Macroinvertebrate-Based Biomonitoring of Coastal Wetlands in Mediterranean Chile: Testing Potential Metrics Able to Detect Anthropogenic Impacts
Abstract
:1. Introduction
2. Materials and Methods
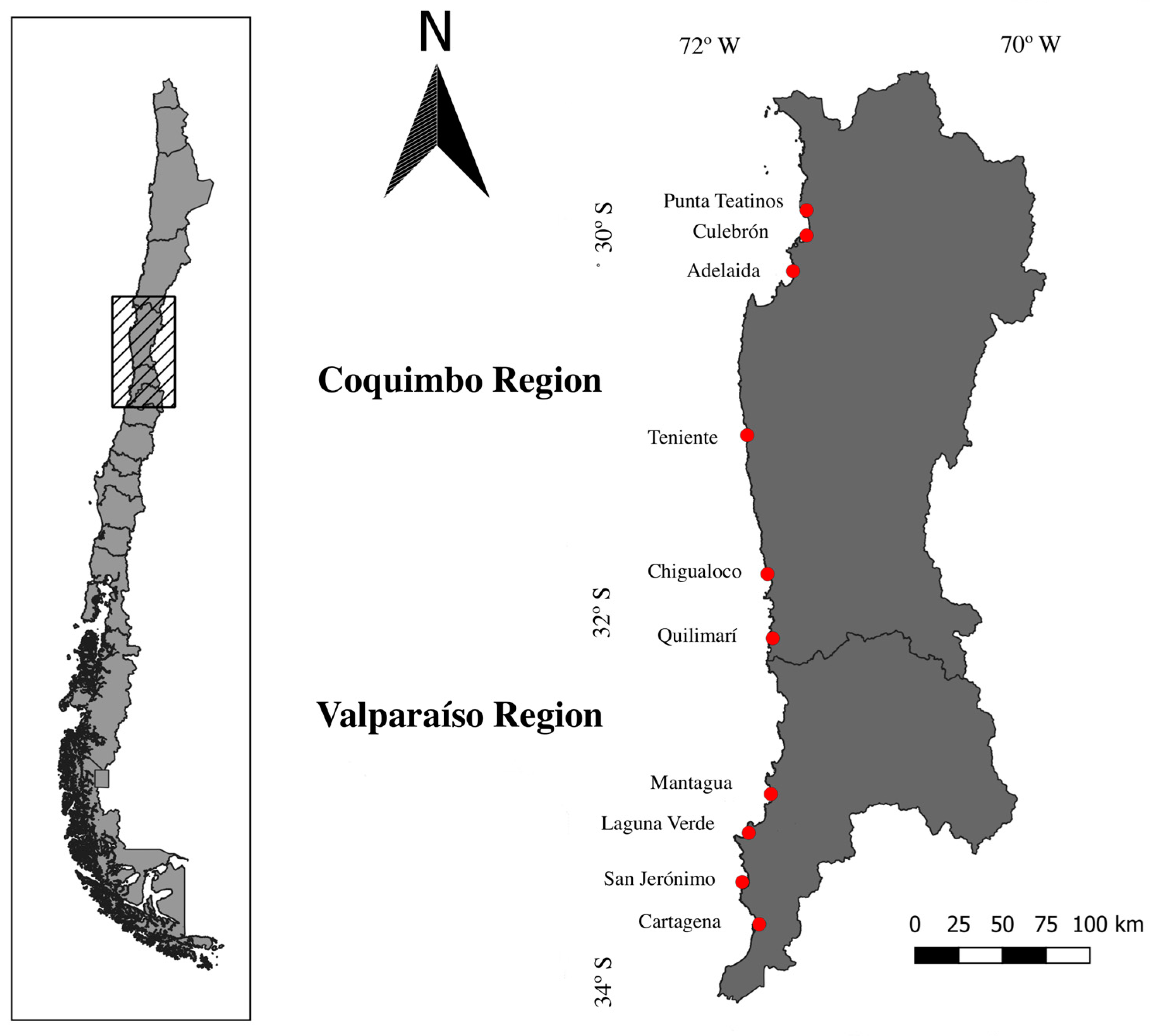
2.1. Study Sites
2.2. Environmental Data
2.3. Macroinvertebrate Data
2.4. Classifying Wetlands Sites According to Their Human Disturbance
2.5. Candidate Metrics
2.6. Metric Selection
3. Results
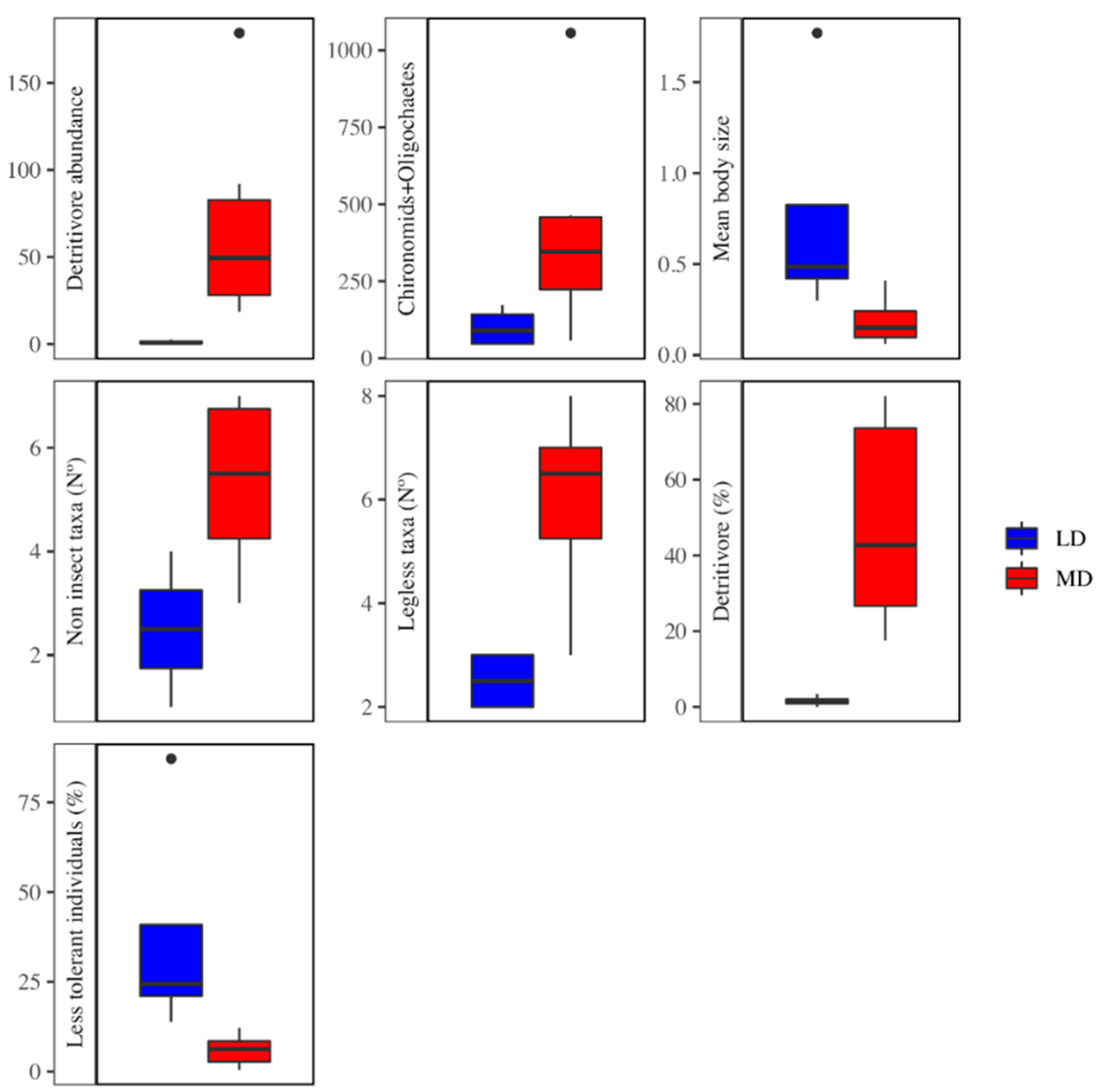
3.1. Disturbance Classification
3.2. Metric Selection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Camacho, V.V.; Ruiz-Luna, A.; Berlanga-Robles, A.C. Effects of land use changes on ecosystem services value provided by coastal wetlands: Recent and future landscape scenarios. J. Coast. Zone Manag. 2016, 19, 418. [Google Scholar]
- Lee, S.Y.; Dunn, R.J.K.; Young, R.A.; Connolly, R.M.; Dale, P.E.R.; Dehayr, R.; Lemckert, C.J.; McKinnon, S.; Powell, B.; Teasdale, P.R.; et al. Impact of urbanization on coastal wetland structure and function. Austral Ecol. 2006, 31, 149–163. [Google Scholar] [CrossRef]
- Pardo, M.T.; Esteve, M.A.; Giménez, A.; Martínez-Fernández, J.; Carreño, M.F.; Serrano, J.; Miñano, J. Assessment of hydrological alterations on wandering beetle assemblages (coleoptera: Carabidae and Tenebrionidae) in coastal wetlands of arid Mediterranean systems. J. Arid Environ. 2008, 72, 1803–1810. [Google Scholar] [CrossRef]
- Carreño, M.F.; Esteve, M.A.; Martinez, J.; Palazón, J.A.; Pardo, M.T. Habitat changes in coastal wetlands associated to hydrological changes in the watershed. Estuar. Coast. Shelf Sci. 2008, 77, 475–483. [Google Scholar] [CrossRef]
- Novoa, V.; Rojas, O.; Ahumada-Rudolph, R.; Sáez, K.; Fierro, P.; Rojas, C. Coastal wetlands: Ecosystems affected by urbanization? Water 2020, 12, 698. [Google Scholar] [CrossRef] [Green Version]
- Davidson, N.C. How much wetland has the world lost? Long-term and recent trends in global wetland area. Mar. Freshwater Res. 2014, 65, 934–941. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhang, X.; Zhao, X.; Ma, S.; Cao, H.; Cao, J. Assessing spatial vulnerability from rapid urbanization to inform coastal urban regional planning. Ocean Coast. Manag. 2016, 123, 53–65. [Google Scholar] [CrossRef]
- Borja, A.; Bricker, S.B.; Dauer, D.M.; Demetriades, N.T.; Ferreira, J.G.; Forbes, A.T.; Hutchings, P.; Xiaoping, J.; Kenchington, R.; Marques, J.C.; et al. Overview of integrative tools and methods in assessing ecological integrity in estuarine and coastal systems worldwide. Mar. Pollt. Bull. 2008, 56, 1519–1537. [Google Scholar] [CrossRef]
- Birk, S.; Bonne, W.; Borja, A.; Brucet, S.; Courrat, A.; Poikane, S.; Solimini, A.; van de Bund, W.; Zampoukas, N.; Hering, D. Three hundred ways to assess Europe’s surface waters: An almost complete overview of biological methods to implement the Water Framework Directive. Ecol. Indic. 2012, 18, 31–41. [Google Scholar] [CrossRef]
- Rosenberg, D.M.; Resh, V.H. Introduction to freshwater biomonitoring and benthic macroinvertebrates. In Freshwater Biomonitoring and Benthic Macroinvertebrates; Rosenberg, D.M., Resh, V.H., Eds.; Chapman and Hall: New York, NY, USA, 1993; pp. 1–9. [Google Scholar]
- Barbour, M.T.; Gerritsen, J.; Griffith, G.E.; Frydenborg, R.; McCarron, E.; White, J.S.; Bastian, M.L. A framework for biological criteria for Florida streams using benthic macroinvertebrates. J. N. Am. Benth. Soc. 1996, 15, 185–211. [Google Scholar] [CrossRef]
- Menetrey, N.; Oertli, B.; Lachavanne, J.B. The CIEPT: A macroinvertebrate-based multimetric index for assessing the ecological quality of Swiss lowland ponds. Ecol. Indic. 2011, 11, 590–600. [Google Scholar] [CrossRef]
- Mereta, S.T.; Boets, P.; De Meester, L.; Goethals, P.L. Development of a multimetric index based on benthic macroinvertebrates for the assessment of natural wetlands in Southwest Ethiopia. Ecol. Indic. 2013, 29, 510–521. [Google Scholar] [CrossRef]
- Fierro, P.; Arismendi, I.; Hughes, R.M.; Valdovinos, C.; Jara-Flores, A. A benthic macroinvertebrate multimetric index for Chilean Mediterranean streams. Ecol. Indic. 2018, 91, 13–23. [Google Scholar] [CrossRef]
- Sapounidis, A.S.; Koutrakis, E.T. Development of a fish-based multimetric index for the assessment of Lagoons’ ecological quality in northern Greece. Water 2021, 13, 3008. [Google Scholar] [CrossRef]
- Vadas, R.L., Jr.; Hughes, R.M.; Bae, Y.J.; Baek, M.J.; Gonzáles, O.C.B.; Callisto, M.; de Carvalho, D.R.; Chen, K.; Ferreira, M.; Fierro, P.; et al. Assemblage-based biomonitoring of freshwater ecosystem health via multimetric indices: A critical review and suggestions for improving their applicability. Water Biol. Sec. 2022, 100054. [Google Scholar] [CrossRef]
- Karr, J.R.; Chu, E.W. Restoring Life in Running Waters; Island Press: Washington, DC, USA, 1999. [Google Scholar]
- Borja, A.; Barbone, E.; Basset, A.; Borgersen, G.; Brkljacic, M.; Elliott, M.; garmendia, J.M.; Marques, J.C.; Mazik, K.; Muxika, I.; et al. Response of single benthic metrics and multi-metric methods to anthropogenic pressure gradients, in five distinct European coastal and transitional ecosystems. Mar. Pollt. Bull. 2011, 62, 499–513. [Google Scholar] [CrossRef]
- Vásquez, D.; Sandoval, N.; Fierro, P.; Valdovinos, C. Morphological impacts of the Chilean megathrust earthquake Mw 8.8 on coastal wetlands of high conservation value. Estuar. Coast. Shelf Sci. 2020, 245, 106922. [Google Scholar] [CrossRef]
- Marín, A.; Gelcich, S.; Castilla, J.C. Ecosystem services and abrupt transformations in a coastal wetland social-ecological system: Tubul-Raqui after the 2010 earthquake in Chile. Ecol. Soc. 2014, 19, 22. [Google Scholar] [CrossRef]
- Rojas, O.; Mardones, M.; Martínez, C.; Flores, L.; Sáez, K.; Araneda, A. Flooding in central Chile: Implications of tides and sea level increase in the 21st century. Sustainability 2018, 10, 4335. [Google Scholar] [CrossRef] [Green Version]
- Bertrán, C.; Fierro, P.; Encalada, E.; Peña-Cortés, F.; Tapia, J.; Hauenstein, E.; Vargas-Chacoff, L. Macrobenthos of the coastal Budi lagoon, southern Chile: Changes associated with seasonal environment variation. Braz. J. Oceanogr. 2016, 64, 239–248. [Google Scholar] [CrossRef]
- Arriagada, L.; Rojas, O.; Arumí, J.L.; Munizaga, J.; Rojas, C.; Farias, L.; Vega, C. A new method to evaluate the vulnerability of watersheds facing several stressors: A case study in Mediterranean Chile. Sci. Total Environ. 2019, 651, 1517–1533. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Buitrago, N.; Contreras-Lopez, M.; Martinez, C.; Williams, A. Can coastal scenery be managed? The Valparaíso region, Chile as a case study. Ocean Coast. Manag. 2018, 163, 383–400. [Google Scholar] [CrossRef]
- Fariña, J.M.; He, Q.; Silliman, B.R.; Bertness, M.D. Biogeography of salt marsh plant zonation on the Pacific coast of South America. J. Biogeogr. 2018, 45, 238–247. [Google Scholar] [CrossRef]
- Contreras López, M.; Figueroa Sterquel, R.; Salcedo Castro, J.; Vergara Cortés, H.; Zuleta, C.; Bravo, V.; Piñones, C.; Cortés Molina, F. Vulnerabilidad de Humedales y Dunas Litorales en Chile Central. In Vulnerabilidad de las Zonas Costeras de Latinoamérica al Cambio Climático; Botello, A., Villanueva, S., Gutiérrez, J., Rojas, J.L., Eds.; Editorial Universidad Juárez Autónoma de Tabasco (UJAT), Universidad Nacional Autónoma de México (UNAM), Universidad Autónoma de Campeche (UAC): Campeche, Mexico, 2017; pp. 227–246. [Google Scholar]
- Zuleta-Ramos, C.; Contreras-López, M. Humedales Costero de Coquimbo: Bio-Diversidad, Vulnerabilidades & Conservación; Universidad de La Serena & Ministerio del Medio Ambiente: La Serena, Chile, 2019. [Google Scholar]
- EPA. Methods for Chemical Analysis of Water and Wastes; EPA-600/4-79-020; US Environmental Protection Agency: Washington, DC, USA; Office of Research and Development: Washington, DC, USA; Environmental Monitoring and Support Laboratory: Cincinnati, OH, USA, 1983; Method 353.2.
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Strickland, J.D.H.; Parsons, T.R. Determination of Reactive Silicate. In A Practical Handbook of Seawater Analysis, 2nd ed.; Fisheries Research Board of Canada: Ottawa, Canada, 1972; Volume 167, pp. 65–70. [Google Scholar]
- Domínguez, E.; Fernández, H.R. Macroinvertebrados Bentónicos Sudamericanos. Sistemática y Biología; Fundación Miguel Lillo: Tucumán, Argentina, 2009; Volume 656. [Google Scholar]
- Rogers, D.C.; Thorp, J.H. Thorp and Covich’s Freshwater Invertebrates (Fourth Edition) Keys to Palaearctic Fauna, 4th ed.; Academic Press: New York, NY, USA, 2019. [Google Scholar]
- Heckman, C.W. Encyclopedia of South American Aquatic Insects: Hemiptera-Heteroptera; Illustrated Keys to Known Families, Genera, and Species in South America; Springer: Washington, DC, USA, 2011; pp. 1–679. [Google Scholar]
- Coccia, C.; Contreras-López, M.; Farina, J.M.; Green, A.J. Comparison of taxonomic and size-based approaches to determine the effects of environment and disturbance on invertebrate communities in coastal Chile. Ecol. Indic. 2022, 143, 109356. [Google Scholar] [CrossRef]
- Ligeiro, R.; Hughes, R.M.; Kaufmann, P.R.; Macedo, D.R.; Firmiano, K.R.; Ferreira, W.R.; Oliveira, D.; Melo, A.S.; Callisto, M. Defining quantitative stream disturbance gradients and the additive role of habitat variation to explain macroinvertebrate taxa richness. Ecol. Indic. 2013, 25, 45–57. [Google Scholar] [CrossRef] [Green Version]
- Sala, J.; Gascón, S.; Boix, D.; Gesti, J.; Quintana, X.D. Proposal of a rapid methodology to assess the conservation status of Mediterranean wetlands and its application in Catalunya (NE Iberian Peninsula). Arch. Sci. 2004, 57, 141–152. [Google Scholar]
- Gascón, S.; Boix, D.; Sala, J.; Quintana, X.D. Patterns in size and species diversity of benthic macroinvertebrates in Mediterranean salt marshes. Mar. Ecol. Progr. Ser. 2009, 391, 21–32. [Google Scholar] [CrossRef]
- Petchey, O.L.; Belgrano, A. Body-size distributions and size-spectra: Universal indicators of ecological status? Biol. Lett. 2010, 6, 434–437. [Google Scholar] [CrossRef] [Green Version]
- Figueroa, R.; Palma, A.; Ruiz, V.; Niell, X. Comparative analysis of biotic indexes used to evaluate water quality in a Mediterranean river of Chile: Chillan River, VIII Region. Rev. Chil. Hist. Nat. 2007, 80, 225–242. [Google Scholar]
- Merrit, R.W.; Cummins, K.W. Trophic relations of macroinvertebrates. In Methods in Stream Ecology; Hauer, R.F., Lamberti, G.A., Eds.; Academic Press: San Diego, DC, USA, 1996; pp. 453–473. [Google Scholar]
- Fierro, P.; Bertrán, C.; Mercado, M.; Peña-Cortés, F.; Tapia, J.; Hauenstein, E.; Caputo, L.; Vargas-Chacoff, L. Landscape composition as a determinant of diversity and functional feeding groups of aquatic macroinvertebrates in southern rivers of the Araucanía, Chile. Latin American, J. Aquat. Res. 2015, 43, 186–200. [Google Scholar] [CrossRef]
- Solis, M.; Arias, M.; Fanelli, S.; Bonetto, C.; Mugni, H. Agrochemicals’ effects on functional feeding groups of macroinvertebrates in Pampas streams. Ecol. Indic. 2019, 101, 373–379. [Google Scholar] [CrossRef]
- Quintana, X.D.; Sandra, B.; Dani, B.; López-Flores, R.; Stéphanie, G.; Badosa, A.; Jordi, S.; Moreno-Amich, R.; Egozcue, J.J. A nonparametric method for the measurement of size diversity with emphasis on data standardization. Limnol. Oceanogr. Methods 2008, 6, 75–86. [Google Scholar] [CrossRef]
- Quintana, X.D.; Egozcue, J.J.; Martínez-Abella, O.; López-Flores, R.; Gascón, S.; Brucet, S.; Boix, D. Update: A non-parametric method for the measurement of size diversity, with emphasis on data standardization. The measurement of the size evenness. Limnol. Oceanogr. Methods 2016, 14, 408–413. [Google Scholar] [CrossRef] [Green Version]
- Edwards, A.M.; Robinson, J.P.W.; Plank, M.J.; Baum, J.K.; Blanchard, J.L. Testing and recommending methods for fitting size spectra to data. Methods Ecol. Evol. 2017, 8, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, R.B.S.; Castro, C.M.; Baptista, D.F. Desenvolvimento de índices multimétricos para utilização em programas de monitoramento biológico da integridade de escossistemas aquáticos. Oecol. Bras. 2008, 12, 487–505. [Google Scholar] [CrossRef] [Green Version]
- Arias, M.J.; Vaschetto, P.A.; Marchese, M.; Regaldo, L.; Gagneten, A.M. Benthic macroinvertebrates and zooplankton communities as ecological indicators in urban wetland of Argentina. Sustainability 2022, 14, 4045. [Google Scholar] [CrossRef]
- Soria, M.; Gutiérrez-Cánovas, C.; Bonada, N.; Acosta, R.; Rodríguez-Lozano, P.; Fortuño, P.; Burgazzi, G.; Vinyoles, D.; Gallart, F.; Latron, J.; et al. Natural disturbances can produce misleading bioassessment results: Identifying metrics to detect anthropogenic impacts in intermittent rivers. J. Appl. Ecol. 2020, 57, 283–295. [Google Scholar] [CrossRef]
- Šidagytė-Copilas, E.; Arbačiauskas, K. A multimetric macroinvertebrate index for the assessment of the ecological status of Lithuanian rivers. Limnologica 2022, 97, 126010. [Google Scholar] [CrossRef]
- Ruaro, R.; Gubiani, E.A.; Hughes, R.M.; Mormul, R.P. Global trends and challenges in multimetric indices of biological condition. Ecol. Indic. 2020, 110, 105862. [Google Scholar] [CrossRef]
- Kovalenko, K.E.; Brady, V.J.; Ciborowski, J.J.; Host, G.E.; Johnson, L.B. Macroinvertebrate and Fish Community Metrics: Confounding Effects and Consistency over Time. Wetlands 2020, 40, 1107–1116. [Google Scholar] [CrossRef]
- Coccia, C.; Fariña, J.M. Responses of plant-arthropod communities to nutrient enrichment in hyper-arid and semi-arid zones. Estuar. Coast. Shelf Sci. 2022, 272, 107884. [Google Scholar] [CrossRef]
- Simboura, N.; Panayotidis, P.; Papathanassiou, E. A synthesis of the biological quality elements for the implementation of the European Water Framework Directive in the Mediterranean ecoregion: The case of Saronikos Gulf. Ecol. Indic. 2005, 5, 253–266. [Google Scholar] [CrossRef]
- Bunster, T.; Gironás, J.; Rojas, C.; Bonilla, C. Impacts of Urbanization and Land Use Change over Water Resources. In Water Resources of Chile; Springer: Cham, Switzerland, 2021; pp. 365–387. [Google Scholar]
- Carey, J.C.; Fulweiler, R.W. Watershed land use alters riverine silica cycling. Biogeochemistry 2013, 113, 525–544. [Google Scholar] [CrossRef]
- Alavaisha, E.; Lyon, S.W.; Lindborg, R. Assessment of water quality across irrigation schemes: A case study of wetland agriculture impacts in Kilombero Valley, Tanzania. Water 2019, 11, 671. [Google Scholar] [CrossRef] [Green Version]
- Pearson, T.H.; Rosenberg, R. Macrobenthic succession in relation to organic enrichment and pollution of the marine environment. Oceanogr. Mar. Biol. 1978, 16, 229–311. [Google Scholar]
- Marques, J.C.; Nielsen, S.N.; Pardal, M.A.; Jørgensen, S.E. Impact of eutrophication and river management within a framework of ecosystem theories. Ecol. Modell. 2003, 166, 147–168. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, P.G.; Pardal, M.A.; Lillebø, A.I.; Ferreira, S.M.; Raffaelli, D.; Marques, J.C. Dynamic changes in seagrass assemblages under eutrophication and implications for recovery. J. Exp. Mar. Biol. Ecol. 2004, 302, 233–248. [Google Scholar] [CrossRef] [Green Version]
- Mangadze, T.; Wasserman, R.J.; Froneman, P.W.; Dalu, T. Macroinvertebrate functional feeding group alterations in response to habitat degradation of headwater Austral streams. Sci. Total Environ. 2019, 695, 133910. [Google Scholar] [CrossRef]
- Verdonschot, P.F. Beyond masses and blooms: The indicative value of oligochaetes. Hydrobiologia 2006, 564, 127–142. [Google Scholar] [CrossRef]
- Asha, C.V.; Retina, I.C.; Suson, P.S.; Bijoy Nandan, S. Ecosystem analisis of the degrading Vembanad wetland ecosystem, the largest Ramsar site on the South West Coast of India – Mesaures for its sustainable management. Reg. Stud. Mar. Sci. 2016, 8, 408–421. [Google Scholar]
- Onyena, A.P.; Nkwoji, J.A.; Chukwu, L.O. Evaluation of hydrochemistry and benthic macroinvertebrate in Chanomi Creek, Niger Delta Nigeria. Reg. Stu. Mar. Sci. 2021, 46, 101907. [Google Scholar] [CrossRef]
- Surugiu, V. The use of polychaetes as indicators of eutrophication and organic enrichment of coastal waters: A study case–Romanian Black Sea coast. Analele Ştiinţifice ale Universităţii “Al. I. Cuza” Iaşi, s. Biol. Animal. 2005, 51, 55–62. [Google Scholar]
- Wildsmith, M.D.; Rose, T.H.; Potter, I.C.; Warwick, R.M.; Clarke, K.R. Benthic macroinvertebrates as indicators of environmental deterioration in a large microtidal estuary. Mar. Pollut. Bull. 2011, 62, 525–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Townsend, C.R.; Hildrew, A.G. Species traits in relation to a habitat templet for river systems. Freshw. Biol. 1994, 31, 265–275. [Google Scholar] [CrossRef]
- Benzina, I.; Bachir, A.S.; Santoul, F.; Céréghino, R. Macroinvertebrate functional trait responses to environmental gradients and anthropogenic disturbance in arid-land streams of North Africa. J. Arid Environ. 2021, 195, 104626. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Z.; Castro, D.M.; Tan, X.; Jiang, X.; Meng, X.; Ge, Y.; Xie, Z. Effects of different types of land-use on taxonomic and functional diversity of benthic macroinvertebrates in a subtropical river network. Environ. Sci. Pollut. Res. 2021, 28, 44339–44353. [Google Scholar] [CrossRef]
- Gardner, J.L.; Peters, A.; Kearney, M.R.; Joseph, L.; Heinsohn, R. Declining body size: A third universal response to warming? Trends Ecol. Evol. 2011, 26, 285–291. [Google Scholar] [CrossRef]
- Verberk, W.C.; Atkinson, D.; Hoefnagel, K.N.; Hirst, A.G.; Horne, C.R.; Siepel, H. Shrinking body sizes in response to warming: Explanations for the temperature–size rule with special emphasis on the role of oxygen. Biol. Rev. 2021, 96, 247–268. [Google Scholar] [CrossRef]
- Figueroa, R.; Suarez, M.L.; Andreu, A.; Ruiz, V.H.; Vidal-Abarca, M.R. Wetlands ecological characterization of central Chile semi-dry area. Gayana 2009, 73, 76–94. [Google Scholar]
- Chen, K.; Hughes, R.M.; Xu, S.; Zhang, J.; Cai, D.; Wang, B. Evaluating performance of macroinvertebrate-based adjusted and unadjusted multi-metric indices (MMI) using multi-season and multi-year samples. Ecol. Indic. 2014, 36, 142–151. [Google Scholar] [CrossRef]
- Martínez-Megías, C.; Rico, A. Biodiversity impacts by multiple anthropogenic stressors in Mediterranean coastal wetlands. Sci. Total Environ. 2021, 818, 151712. [Google Scholar] [CrossRef] [PubMed]

| Environmental Variables | Least Disturbed N = 4 | Intermediate N = 15 | Most Disturbed N = 6 |
|---|---|---|---|
| Temperature (°C) | 18.5 (15.2–23.6) | 18.4 (12.0–22.8) | 15.7 (12.2–17.6) |
| Dissolved oxygen (mg/L) | 10.6 (9.3–11.3) | 10.0 (5.3–17.1) | 6.1 (2.2–10.0) |
| pH | 10.1 (7.9–11.4) | 8.9 (7.5–11.6) | 8.1 (7.7–9.1) |
| Chlorophyll-a (µg/L) | 4.1 (0.7–6.7) | 6.5 (1.5–16.0) | 12.2 (1.3–29.6) |
| Depth (cm) | 31.5 (20.0–45.4) | 38.6 (22.4–67.0) | 34.5 (16.2–53.8) |
| Phosphates (µM) | 1.5 (0.7–2.6) | 1.2 (0.3–2.6) | 3.2 (2.8–3.6) |
| Silicates (µM) | 158.8 (103.1–200.4) | 116.7 (75.5–200.4) | 190.5 (173.0–208.0) |
| Nitrates (µM) | 3.3 (1.9–6.5) | 16.9 (0.2–77.7) | 5.4 (0.1–10.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coccia, C.; Vega, C.; Fierro, P. Macroinvertebrate-Based Biomonitoring of Coastal Wetlands in Mediterranean Chile: Testing Potential Metrics Able to Detect Anthropogenic Impacts. Water 2022, 14, 3449. https://doi.org/10.3390/w14213449
Coccia C, Vega C, Fierro P. Macroinvertebrate-Based Biomonitoring of Coastal Wetlands in Mediterranean Chile: Testing Potential Metrics Able to Detect Anthropogenic Impacts. Water. 2022; 14(21):3449. https://doi.org/10.3390/w14213449
Chicago/Turabian StyleCoccia, Cristina, Carlos Vega, and Pablo Fierro. 2022. "Macroinvertebrate-Based Biomonitoring of Coastal Wetlands in Mediterranean Chile: Testing Potential Metrics Able to Detect Anthropogenic Impacts" Water 14, no. 21: 3449. https://doi.org/10.3390/w14213449






