of triphenyl methane dyes, crystal violet is considered one of the most carcinogenic and toxic in nature [8] due to its complex aromatic structure [9,10]. This cationic dye, due to its mutagenic and non-biodegradable nature, is considered a major pollutant when directly discharged into water and poses a major threat to aquatic life as well as human life. The major health issues caused by CV are vomiting, diarrhea, headache, dizziness, as well as damage to the gastrointestinal tract by excessive ingestion of CV [11].
Crystal violet (methyl violet) is a cationic dye with basic monocovalent in nature, considered a mixture of three basic compounds in its structure commonly referred to as tetramethyl, pentamethyl and hexamethyl, which differ from each other on the basis of the number of methyl groups in their amine functional group [12]. There are various applications of CV in different fields like textile industries and pharmaceutical industries along with their diverse range of implementation in each industry, such as dye paper, printing inks, fertilizers, disinfectants, anti-freeze and leather jackets [13,14,15] for an intravenous agent, veterinary products, in the staining of biological process, a supplement of poultry feed to minimize the dispersion of fungus, forge and parasites of the intestine, industrial dying, the coloring of wood, silk, paper, and inks as biological stain paper [16], and in the cosmetic and food industries. In addition, triphenylmethane dyes are employed as staining agents in bacteriological and histopathological applications and for the coloration of textile products [17]. The removal of crystal violet from wastewater due to its toxicity and high solubility in water is thus necessary [18].
There are various classical methods used for wastewater treatments, including flocculation, reverse osmosis, biological methods, and adsorption and chemical methods, but there are some limitations related to these methods [19,20], such as flocculation, reverse osmosis and adsorption, and often transmit the organic contaminants to other media, thus leading to secondary pollution [21]. Similarly, chemical methods are usually high-priced and also the formation of sludge creates a large issue of removal. In addition, there is an unnecessary use of chemicals during this process which cause environmental pollution. In addition, large amounts of electricity and other chemicals are consumed during the process and are usually costly [22].
With the passage of time, various technological developments have been applied for the removal of these contaminants. Among these technologies, advanced oxidation processes (AOPs) are one of the most important processes, which are highly selective technologies for removing and demounting the means of disintegration of those pollutants, which are relatively stable in nature [22,23]. Currently, the advanced oxidation technologies (AOTs) used are environmentally compatible and capable of oxidizing a number of contaminants that rely on the production and use of hydroxyl radical (OH) [24] and sulfate radical (SO4•−), which can more efficiently and selectively oxidize rapidly into CO2, H2O and inorganic species by the process of mineralization to process an elevated number of organic pollutants [25,26].
One of the most important and highly efficient oxidants is peroxymonosulfate (PMS, HSO5•−), which is considered an active agent of oxone in aqueous solutions with a general formula of 2KHSO5•KHSO4•K2SO4. This formula shows that it has the triple salt of potassium, is also eco-friendly in nature, and is considered versatile in nature with high efficiency [27,28], which is one of the most important resources of highly reactive radicals, such as hydroxyl and sulfate radicals, respectively. The hydroxyl radicals (•OH) are the most powerful and non-selective oxidants and have a value of bimolecular rate constants of 107 to 1010 M−1 s−1 for organic pollutants [29], a high standard redox potential such as (E° = 2.7 V) [30], and thus, carried out complete the degradation of organic chemicals and pollutants, which are difficult to detoxify with traditional approaches [31]. The sulfate radicals (SO4•−) are the most active radicals with a standard redox potential of 2.5–3.1 V [32,33] and bimolecular rate constants for organic pollutants in the range of 105–109 M−1 s−1 [34]. PMS can be activated by UV radiation into radicals, i.e., •OH and SO4•−, through the hemolytic breakage of the peroxide bond (-O-O-) [35,36].
The UV/PMS AOT is, relatively, the most successful and highly energetic technology for the decolourization and decontamination of aquatic environments due to their excellent water solubility, low cost, ease of storage, ease of transportation, low environmental impact, high rate of oxidation, simplicity, and no resultant sludge formation [36]. The activation of PMS by ultraviolet radiation is considered one of the most feasible methods [37] because of its activation energy [38,39]. The activation of PMS generates two types of radicals, such as •OH and SO4•−, which are the most efficient radicals for the degradation of organic contaminants. As sulfate radicals, (SO4•−) radicals are the most effective for the degradation of organic contaminants in aquatic environments with a high amount of natural organic matter (NOM) due to their relatively low tendency for NOM [36].
The degradation of crystal violet by ultraviolet radiation (UV-254 nm) is also highly effective due to the formation of hydroxyl radicals and hydrogen radicals due to the decomposition of water molecules [40,41]. Such radicals further degrade the organic contaminants into less toxic products. Owing to such factors, sulfate radicals (SR-AOTs) are considered the most effective for the degradation of carcinogenic and toxic organic contaminants in aquatic environments, such as dyes [42], pesticides [43], sunscreen agents and pharmaceuticals [44,45] into less toxic by-products.
This work is mostly focused on introducing the most successful, highly energetic and environmentally friendly treatments, such as hydroxyl and sulfate radicals, which are advanced oxidation technologies with different initial doses of oxidants as well as target organic contaminants. The effects of various parameters were also investigated for these systems, such as the effect of an initial dose of CV, initial concentration of PMS, pH, typical inorganic ions, etc. The effect of pH was investigated in the range of 1.92 to 12.07. Similarly, the effects of various anions such as NO2•−, HCO3•−, CO3•2−, SO4•2− and CH3COO•− were investigated for the degradation of target pollutants. Total carbon (TC) analysis was carried out by ECOSAR. Degradation of by-products of CV were detected by HPLC-MS to establish new potential degradation pathways of CV. Kinetic study was carried out in the UV-254 nm, PMS, and UV/PMS systems, which follow the pseudo-first-order kinetics.
The aim of the present work is to analyze the possibility of decolorization of CV by different AOPs, such as UV-254 nm, PMS, and UV/PMS. Commonly referred to as AOPs, advanced oxidation processes are used to oxidize the complex organic in wastewater and that are difficult to decompose into simpler form end-products with biological processes [46,47].
2. Materials and Methods
2.1. Materials
The target pollutant under study is crystal violet (2-chloro-4-ethylamino-6-isopropylamino-s-triazine) (99.9%), which was purchased from Sigma Aldrich. The other chemicals, including potassium Peroxymonosulfate (PMS), sodium nitrate (NaNO3), sodium nitrite (NaNO2), sodium sulfate (Na2SO4), and sodium acetate (CH3COONa), were purchased from Fisher Scientific. Sodium hydrogen carbonates (NaHCO3), and sodium carbonate (Na2CO3) were purchased from Scharlau. To study the effect of pH, hydrochloric acid and sodium hydroxide, buffer solutions of 4.0 and 7.0 of sodium acetate were used to study the calibration process—these substances were purchased from Sigma Aldrich.
2.2. Analytical Methods
SpectroVis® Plus (OrderCodeGDX-SVISPL) was used for the evaluation of crystal violet under the degeneration of its mechanistic approach of photolysis, UV only, PMS only and homogenous UV/PMS processes. Go Direct SpectroVis Plus is a portable, visible-to-near-IR spectrophotometer and fluorometer; the instrument was equipped with the following specifications. The light source was incandescent with LED support, the detector was linear CCD, the wavelength range was usually 380–950 nm, the wavelength reporting interval was approximately ~1 nm, the optical resolution (FWHM) was in the range of 5.0 nm with a wavelength accuracy ± 4.0 nm, the photometric accuracy was ±0.10 A.U., the typical scan time was ~2 s, and the operating temperature was 15–35 °C [48]. The analysis was performed under ambient conditions by calibrating the Go Direct SpectroVis Plus with distilled water and a full-spectrum of the sample was taken in a cuvette about 3/4 full with the sample solution to be tested and then placed in the spectrophotometer where the spectrum absorbance vs. wavelength was obtained.
2.3. Experimental Set-Up for Photochemical Procedure
To carry out the oxidation process by means of an ultraviolet lamp, a schematic procedure was established. The experimental setup of this process usually involves a photochemical reactor along with a Petri dish made of Pyrex with dimensions of 60 mm (diameter) × 15 mm (height) with a quartz cover. Sample solutions were prepared with Milli-Q water (resistivity of 18.2 MΩ cm). The solutions of acidic and basic pH were prepared using the hydrochloric acid and NaOH solutions, respectively. In the end, solutions were examined for their TOC, where the sample solutions were allowed to irradiate at different intervals without the addition of a quenching compound. All experiments were carried out in triplicate.
3. Results and Discussion
3.1. Removal of CV by UV, PMS and UV/PMS Process
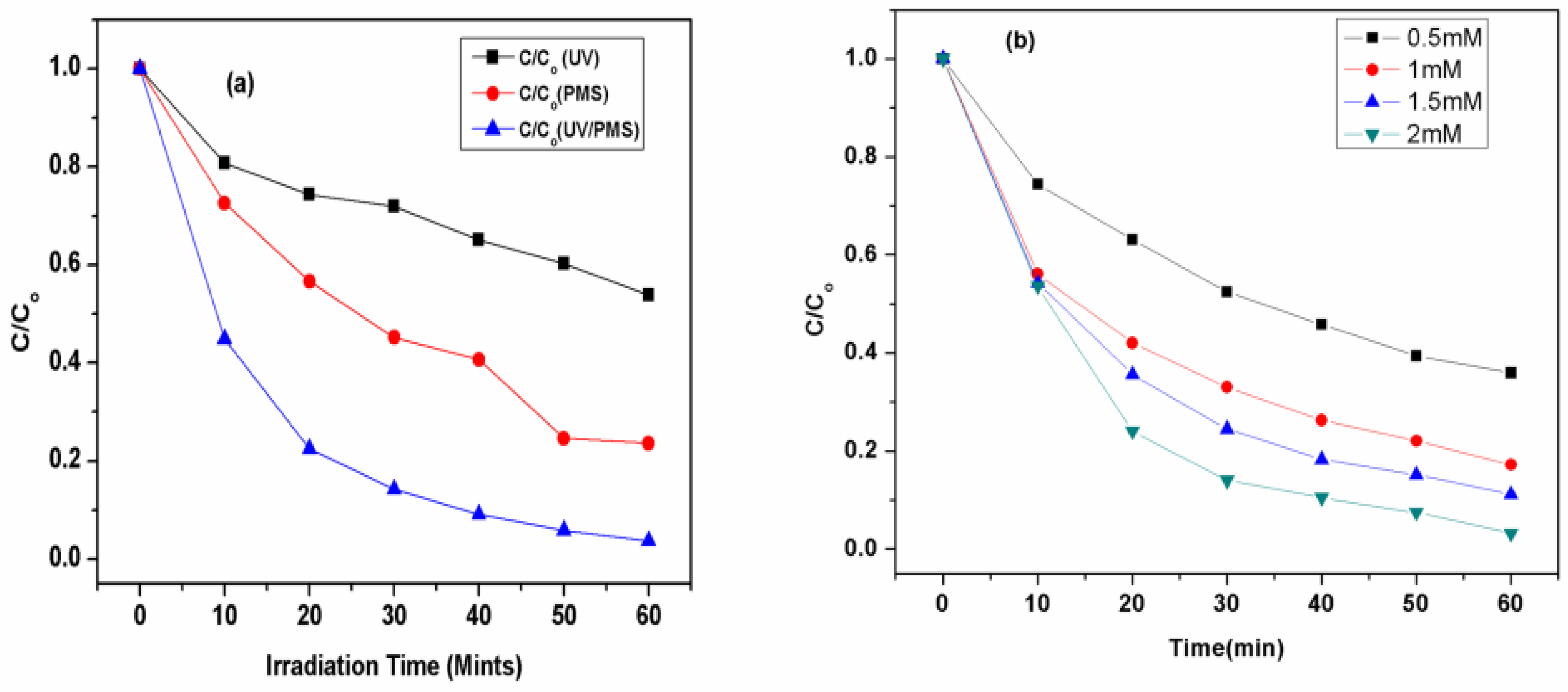
A comparative study for the removal of CV was carried out by UV-254 nm, PMS and UV/PMS system; the results are shown in Figure 1a. To investigate the effect of the oxidant, the degradation of CV was carried out in the presence of UV radiation only. Due to the presence of the pi-bonds in CV molecules, their degradation by UV radiation could be expected. However, only a 42% removal was observed under UV irradiation, reflecting its persistent nature. Similarly, only a 76% removal with PMS shows a higher removal efficiency compared to UV radiation only.
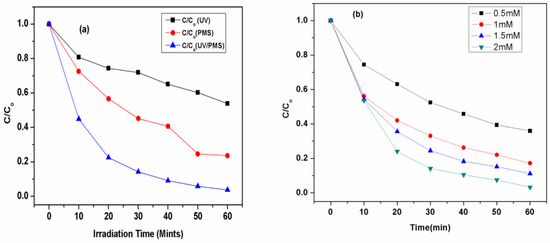
Figure 1.
(a). Removal of CV by UV, PMS and UV/HSO5•− AOTs. Experimental conditions: [CV]ₒ = 0.01 mM, [oxidant]ₒ = 2.0 mM, [pH]ₒ = 5.88 for PMS. (b). Effect of initial concentrations of PMS on its Kobs (mint)−1 and removal rate (mM mint)−1 by UV/PMS process. Experimental conditions: [PMS]ₒ = 2.0 mM, [pH]ₒ = 5.88.
The coupling of PMS with a UV system significantly enhanced the degradation as well as the removal of CV up to 97%, which indicates the formation of reactive species such as •OH and SO4•−, as shown in Equation (1) [49].
HSO5•− + hv → •OH + SO4•− Φ of •OH + SO4•− = 1.04
Figure 1a shows that higher removal efficiency of CV was obtained by UV/PMS, followed by the PMS and UV processes alone, respectively. The highest efficiency of UV/PMS process might be due to a higher quantum yield, such as (Φ) of 1.04, as compared to the PMS system. Thus, there are two pathways for the generation of oxidant-free radicals by the UV activation of the PMS. In the first pathway, which is considered the main pathway, there is a fission of an O-O bond by UV energy, as shown in Equation (2):
HSO5•− + hv → •OH + SO4•−
The second pathway is where PMS is activated by electron conduction via the generation of electrons that interact with the UV radiation in water. The possible mechanism is shown in Equations (3) and (4):
H2O + hv→ OH• + H•
HSO5•− + H• → SO4•− + H2O
In both mechanisms, the oxidant-free species generated both sulfate and hydroxyl radical attacks onto the pollutants and degraded them totally or partially, as shown in Equation (5) [50,51]:
SO4•− + OH• + organic compound → organic by-products + CO2 + H2O + SO4•2−
Various studies [52] showed that only those organic molecules can go through the process of direct photolysis when they fulfill two conditions: (1) all organic molecules should present in the ground state must absorb the radiation energy of suitable wavelength to undergo into an excited state, and (2) molecules in the excited state undergo a process of chemical disintegration, which is highly competitive with the non-excited state of molecules by physical infectiveness. This process can be represented by Equations (6)–(8) [53];
This series of reactions shows that crystal violet undergoes degradation resulting in the formation of degradation of by-products along with OH• radical formation, which further degrades the products of CV [52]. Thus, the removal of CV by reactive radicals depends upon the concentration of radicals, the nature of degradation products, and second-order rate constants.
3.2. Electrical Energy Comparison of the UV/PS, UV/PMS and UV/H2O2 Processes
The oxidation process is used for the removal of CV, and these processes are UV and UV/PMS and the peroxymonosulfate process. Their efficiency was determined by means of a comparison of their consumption of electrical energy per order (EE/O), which is defined as “the electrical energy in kilowatt hours (kWh) consumed for the removal of a particular pollutant by one order of magnitude in a unit volume (1 m3) of polluted water”. The results of the kinetics collected in a recent study may be applied to making the comparison of the EE/O of the studied AOPs, which may be deliberate by using the following Equation (9) [54].
where, t is photolysis time (h), V represents total treated volume (m3), and P is the total electrical power of the UV lamp (kW), correspondingly. The study of the removal of crystal violet by calculating their time of photolysis (t), which is needed to eliminate crystal violet by calculating the value of first- and fourth-order removals of crystal violet, usually given as ln (10)/(Kobs) and 4 × ln (10)/(Kobs), correspondingly. Their efficiency was measured in terms of their photolysis time, which is mandatory for the first-order removal of crystal violet.
EE/O = Pt/V
The oxidation process brings about 1.0 × 10−6 mM (0.000408 mg L−1) removal of CV, which is a fourth-order removal of CV with a starting dose of 0.01 Mm (4.08 mg/L). The value of fourth-order removal indicates that this value is too small compared to the lethal dose of the CV present in freshwater used for drinking purposes [55]. Table 1 shows the data information about the first-order and fourth-order removal of CV through different processes, such as UV, PMS and UV/PMS systems for the oxidation of CV. These results indicate that UV/PMS is the most efficient and cost-effective process for the degradation of CV among the studied AOPs, as it has the lowest value of EE/O, and is a better system based on the results. The electrical energy for first-order removal and electrical energy for fourth-order removal value to UV/PMS system is lower, such as 1.68 and 6.48, respectively than PMS and UV only. Thus, the UV/PMS system is most economically suitable compared to the use of UV and PMS alone, as shown in Table 1.

Table 1.
Electrical energy comparison of UV and UV/PMS processes for removal of CV. Experimental conditions: [CV]ₒ = 0.01 mM and [PMS]ₒ = 2 mM, [pH]ₒ = 5.88.
3.3. Effect of Various Initial Doses of Oxidant
Table 2 and Figure 1b depict the effect of the initial dose of PMS to analyze the effect of peroxymonosulfate (HSO5•−) on the degradation of CV, by altering the initial concentration of HSO5•− from 0.5 mM to 2 mM [56]. In order to study the kinetics for the degradation of crystal violet through UV/PMS, the observed pseudo-first-order rate constant (Kobs) was calculated from Equation (10) to (13), and the resulting values are depicted in Table 2.
ln (C/C) = Kobs × t
−d [CV/dt] = k [CV]
−d [CV]/[CV] = k × dt
−ln [CV]/[CV] = Kobs × t

Table 2.
The effect of the initial concentrations of PMS on the removal efficiency, Kobs, and degradation rate of CV dye by the UV/PMS system at [CV]ₒ = 5 ppm, [PMS] = 0.5–2 mM (12 min).
These results showed that the degradation efficiency of CV increases with the increase in oxidant concentration, and the Kobs obtained by using Equation (10) were also found to increase with the increase in the initial concentration of PMS from 0.0939 to 0.2857 min−1 for 0.5 to 2 mM, respectively, as shown in Table 2. These results were also consistent with findings in previously reported studies [56,57,58].
The possible increase of kobs (min−1) is directly related to the generation of hydroxyl and sulfate radicals (•OH, SO4•−), which increases with an increase in the rate of generation of both radicals when a higher [PMS]ₒ was used, thus causing the increase in value of kobs at a high dose of PMS. It was also observed that there was a decrease of kobs when the initial dose of peroxymonosulfate was decreased due to the low concentrations of •OH and SO4•. To compute the time needed for first- and fourth-order removal of crystal violet, the initial dose of PMS was varied, as there have been implications of an observed pseudo-first-order rate constant, which is calculated by Equations (14) and (15):
Time required for first-order removal (min/O) = ln10/kobs
Time required for fourth-order removal (min/fourth-order) = 4ln10/kobs
To calculate the degradation rate for crystal violet, there is a need to define the degradation rate, which can be defined as “at different initial doses of peroxymonosulfate, there is a variation of concentration with respect to time (ppm/min)”. In addition, the degradation rate was calculated for the initial duration of treatment, which is usually for 0 to 2 min. It was observed that the degradation rate was obtained to be 0.057 mM/min for [PMS] = 0.5 mM and 0.099 mM/min for [PMS] = 2 mM, as shown in Table 2. It was observed that the degradation rate increases when the initial dose of peroxymonosulfate [PMS] was increased because the high dose of peroxymonosulfate provides a higher rate of active radical generation, i.e., •OH and SO4•−, which causes the rapid rate of collision with the target molecules (crystal violet), which enhance the rate of degradation of CV. The results showed that the degradation efficiency of the CV dye increased when the concentration of the oxidants increased, as shown in Table 2. When the oxidant is at a low concentration, the degradation efficiency of CV is also low. This is because, at a low dose of peroxymonosulfate, the generation of hydroxyl and sulfate radicals will be lower, which is the main reason for the low value of Kobs for the degradation of CV [56,57,58]. Others studies support these results [56,59]. Thus, the UV/PMS process is the most successful process for the degradation of crystal violet and the efficiency of such a process is further expanded with the study of different initial concentrations of CV, solution pH, and different inorganic ions.
The removal efficiency shown herein was determined after a 12 min reaction time. The degradation rate was determined for the initial reaction time from 0 to 2 min
3.4. Effect of Initial Dye Concentration
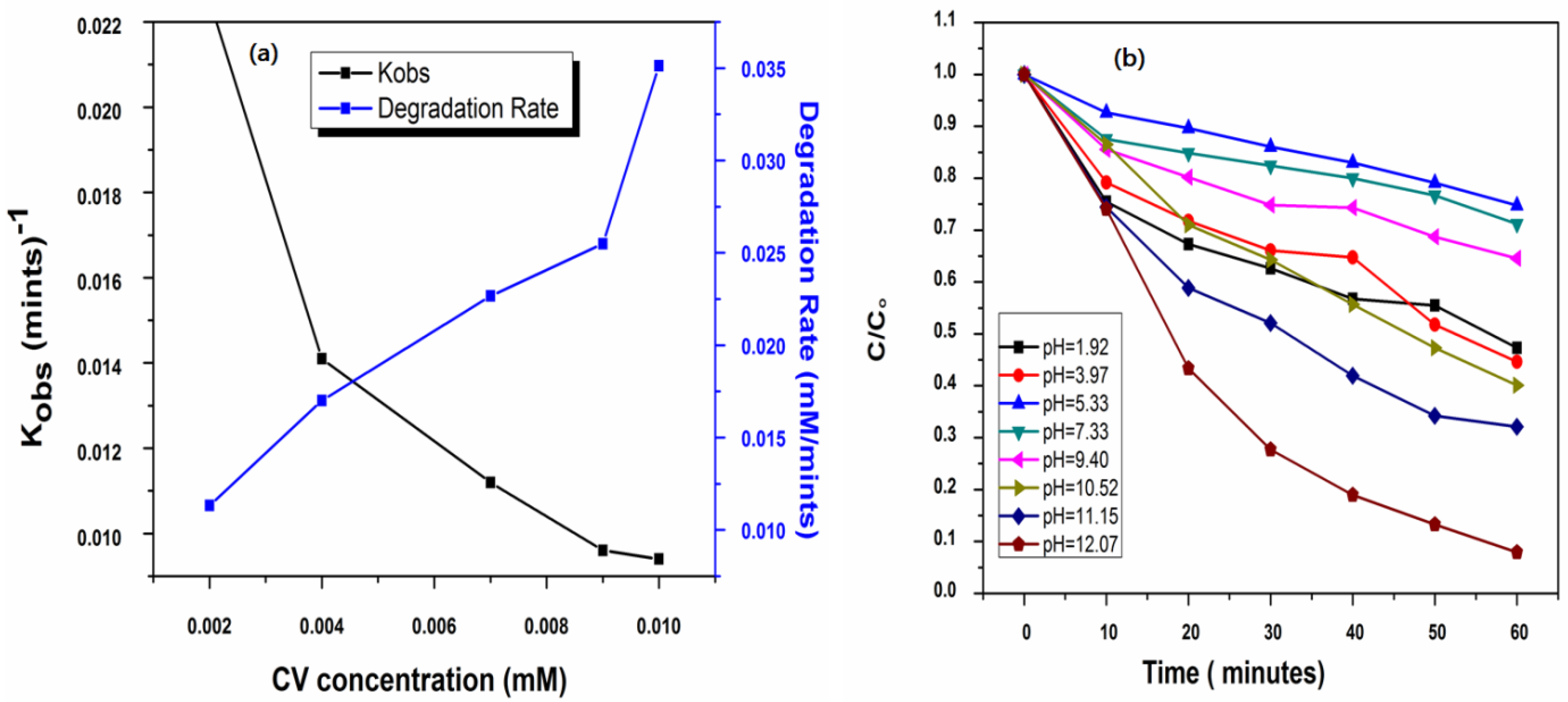
To investigate the practical applications of UV/PMS process and to find out the optimum dose of UV radiation applied to activate the PMS in UV/HSO5•− system for the efficient removal of crystal violet, the effects of the different initial doses of crystal violet was studied. Hence, the degradation rates and Kobs for crystal violet were investigated at different initial doses of crystal violet ranging from 1–5 ppm at constant irradiation for 60 min and an initial dose of peroxymonosulfate [PMS] of 1 mM (Figure 2a). The results are shown in Table 3, which indicates that the maximum removal efficiency of crystal violet was 77% for 1 ppm when the reaction was run for 60 min, while there was only 42% removal of crystal violet for 5 ppm of the crystal violet. Moreover, other results in the literature [52,60,61] are consistent with these results. Peroxymonosulfate undergoes oxidation by ultraviolet radiation and produces active radicals, i.e., •OH and SO4•−, which cause the degradation of crystal violet, resulting in various kinds of degradation by-products (DPs), which are assumed to have high efficiency with hydroxyl and sulfate radicals. When the initial dose of crystal violet increased, there was a rise in the amount of degradation by-products, which created a competitive environment for active radicals of hydroxyl and sulfate. Thus, there is a competency between parent pollutants and degradation by-products for both radicals. This competency will be more likely at high doses of parent pollutants compared to small doses of CV.
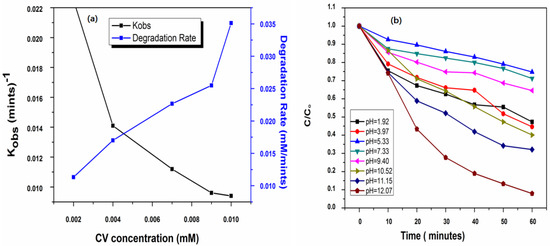
Figure 2.
Effect of initial (a) concentration of CV on its Kobs (mint)−1 and removal rate (mM mint)−1 by UV/PMS process at [PMS]ₒ = 2.0 mM, [pH]ₒ = 5.88 (b) solution pH on the removal efficiency of CV by U/PMS on removal efficiency of CV by UV/PMS process at [CV]ₒ = 0.01 mM (5 ppm), [pH]ₒ = 1.92–12.07.

Table 3.
Effects of the initial concentration of CV on the removal efficiency, Kobs and degradation rate of CV dye by UV only. Experimental conditions: [CV]ₒ = 1–5 ppm (0.002–0.01 mM), molar ratio when [CV]ₒ = 1 ppm (0.002 mM).
The main cause of low degradation rates at higher concentrations of contaminants might be due to the molar ratios (proportion of peroxymonosulfate to CV), which reduce at lowers the concentrations of target contaminants and is considered the main cause of the lower degradation efficiency for CV at a higher dose compared to a small dose of CV [56,62]. For example, at 1 ppm, the molar ratio varies from 250 to 1000, but when the contaminant concentration is high, e.g., 5 ppm, the molar ratio range is 50–200.
The effect of the initial dose of crystal violet on the kobs was calculated, which was found to decrease with the rise in the initial dose of crystal violet. The results are shown in Table 3 and Figure 2a. These show that the value of Kobs reduced from 0.0225 to 0.0094 min−1 as the initial dose of crystal violet raised from 1 ppm to 5 ppm. These results are due to the high competency of crystal violet and their degradation by-products for active radicals along with the low ratio of crystal violet and of hydroxyl and sulfate radicals when the initial dose of crystal violet increased, which is the main cause for the low value of kobs with a high dose of crystal violet [44]. Another possible reason for lowering the removal efficiency of CV with an increase in [CV]ₒ also increases the adsorption rate and consequently, light travels a smaller distance. Thus, a higher initial CV concentration reduces the proportion of irradiation energy required for the oxidation of PMS to generate the SO4•− formation by the filtering/shading effect. This results in a decrease in the value of Kobs for CV through the ultraviolet-mediated peroxymonosulfate method. Similarly, the power function was obtained by a linear relationship between the initial dose of crystal violet and its observed pseudo-first-order rate constant. Moreover, the calculation of time that need to bring about the first-order and fourth-order removal of the target compound by using kobs at various doses of crystal violet by using Equations (15) and (16). The results are provided in Table 3.
Removal efficiency was determined at a 60 min reaction time, and the degradation rate for varying doses of crystal violet was determined for 0 to 2 min, which is 0.011 ppm/min for [CV] = 1 ppm and 0.035 ppm/min for [CV] = 5 ppm. The results are shown in Table 3. It is clear from these results that an increase in the initial concentration of CV resulted in an increase in the initial degradation rate because at a maximum dose of CV, a maximum amount of contaminants will be subjected to the •OH and SO4•− active radicals, which might be a reason for the high degradation rate at a higher initial concentration of CV [56,57]. Other studies have reported similar results [43,56,58,59,63].
3.5. Effects of Initial pH
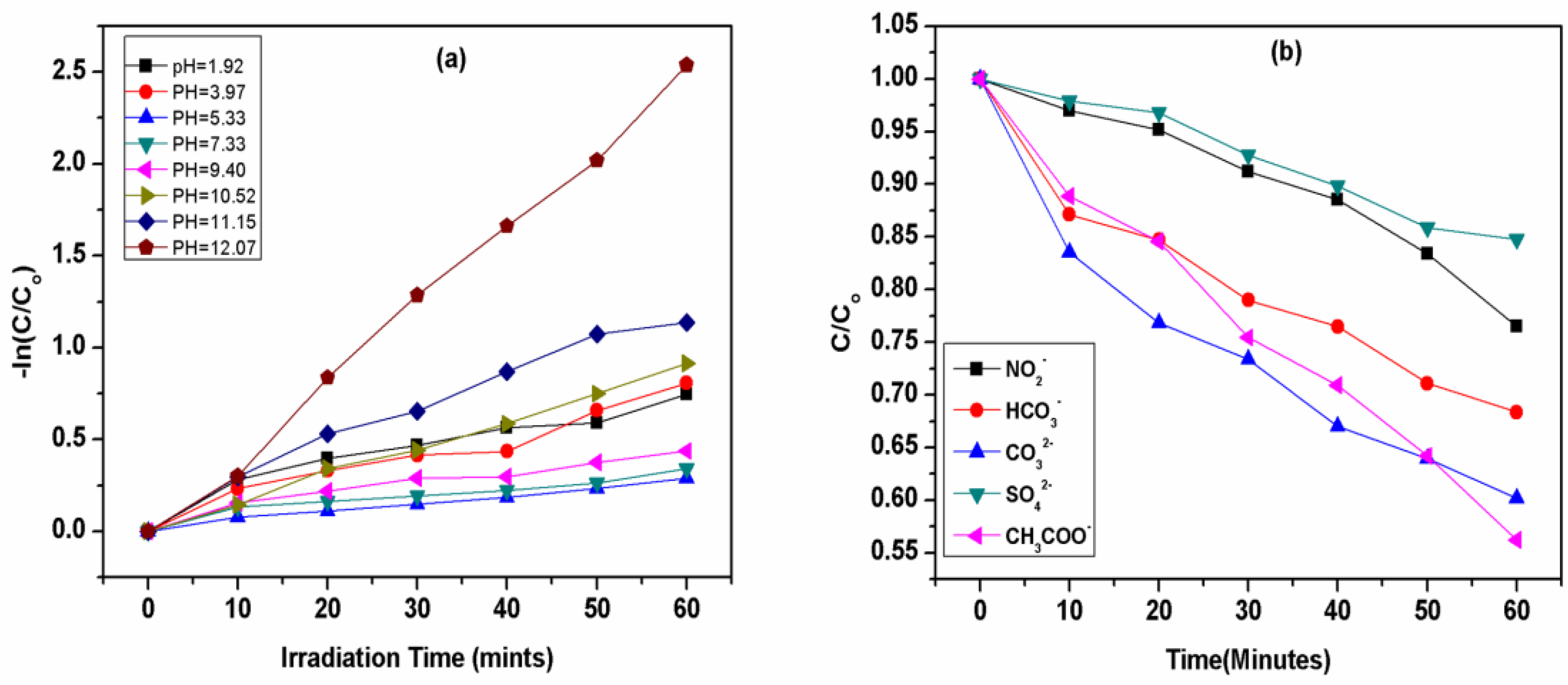
One of the most important factors in this process is pH, which controls the whole performance of advanced oxidation process. The pH values of wastewater vary widely from acidic to alkaline through to neutral. Thus, investigating the effect of pH in our study was considered vital for the potential applications of the UV-254/PMS system on a large scale-treatment of CV-contaminated waters due to the generation of active radicals of hydroxyl and SO4•- along with the chemical structure of CV. In addition to these effects, the variation in pH values directly affects the degradation of CV through UV/PMS. Thus, pH values play an important role in any chemical process involving the removal of pollutants from the water through an increase or decrease in its removal capability [64]. Hence, to examine the efficiency of the oxidation process, the effects of pH were studied in the range of 1.92–12.07 to explore the effect on the removal of CV by the UV/PMS system. The results are shown in Figure 2b and Figure 3a.
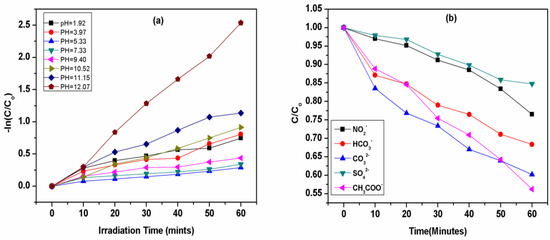
Figure 3.
Effect of different (a) pH on degradation of crystal violet (b). inorganic anions (NO2−, CO32−, HCO3−, SO42−, CH3COO−) on the removal efficiency of CV by UV/PMS at [CV]ₒ = 0.01 mM, [NO2]ₒ = [CO32−]ₒ = [HCO3−]ₒ = [SO42−] = [CH3COO−]ₒ = 1 ppm, [pH]ₒ = 5.88.
The estimated degradation efficiency is determined by using Equation (16):
where Cₒ = Initial concentration of dye at time t = 0, Ct = Concentration of dye at time t.
Degradation Efficiency (%) = (Cₒ − Ct)/Cₒ
It is clear from Figure 4 that an increase in pH initially decreases the removal efficiency and the maximum removal efficiency was obtained at pH 12.07. Crystal violet undergoes a decadence process at various pH levels by going through the series of initial pH = 12.07(Kobs = 0.0415 mints−1) > pH = 11.15(Kobs = 0.0208 mints−1) > pH = 10.52(Kobs = 0.0151 mints−1) > pH = 1.92(Kobs = 0.0134 mints−1) > pH = 3.97(Kobs = 0.0132 mints−1) > pH = 9.40(Kobs = 0.0079 mints−1) > pH = 7.33(Kobs = 0.0058 mints−1) > pH = 5.33(Kobs = 0.0048 mints−1). It is clear that the removal efficiency of CV first decreases from 53 to 29% at pH 1.92 to 7.33, then increases from 36 to 92% at pH 9.40 to 12.07, suggesting that at an acidic pH, the removal efficiency decreases while alkaline conditions favor the removal efficiency of CV dye. It is clear that pH plays an important role in the removal of pollutants by regulating the rate of generation of different radicals, such as the formation of the hydroxyl radical (OH•).
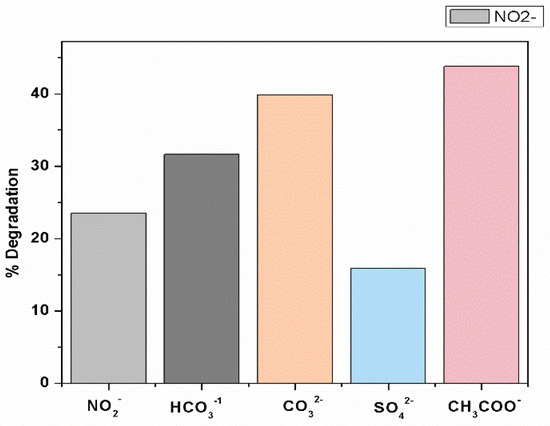
Figure 4.
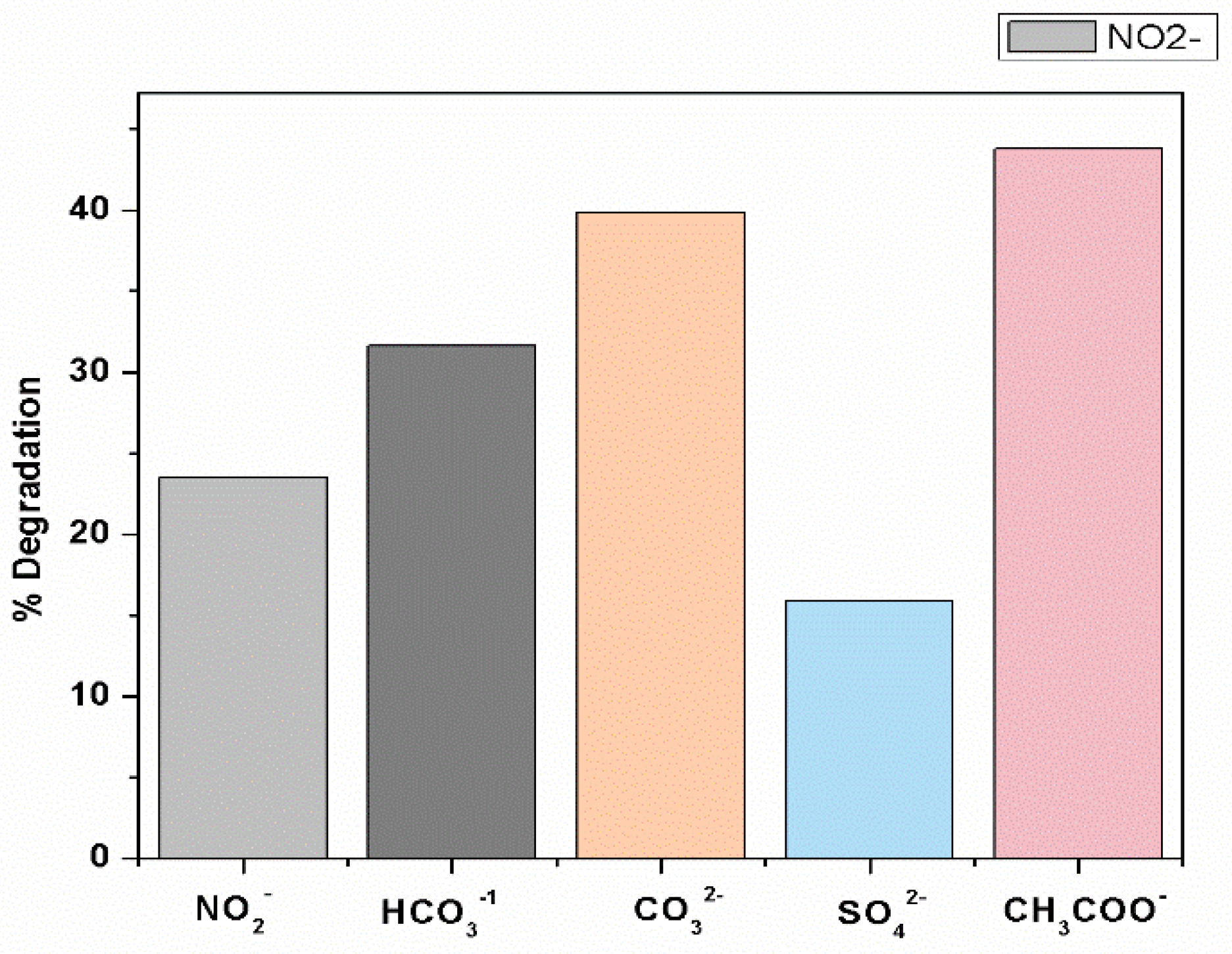
Effects of inorganic anions (NO2−, CO32−, HCO3−, SO42−, CH3 COO−) on the removal efficiency of CV by UV/PMS at [CV]ₒ = 0.01 mM, [NO2− ]ₒ = [CO32−]ₒ = [HCO3−]ₒ = [SO42−]ₒ = [CH3COO−]ₒ = 1 ppm, [pH]ₒ = 5.88.
Figure 2b shows that there is the maximum removal of CV at extreme conditions of pH in both conditions, i.e., under highly acidic as well as under highly alkaline pH, which can be explained on the basis of the rate of formation of both types of ions and radicals under acidic and alkaline pH values, such as with H+, H•, OH•−, and •OH [65]. When the pH of the solution is acidic, such as 1.92–3.97, there is maximum availability of H+ and H•, which shows the maximum degradation of CV, and hence, increases the removal efficiency of CV from an aqueous solution [66]. When the pH of the solution further increases from 5.33–7.33, the rate of degradation/decolorization of CV becomes low, which might be attributed to a decrease in the rate of formation of H+ and H• at this pH range, as shown in Figure 3a [67].
CV undergoes degradation under acidic pH by means of the following acidic mechanism of hydrolysis as shown in Equation (17):
CV + H+ → CVH+ + H2O → CVOH + 2H+
It has been reported that there was a better removal efficiency of dyes with maximum degradation in solutions when the pH of the solution was maintained between 2.0–4.0. Similarly, in the UV/PMS system, the removal of CV is greatly influenced by the pH of the solution. The H+ and OH•− concentrations in the solution are greatly influenced by the change in the pH of the solution. Possible reasons for the decline in the removal efficiency of the dye include the scavenging of •OH by OH•−, and the low redox potential of •OH. •H and •OH radicals reacts with OH•−, which are already at a low concentration due to their highly basic pH (i.e., 10.52, 11.15 and 12.02), where OH•− are already in high concentration and thus, the effective concentration of •H and •OH [Equations (18) and (19)] radicals to reacting with the CV correspondingly decreases [68].
•H + HO•− → eaq− + H2O
•OH + −•OH → H2O + O•−
The high removal efficiency of CV at pH 1.92 and 3.97 could also be due to lower scavenging of eaq− and •OH. Thus, in acidic conditions (pH 1.92 and pH 3.97), the recombination of eaq− and the •OH radical is avoided (Equation (20)), thus allowing the •OH radical to react with CV [69]:
eaq⁻ + •OH → OH•−
Moreover, the better removal efficiency at pH 1.92 and 3.97 might be due to the degradation of the crystal violet, which increases the elimination of crystal violet due to the high exposure of H2O to UV radiation, which undergoes a disintegration process with the generation of OH•−, H+, and •OH radicals at high rates [70]. Secondly, the high degradation rate of CV with better removal capabilities at pH 1.92 and 3.97 can be attributed to the bubbling phenomenon of CV pollutants for hydroxyl radicals [58].
This can be attributed due to acidic conditions, i.e., (pH 5.33), wherein there is a significant possibility of a scavenging reaction of hydrogen ion (H+) by hydroxyl radicals (•OH), which can be represented by Equation (21) [61,71,72,73,74]:
•OH + H+ + e⁻ → H2O2
It is reported that at pH 5.33, there is a decline in the concentration of hydroxyl radicals (•OH) because of the generation of hydrogen peroxide, as shown in Equation (21), easily undergoes its disintegration process. Because of the disintegration of hydrogen peroxide, there will be a remarkable decrease in the formation of hydroxyl radicals due to formation of the complex compound with dye, which results in the lowering in removal efficiency when the pH is 5.33.
It has also been reported that when the pH of the solution is maintained in the range of 5.33 and 7.33, there is the formation of chloride ions from HCl, which have antagonistic conditions for the hydroxyl radicals, as shown in Equation (22). There will be a formation of ClOH•− species with the reaction of chloride ions with hydroxyl radicals, which has a high tendency to react with H+, resulting in the formation of Cl•. These Cl• react with each other at a high rate with the formation of Cl2•−, as shown in Equations (23)–(25). These Cl2•− have a very low tendency to react with organic compounds to make their degradation possible compared to •OH, which has a greater tendency towards organic compounds [51,52,75].
Cl− + •OH → ClOH•−
ClOH•− → •OH + Cl−
ClOH•− + H+ → Cl• + H2O
Cl− + Cl• → Cl2•−
In addition, at moderate basic pH, the formation of HCO3−/CO32− should be considered, as two well-known radical scavengers can reduce the degradation of the pollutant in the pH range of 7.33 to 9.33. Thus, due to the formation of these radical scavengers in the pH range of 7.33 and 9.33, the removal efficiency of CV dye due to the unavailability of •OH and •H is lowered.
CVH+ + H2O → CVOH + 2OH− + 2H+ → CVOH + 2H2O
In the above Equation (26), CV stands for crystal violet, while CVH+ is crystal violet in its protonic form.
It has been observed that the removal efficiency of dyes, along with their degradation process, is at a maximum when the pH of the solution is kept in the range of 10.50 to 12.07 [76]. The high removal of CV dye at an alkaline pH could be associated with the chemical instability of CV under an alkaline pH [77,78]. The possible reason for the high degradation of CV dye under these conditions is due to the remarkably high rate of the formation of hydroxyl radicals, which have a strong potential to react with the target pollutant, thus enhancing degradation efficiency [79,80,81,82].
Thus, the process of decomposition of CV under basic conditions can be represented by Equation (27)
CV + OH− → CVOH− + H2O → CVH+ + 2OH−
3.6. Effect of Typical Inorganic Ions
The effects of typical inorganic ions were studied to examine their effect on removal efficiency. The inorganic anions commonly exist in natural water, which includes Cl−, NO2−, CO32−, HCO3−, SO42− and CH3COO−, etc., and make up the water system, which is generally found in the range of 10−5 to 10−3 M. It was observed that there is a high tendency of inorganic ions to react with hydroxyl and sulfate active radicals, as shown in Equations (28)–(37). Due to such features, the effect of ions such as nitrates, carbonates, bicarbonates, sulfate, acetates, etc., have been reported in terms of the degradation of crystal violet to demonstrate the efficiency of AOPs for the treatment of wastewater as well as for organic contaminants from industrial effluent.
Equations (30) and (34) show that the hydroxyl and sulfate radicals have a high tendency to react with sulfate and nitrate ions, and are usually considered to be the cause for the prohibition of efficient removal of crystal violet dye by the formation of inorganic ions. The decrease in the degradation rate was found in the following decreasing order: SO42− > NO2− > HCO3− > CO32− > CH3COO−. The degradation rate of CV is highly affected by radical scavenging counteracting the capability of hydroxyl radicals (•OH) and eaq−, which play major roles in the removal of dyes by increasing their removal efficiency. Thus, CO32− [61,83] and NO2− ions are considered the most notable scavengers for hydroxyl radicals (•OH). eaq−, as represented in Equations (28), (30) and (31), respectively, subsequently influence the degradation rate by lowering the rate of reaction of hydroxyl radicals with CV [84,85].
The relative higher inhibition of CV degradation by SO4 2− and NO2− ions could be due to its fast reaction with •OH radicals, as shown by Equations (30) and (34). In the presence of these ions, there is competition between these ions and CV for an •OH radical. Since the reaction between the NO2− and •OH radicals is very fast (Equation (30)), a smaller amount of the •OH radical is available to react with CV [86], resulting in lower rates of degradation compared to the degradation in the absence of any additives (Figure 3b). Moreover, the greater inhibitory effect of SO42−, and NO2− is due to its high second-order rate constant with •OH (reaction (30) and (34), which may have led to a greater competition of the SO42− and NO2− with CV for •OH and, consequently, higher inhibition was observed in the presence of SO4 2−, and NO2− in the present study.
In addition, NO2− ions are also strong scavengers of eaq−, as shown in Equation (31) [58]. The removal efficiency of CV by UV/PMS was also inhibited to a greater extent in the presence of HCO3− and CO32− ions. However, HCO3− has a greater inhibiting effect than CO32−. The removal of CV by •OH was inhibited by CO32− but at a lower rate might be due to its low reactivity with •OH (reaction 28). As the HCO3− ions have faster kinetic energy towards •OH (reaction (29), they are expected to decrease the probability of the reaction of •OH with CV [27]. The addition of CO32− has been reported to have lower inhibiting effects than SO42−, NO2−, and HCO3− and, thus, CO32− is more efficient in the removal of CV dye than all other ions in the solution. The main cause of the efficient elimination of CV by CO32− is due to its low second-order rate constant with •OH (reaction (32)), which might have led to the lower competition of the CO32− with CV for •OH and a higher removal of CV by UV/PMS in the presence of CO32− and HCO3−. The effect of CO32− has been reported to raise the pH of a solution, and a higher pH has been reported to affect the reactivity of •OH and enhances removal efficiency [87]. These results are supported by other studies reported in the literature [88,89].
A decrease in the removal efficiency in the presence of either of such anions is due to the scavenging effect of •OH and SO4 •−. The results also show that NO2− and SO42− have more effect on decreasing the removal efficiency and thus have more of an inhibition effect on the removal efficiency compared to HCO3−, CO32−, and CH3COO− [30,61]. The study of kinetics for these inorganic anions reported in the literature [30] and their corresponding values are given in Equations (28) to (37) [30,90], and these results are also consistent with the present study [62].
•OH + CO32− → CO3•− + •OH → k = 3.9 × 108 M−1 S−1
•OH + HCO3−→ CO3 •− + H2 O → k = 8.6 × 106 M−1 S−1
•OH + NO2− → NO2 • + OH− → k = 8.0 × 109 M−1 S−1
NO2− + eaq− → NO22− →k = 4.1 × 109 M−1 S−
SO4•− + CO32− → CO3•− + SO42− → k = 6.1 × 106 M−1 S−1
SO4 •− + HCO3− → CO3 •− + HSO4− → k = 2.8 × 106 M−1 S−
SO42− + •OH → SO4•− + OH− → k = 6.5 × 107 M−1 S−
•OH + Cl− → ClOH•− → k = 4.3 × 109 M−1 S−1
SO4•− + Cl− → SO42− + Cl• → k = 3.0 × 108 M−1 S−
NO2− + eaq− → NO22− → k = 4.1 × 109 M−1 S−
Figure 4 shows the effects of various ions, such as nitrates, bicarbonates, carbonates, sulfate and acetates in the concentration range of 1.0 mM for anions on the degradation of CV by a UV/PMS system. Among the other reported ions, only SO42− shows the suppression effect for the degradation of crystal violet. The inhibition effect of SO42− is mostly the extermination nature of hydroxyl and sulfate ions [91].
The removal efficiency and reaction time of the developed catalytic system are compared with other reported catalytic systems, and the results are shown in Table 4. These results showed that the presently developed catalyst system has a better removal efficiency in minimum reaction times and can serve as a promising catalyst for the removal of CV and other dyes.

Table 4.
Comparison of removal efficiency for various dyes of different catalyst systems.
4. Conclusions
This study shows that UV/PMS AOTs are one of the most promising and auspicious advanced oxidation technologies for the decolorization of CV, which is directly affected by initial substrate concentration, initial oxidant concentration, pH and different inorganic ions. The comparative removal of CV has corresponding studies showing that better removal of CV has been carried out by a UV/PMS system rather than a PMS and UV system alone. The better removal of CV by UV/PMS system is dependent on the formation of free radical species like •OH and SO4•−, which are formed by PMS by means of UV radiation of 254 nm. Only a 42% removal of CV dye was obtained by UV radiation, while only a 76% removal of CV dye was obtained by the PMS system. However, the coupling of PMS with the UV system significantly enhanced the degradation as well as the removal of CV dye by as much as 97%. The removal efficiency of crystal violet increases as the dosage of oxidant increases and vice versa.
It has been observed that the removal efficiency of CV decreases with the increase of the CV concentration. Meanwhile, the degradation rate increases when the initial concentration of CV increased then the Kobs decreased. It is clear that Kobs decreased first from 0.0134 to 0.0048 mints−1 when the pH increased from 1.92 to 5.33, then the value of Kobs becomes constant from 0.0048 to 0.0079 mints−1 at pH 5.33 to 9.40, suggesting that the acidic condition was more auspicious to the decolorization of CV than neutral conditions. In addition, the value of Kobs increases from 0.0079 to 0.0415 mints−1 when the pH increases from 9.40 to 12.07, suggesting the best removal of CV dye at basic pH conditions and alkaline circumstances greatly accelerated the CV degradation. The maximum removal efficiency of crystal violet dye occurs at pH 12.07. The removal efficiency of CV is found to be 24%, 32%, 39%, 15%, and 44% for nitrates ions, HCO3−, carbonates ions, SO42−, and CH3COO−, correspondingly. The best removal efficiency rate of CV dye was achieved with CH3COO− and CO32− inorganic ions. The studies of the kinetics of the elimination of CV by UV/PMS at varying initial doses of PMS were demonstrated and concluded that the reaction succeeds through pseudo-first-order kinetics. For the elimination of toxic organic contaminants from water and for its conversion into less toxic substances, the method under study is more efficient and beneficial and less costly than other methods. Moreover, such systems can be developed for other pollutants/contaminants found in wastewater and can be installed directly on the site of flow from industrial sources. The present study also reinforces and suggests that future studies perform a comparison of different oxidation technologies and their efficiencies in improving the research scope.
Author Contributions
N.A. conducted the experiments and wrote and revised the manuscript, I.A.K. supervised and revised the manuscript, M.W. performed the visualization and validation of the manuscript, A.A.K. conducted the formal analysis in the manuscript, S.U.D. assisted with the methodology, S.A.Q. helped to review the manuscript, A.M.K. reviewed the manuscript and assisted with English editing, M.U.H. reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data will be provided on request.
Conflicts of Interest
The authors have no conflict of interest for this study.
References
- Gessner, T.; Mayer, U. Triarylmethane and diarylmethanedyes. In Ullmann’s Encyclopedia of Industrial Chemistry, 6th ed.; Mayer: NewYork, NY, USA, 2002; Volume 27, pp. 166–173. [Google Scholar]
- Manna, S.; Roy, D.; Saha, P.; Gopakumar, D.; Thomas, S. Rapid methylene blue adsorption using modified lignocellulosic materials. Process Saf. Environ. Prot. 2017, 107, 346–356. [Google Scholar] [CrossRef]
- Iqbal, J.; Shah, N.S.; Sayed, M.; Rauf, S.; Khan, Z.U.H.; Niazi, N.K.; Polychronopoulou, K.; Howari, F.; Rehman, F. Efficient removal of norfloxacin using nano zerovalent cerium composite biochar-catalyzed peroxydisulfate. J. Clean. Prod. 2022, 377, 134405. [Google Scholar] [CrossRef]
- Roselin, L.S.; Selvin, R. Photocatalytic treatment and reusability of textile dyeing effluents from cotton dyeing industries. Sci. Adv. Mater. 2011, 3, 113–119. [Google Scholar] [CrossRef]
- Nassar, M.M.; Magdy, Y.H. Removal of different basic dyes from aqueous solutions by adsorption on palm-fruit bunch particles. Chem. Eng. J. 1997, 66, 223–226. [Google Scholar] [CrossRef]
- Pagga, U.; Brown, D. The degradation of dyestuffs: Part II Behaviour of dyestuffs in aerobic biodegradation tests. Chemosphere 1986, 15, 479–491. [Google Scholar] [CrossRef]
- Arslan, I.; Balcioǧlu, I.A.; Bahnemann, D.W. Advanced chemical oxidation of reactive dyes in simulated dyehouse effluents by ferrioxalate-Fenton/UV-A and TiO2/UV-A processes. Dye. Pigment. 2000, 47, 207–218. [Google Scholar] [CrossRef]
- Durango-Usuga, P.; Guzmán-Duque, F.; Mosteo, R.; Vazquez, M.V.; Peñuela, G.; Torres-Palma, R.A. Experimental design approach applied to the elimination of crystal violet in water by electrocoagulation with Fe or Al electrodes. J. Hazard. Mater. 2010, 179, 120–126. [Google Scholar] [CrossRef]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Adak, A.; Bandyopadhyay, M.; Pal, A. Removal of crystal violet dye from wastewater by surfactant-modified alumina. Sep. Purif. Technol. 2005, 44, 139–144. [Google Scholar] [CrossRef]
- Ghosh, D.; Bhattacharyya, K.G. Adsorption of methylene blue on kaolinite. Appl. Clay Sci. 2002, 20, 295–300. [Google Scholar] [CrossRef]
- Suhail, F.; Mashkour, M.S.; Saeb, D. The study on photo degradation of crystal violet by polarographic technique. Int. J. Basic Appl. Sci. 2015, 15, 12–21. [Google Scholar]
- Senthilkumaar, S.; Porkodi, K. Heterogeneous photocatalytic decomposition of crystal violet in UV-illuminated sol–gel derived nanocrystalline TiO2 suspensions. J. Colloid Interface Sci. 2005, 288, 184–189. [Google Scholar] [CrossRef]
- Singh, K.P.; Gupta, S.; Singh, A.K.; Sinha, S. Optimizing adsorption of crystal violet dye from water by magnetic nanocomposite using response surface modeling approach. J. Hazard. Mater. 2011, 186, 1462–1473. [Google Scholar] [CrossRef]
- Habibi, M.H.; Hassanzadeh, A.; Mahdavi, S. The effect of operational parameters on the photocatalytic degradation of three textile azo dyes in aqueous TiO2 suspensions. J. Photochem. Photobiol. A Chem. 2005, 172, 89–96. [Google Scholar] [CrossRef]
- Salem, I.A. Activation of H2O2 by Amberlyst-15 resin supported with copper (II)-complexes towards oxidation of crystal violet. Chemosphere 2001, 44, 1109–1119. [Google Scholar] [CrossRef]
- Akyol, A.; Yatmaz, H.; Bayramoglu, M. Photocatalytic decolorization of Remazol Red RR in aqueous ZnO suspensions. Appl. Catal. B Environ. 2004, 54, 19–24. [Google Scholar] [CrossRef]
- Pielesz, A. The process of the reduction of azo dyes used in dyeing textiles on the basis of infrared spectroscopy analysis. J. Mol. Struct. 1999, 511, 337–344. [Google Scholar] [CrossRef]
- Vijayakumar, G.; Tamilarasan, R.; Dharmendirakumar, M. Adsorption, Kinetic, Equilibrium and Thermodynamic studies on the removal of basic dye Rhodamine-B from aqueous solution by the use of natural adsorbent perlite. J. Mater. Environ. Sci. 2012, 3, 157–170. [Google Scholar]
- Al-Shahrani, S. Phenomena of removal of crystal violet from wastewater using Khulays natural bentonite. J. Chem. 2020, 2020, 4607657. [Google Scholar] [CrossRef]
- Sahoo, C.; Gupta, A.; Pal, A. Photocatalytic degradation of Crystal Violet (CI Basic Violet 3) on silver ion doped TiO2. Dye. Pigment. 2005, 66, 189–196. [Google Scholar] [CrossRef]
- Bafana, A.; Devi, S.S.; Chakrabarti, T. Azo dyes: Past, present and the future. Environ. Rev. 2011, 19, 350–371. [Google Scholar] [CrossRef]
- Glaze, W.H.; Kang, J.-W.; Chapin, D.H. The chemistry of water treatment processes involving ozone, hydrogen peroxide and ultraviolet radiation. Ozone Sci. Eng. 1987, 9, 335–352. [Google Scholar] [CrossRef]
- Iqbal, J.; Shah, N.S.; Sayed, M.; Niazi, N.K.; Imran, M.; Khan, J.A.; Khan, Z.U.H.; Hussien, A.G.S.; Polychronopoulou, K.; Howari, F. Nano-zerovalent manganese/biochar composite for the adsorptive and oxidative removal of Congo-red dye from aqueous solutions. J. Hazard. Mater. 2021, 403, 123854. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, H. Manganese Oxides as Catalysts for Water and Wastewater Treatment: A Critical Review. Environ. Sci. Nano 2019, 133, 105141. [Google Scholar] [CrossRef]
- Trojanowicz, M.; Bojanowska-Czajka, A.; Bartosiewicz, I.; Kulisa, K. Advanced oxidation/reduction processes treatment for aqueous perfluorooctanoate (PFOA) and perfluorooctanesulfonate (PFOS)–a review of recent advances. Chem. Eng. J. 2018, 336, 170–199. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, T.; Zhou, Y.; Fang, L.; Shao, Y. Degradation of atenolol by UV/peroxymonosulfate: Kinetics, effect of operational parameters and mechanism. Chemosphere 2013, 93, 2717–2724. [Google Scholar] [CrossRef] [PubMed]
- Anipsitakis, G.P.; Tufano, T.P.; Dionysiou, D.D. Chemical and microbial decontamination of pool water using activated potassium peroxymonosulfate. Water Res. 2008, 42, 2899–2910. [Google Scholar] [CrossRef]
- Haag, W.R.; Yao, C.D. Rate constants for reaction of hydroxyl radicals with several drinking water contaminants. Environ. Sci. Technol. 1992, 26, 1005–1013. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (OH/⋅ O− in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Babu, D.S.; Srivastava, V.; Nidheesh, P.; Kumar, M.S. Detoxification of water and wastewater by advanced oxidation processes. Sci. Total Environ. 2019, 696, 133961. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Z.; Li, S.; Liu, J.; Yao, L.; Li, Y.; Zhang, H. Insights into the mechanism of heterogeneous activation of persulfate with a clay/iron-based catalyst under visible LED light irradiation. Appl. Catal. B Environ. 2016, 185, 22–30. [Google Scholar] [CrossRef]
- Neta, P.; Huie, R.E.; Ross, A. Rate constants for reactions of inorganic radicals in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 1027–1284. [Google Scholar] [CrossRef]
- Oh, W.-D.; Dong, Z.; Lim, T.-T. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects. Appl. Catal. B Environ 2016, 194, 169–201. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Transition metal/UV-based advanced oxidation technologies for water decontamination. Appl. Catal. B Environ. 2004, 54, 155–163. [Google Scholar] [CrossRef]
- He, X.; Armah, A.; Dionysiou, D.D. Destruction of cyanobacterial toxin cylindrospermopsin by hydroxyl radicals and sulfate radicals using UV-254 nm activation of hydrogen peroxide, persulfate and peroxymonosulfate. J. Photochem. Photobiol. A Chem. 2013, 251, 160–166. [Google Scholar] [CrossRef]
- Khan, S.; Naushad, M.; Al-Gheethi, A.; Iqbal, J. Engineered nanoparticles for removal of pollutants from wastewater: Current status and future prospects of nanotechnology for remediation strategies. J. Environ. Chem. Eng. 2021, 9, 106160. [Google Scholar] [CrossRef]
- Li, L.; Wei, D.; Wei, G.; Du, Y. Transformation of cefazolin during chlorination process: Products, mechanism and genotoxicity assessment. J. Hazard. Mater. 2013, 262, 48–54. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M.; Witek-Krowiak, A.W. Agricultural waste peels as versatile biomass for water purification–A review. Chem. Eng. J. 2015, 270, 244–271. [Google Scholar] [CrossRef]
- Rehman, F.; Parveen, N.; Iqbal, J.; Sayed, M.; Shah, N.S.; Ansar, S.; Khan, J.A.; Shah, A.; Jamil, F.; Boczkaj, G. Potential degradation of norfloxacin using UV-C/Fe2+/peroxides-based oxidative pathways. J. Photochem. Photobiol. A Chem. 2022, 435, 114305. [Google Scholar] [CrossRef]
- Iqbal, J.; Shah, N.S.; Khan, Z.U.H.; Rizwan, M.; Murtaza, B.; Jamil, F.; Shah, A.; Ullah, A.; Nazzal, Y.; Howari, F. Visible light driven doped CeO2 for the treatment of pharmaceuticals in wastewater: A review. J. Water Process Eng. 2022, 49, 103130. [Google Scholar] [CrossRef]
- Ghauch, A.; Tuqan, A.M.; Kibbi, N.; Geryes, S. Methylene blue discoloration by heated persulfate in aqueous solution. Chem. Eng. J. 2012, 213, 259–271. [Google Scholar] [CrossRef]
- Shah, N.S.; He, X.; Khan, H.M.; Khan, J.A.; O’Shea, K.E.; Boccelli, D.L.; Dionysiou, D.D. Efficient removal of endosulfan from aqueous solution by UV-C/peroxides: A comparative study. J. Hazard. Mater. 2013, 263, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Ghauch, A.; Tuqan, A.M.; Kibbi, N. Ibuprofen removal by heated persulfate in aqueous solution: A kinetics study. Chem. Eng. J. 2012, 197, 483–492. [Google Scholar] [CrossRef]
- Ghauch, A.; Baalbaki, A.; Amasha, M.; El Asmar, R.; Tantawi, O. Contribution of persulfate in UV-254 nm activated systems for complete degradation of chloramphenicol antibiotic in water. Chem. Eng. J. 2017, 317, 1012–1025. [Google Scholar] [CrossRef]
- Al Mayyahi, A.; Al-Asadi, H. Advanced Oxidation Processes (AOPs) for Wastewater Treatment and Reuse: A Brief Review. Asian J. Appl. Sci. Technol. 2018, 2, 18–30. [Google Scholar]
- Oturan, M.A.; Aaron, J.J. Advanced oxidation processes in water/wastewater treatment: Principles and applications. A review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Khodadad, I.; Abedzadeh, N.; Lakshminarayan, V.; Saini, S.S. Low Cost Spectrometers and Learning Applications for Exposing Kids to Optics. In Proceedings of the Education and Training in Optics and Photonics, Bordeaux, France, 29 June–2 July 2015; p. OUT03. [Google Scholar]
- Guan, Y.-H.; Ma, J.; Li, X.-C.; Fang, J.-Y.; Chen, L.-W. Influence of pH on the formation of sulfate and hydroxyl radicals in the UV/peroxymonosulfate system. Environ. Sci. Technol. 2011, 45, 9308–9314. [Google Scholar] [CrossRef]
- Rodríguez-Chueca, J.; Alonso, E.; Singh, D.N. Photocatalytic mechanisms for peroxymonosulfate activation through the removal of methylene blue: A case study. Int. J. Environ. Res. Public Health 2019, 16, 198. [Google Scholar] [CrossRef]
- Sayed, M.; Khan, J.A.; Shah, L.A.; Shah, N.S.; Shah, F.; Khan, H.M.; Zhang, P.; Arandiyan, H. Solar light responsive poly (vinyl alcohol)-assisted hydrothermal synthesis of immobilized TiO2/Ti film with the addition of peroxymonosulfate for photocatalytic degradation of ciprofloxacin in aqueous media: A mechanistic approach. J. Phys. Chem. C 2018, 122, 406–421. [Google Scholar] [CrossRef]
- Khan, J.A.; He, X.; Shah, N.S.; Khan, H.M.; Hapeshi, E.; Fatta-Kassinos, D.; Dionysiou, D.D. Kinetic and mechanism investigation on the photochemical degradation of atrazine with activated H2O2, S2O82− and HSO5−. Chem. Eng. J. 2014, 252, 393–403. [Google Scholar] [CrossRef]
- Parsons, S. Advanced Oxidation Processes for Water and Wastewater Treatment; IWA Publishing: London, UK, 2004. [Google Scholar]
- Bolton, J.R.; Bircher, K.G.; Tumas, W.; Tolman, C.A. Figures-of-merit for the technical development and application of advanced oxidation technologies for both electric-and solar-driven systems (IUPAC Technical Report). Pure Appl. Chem. 2001, 73, 627–637. [Google Scholar] [CrossRef]
- Ghaedi, M.; Ansari, A.; Bahari, F.; Ghaedi, A.; Vafaei, A. A hybrid artificial neural network and particle swarm optimization for prediction of removal of hazardous dye brilliant green from aqueous solution using zinc sulfide nanoparticle loaded on activated carbon. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 137, 1004–1015. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; He, X.; Khan, H.M.; Boccelli, D.; Dionysiou, D.D. Efficient degradation of lindane in aqueous solution by iron (II) and/or UV activated peroxymonosulfate. J. Photochem. Photobiol. A Chem. 2016, 316, 37–43. [Google Scholar] [CrossRef]
- Rehman, F.; Sayed, M.; Khan, J.A.; Shah, N.S.; Khan, H.M.; Dionysiou, D.D. Oxidative removal of brilliant green by UV/S2O82−, UV/HSO5−and UV/H2O2 processes in aqueous media: A comparative study. J. Hazard. Mater. 2018, 357, 506–514. [Google Scholar] [CrossRef]
- Shah, N.S.; Rizwan, A.D.; Khan, J.A.; Sayed, M.; Khan, Z.U.H.; Murtaza, B.; Iqbal, J.; Din, S.U.; Imran, M.; Nadeem, M.; et al. Toxicities, kinetics and degradation pathways investigation of ciprofloxacin degradation using iron-mediated H2O2 based advanced oxidation processes. Process Saf. Environ. Prot. 2018, 117, 473–482. [Google Scholar] [CrossRef]
- Khan, J.A.; He, X.; Khan, H.M.; Shah, N.S.; Dionysiou, D.D. Oxidative degradation of atrazine in aqueous solution by UV/H2O2/Fe2+, UV/S2O82-/Fe2+ and UV/HSO5-/Fe2+ processes: A comparative study. Chem. Eng. J. 2013, 218, 376–383. [Google Scholar] [CrossRef]
- He, X.; Pelaez, M.; Westrick, J.A.; O’Shea, K.E.; Hiskia, A.; Triantis, T.; Kaloudis, T.; Stefan, M.I.; Armah, A.; Dionysiou, D.D. Efficient removal of microcystin-LR by UV-C/H2O2 in synthetic and natural water samples. Water Res 2012, 46, 1501–1510. [Google Scholar] [CrossRef]
- Shah, N.S.; Khan, J.A.; Nawaz, S.; Khan, H.M. Role of aqueous electron and hydroxyl radical in the removal of endosulfan from aqueous solution using gamma irradiation. J. Hazard. Mater. 2014, 278, 40–48. [Google Scholar] [CrossRef]
- Qi, Y.; Qu, R.; Liu, J.; Chen, J.; Al-Basher, G.; Alsultan, N.; Wang, Z.; Huo, Z. Oxidation of flumequine in aqueous solution by UV-activated peroxymonosulfate: Kinetics, water matrix effects, degradation products and reaction pathways. Chemosphere 2019, 237, 124484. [Google Scholar] [CrossRef]
- Khan, J.A.; He, X.; Shah, N.S.; Sayed, M.; Khan, H.M.; Dionysiou, D.D. Degradation kinetics and mechanism of desethyl-atrazine and desisopropyl-atrazine in water with OH and SO4− based-AOPs. Chem. Eng. J. 2017, 325, 485–494. [Google Scholar] [CrossRef]
- Li, M.; An, Z.; Huo, Y.; Jiang, J.; Zhou, Y.; Cao, H.; Jin, Z.; Xie, J.; Zhan, J.; He, M. Individual and combined degradation of N-heterocyclic compounds under sulfate radical-based advanced oxidation processes. Chem. Eng. J. 2022, 442, 136316. [Google Scholar] [CrossRef]
- Lee, B.-N.; Liaw, W.-D.; Lou, J.-C. Photocatalytic decolorization of methylene blue in aqueous TiO2 suspension. Environ. Eng. Sci. 1999, 16, 165–175. [Google Scholar] [CrossRef]
- Kulkarni, M.R.; Revanth, T.; Acharya, A.; Bhat, P. Removal of Crystal Violet dye from aqueous solution using water hyacinth: Equilibrium, kinetics and thermodynamics study. Resour.-Effic. Technol. 2017, 3, 71–77. [Google Scholar] [CrossRef]
- Rahmat, M.; Rehman, A.; Rahmat, S.; Bhatti, H.N.; Iqbal, M.; Khan, W.S.; Bajwa, S.Z.; Rahmat, R.; Nazir, A. Highly efficient removal of crystal violet dye from water by MnO2 based nanofibrous mesh/photocatalytic process. J. Mater. Res. Technol. 2019, 8, 5149–5159. [Google Scholar] [CrossRef]
- Sayed, M.; Ismail, M.; Khan, S.; Tabassum, S.; Khan, H. Degradation of ciprofloxacin in water by advanced oxidation process: Kinetics study, influencing parameters and degradation pathways. Environ. Technol. 2016, 37, 590–602. [Google Scholar] [CrossRef]
- Sánchez-Polo, M.; López-Peñalver, J.; Prados-Joya, G.; Ferro-García, M.A.; Rivera-Utrilla, J. Gamma irradiation of pharmaceutical compounds, nitroimidazoles, as a new alternative for water treatment. Water Res. 2009, 43, 4028–4036. [Google Scholar] [CrossRef]
- Sayed, M.; Hadi, F.; Khan, J.A.; Shah, N.S.; Shah, L.A.; Khan, H. Degradation of Acetaminophen in Aqueous Media by H2O2 Assisted Gamma Irradiation Process. Z. fur Phys. Chem. 2018, 232, 545–558. [Google Scholar] [CrossRef]
- Shah, N.S.; Khan, J.A.; Sayed, M.; Khan, Z.U.H.; Iqbal, J.; Arshad, S.; Junaid, M.; Khan, H.M. Synergistic effects of H2O2 and S2O82− in the gamma radiation induced degradation of congo-red dye: Kinetics and toxicities evaluation. Sep. Purif. Technol. 2020, 233, 115966. [Google Scholar] [CrossRef]
- Shah, N.S.; Khan, J.A.; Sayed, M.; Khan, Z.U.H.; Ali, H.S.; Murtaza, B.; Khan, H.M.; Imran, M.; Muhammad, N. Hydroxyl and sulfate radical mediated degradation of ciprofloxacin using nano zerovalent manganese catalyzed S2O82−. Chem. Eng. J. 2019, 356, 199–209. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Huang, Y.-F.; Huang, C.-i.; Chen, C.-Y. Efficient decolorization of azo dye Reactive Black B involving aromatic fragment degradation in buffered Co2+/PMS oxidative processes with a ppb level dosage of Co2+-catalyst. J. Hazard. Mater. 2009, 170, 1110–1118. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, F.; Moradi, M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants. Chem. Eng. J. 2017, 310, 41–62. [Google Scholar] [CrossRef]
- Yang, Y.; Pignatello, J.J.; Ma, J.; Mitch, W.A. Comparison of halide impacts on the efficiency of contaminant degradation by sulfate and hydroxyl radical-based advanced oxidation processes (AOPs). Environ. Sci. Technol. 2014, 48, 2344–2351. [Google Scholar] [CrossRef] [PubMed]
- Ruan, W.; Hu, J.; Qi, J.; Hou, Y.; Cao, R.; Wei, X. Removal of crystal violet by using reduced-graphene-oxide-supported bimetallic Fe/Ni nanoparticles (rGO/Fe/Ni): Application of artificial intelligence modeling for the optimization process. Materials 2018, 11, 865. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Muhammad, U.L.; Zango, Z.U.; Kadir, H.A. Crystal violet removal from aqueous solution using corn stalk biosorbent. Sci. World J. 2019, 14, 133–138. [Google Scholar]
- Soliman, A.M.; Elsuccary, S.; Ali, I.M.; Ayesh, A.I. Photocatalytic activity of transition metal ions-loaded activated carbon: Degradation of crystal violet dye under solar radiation. J. Water Process Engl. 2017, 17, 245–255. [Google Scholar] [CrossRef]
- Imran, M.S.; Javed, T.; Areej, I.; Haider, M.N. Sequestration of crystal violet dye from wastewater using low-cost coconut husk as a potential adsorbent. Water Sci. Technol. 2022, 85, 2295–2317. [Google Scholar] [CrossRef]
- Qi, C.; Liu, X.; Lin, C.; Zhang, H.; Li, X.; Ma, J. Activation of peroxymonosulfate by microwave irradiation for degradation of organic contaminants. Chem. Eng. J. 2017, 315, 201–209. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Barbati, S.; Doumenq, P.; Chiron, S. Sulfate radical anion oxidation of diclofenac and sulfamethoxazole for water decontamination. Chem. Eng. J. 2012, 197, 440–447. [Google Scholar] [CrossRef]
- Ghatak, H.R. Advanced oxidation processes for the treatment of biorecalcitrant organics in wastewater. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1167–1219. [Google Scholar] [CrossRef]
- Son, H.-S.; Choi, S.-B.; Khan, E.; Zoh, K.-D. Removal of 1, 4-dioxane from water using sonication: Effect of adding oxidants on the degradation kinetics. Water Res. 2006, 40, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Staehelin, J.; Hoigne, J. Decomposition of ozone in water in the presence of organic solutes acting as promoters and inhibitors of radical chain reactions. Environ. Sci. Technol. 1985, 19, 1206–1213. [Google Scholar] [CrossRef]
- Liu, L.; Lin, S.; Zhang, W.; Farooq, U.; Shen, G.; Hu, S. Kinetic and mechanistic investigations of the degradation of sulfachloropyridazine in heat-activated persulfate oxidation process. Chem. Eng. J. 2018, 346, 515–524. [Google Scholar] [CrossRef]
- Nie, M.; Deng, Y.; Nie, S.; Yan, C.; Ding, M.; Dong, W.; Dai, Y.; Zhang, Y. Simultaneous removal of bisphenol A and phosphate from water by peroxymonosulfate combined with calcium hydroxide. Chem. Eng. J. 2019, 369, 35–45. [Google Scholar] [CrossRef]
- Cao, J.; Lai, L.; Lai, B.; Yao, G.; Chen, X.; Song, L. Degradation of tetracycline by peroxymonosulfate activated with zero-valent iron: Performance, intermediates, toxicity and mechanism. Chem. Eng. J. 2019, 364, 45–56. [Google Scholar] [CrossRef]
- Ahmadi, M.; Ghanbari, F. Organic dye degradation through peroxymonosulfate catalyzed by reusable graphite felt/ferriferrous oxide: Mechanism and identification of intermediates. Mater. Res. Bull. 2019, 111, 43–52. [Google Scholar] [CrossRef]
- Ito, T.; Morimoto, S.; Fujita, S.I.; Nishimoto, S.I. Radical intermediates generated in the reactions of l-arginine with hydroxyl radical and sulfate radical anion: A pulse radiolysis study. Radiat. Phys. Chem. 2009, 78, 256–260. [Google Scholar] [CrossRef]
- Sayed, M.; Khan, J.A.; Shah, L.A.; Shah, N.S.; Khan, H.M.; Rehman, F.; Khan, A.R.; Khan, A.M. Degradation of quinolone antibiotic, norfloxacin, in aqueous solution using gamma-ray irradiation. Environ. Sci. Pollut. Res. 2016, 23, 13155–13168. [Google Scholar] [CrossRef]
- Tripathy, N.; Ahmad, R.; Song, J.E.; Park, H.; Khang, G. ZnO nanonails for photocatalytic degradation of crystal violet dye under UV irradiation. AIMS Mater. Sci. 2017, 4, 267–276. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).