Recent Advances in the Reactor Design for Industrial Wastewater Treatment by Electro-Oxidation Process
Abstract
:1. Introduction
2. Electrochemical Reactors
3. ECRs with Different Cell Architecture
3.1. Plate-Frame ECR
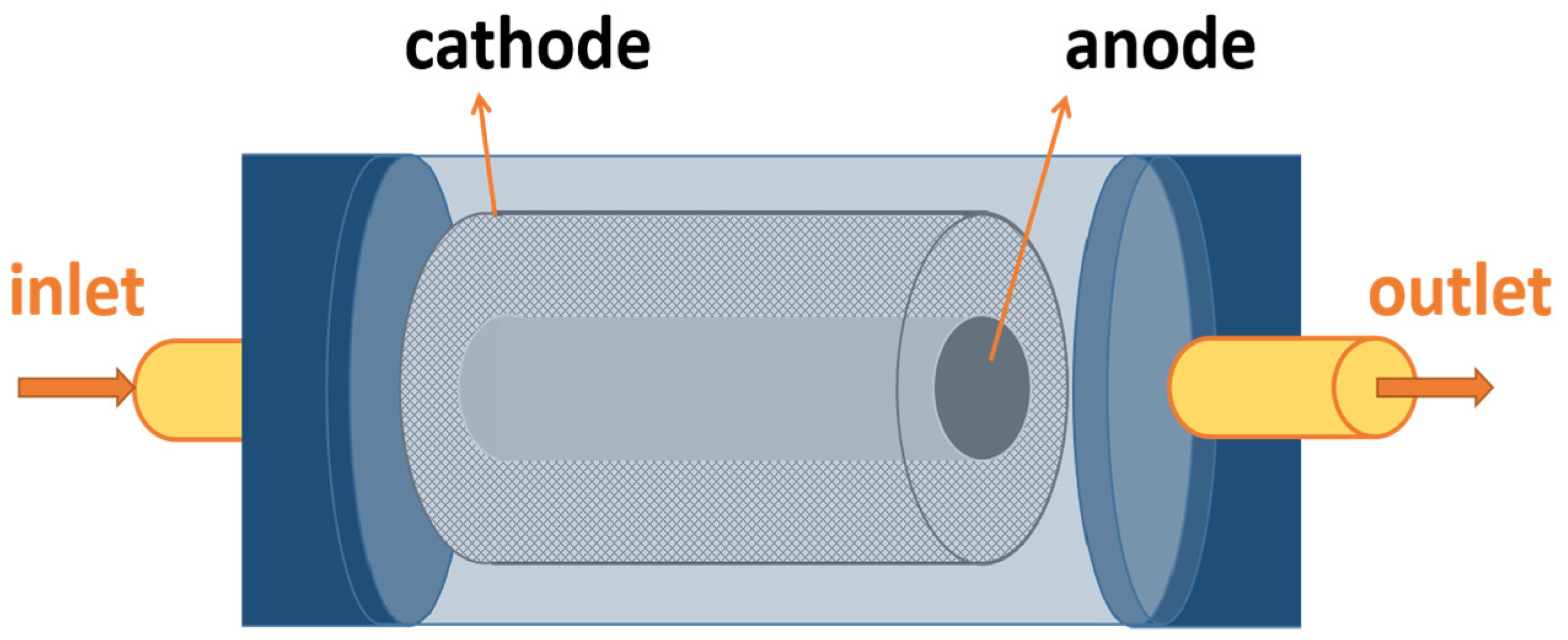
3.2. Tubular ECR
4. Operation Mode of ECR
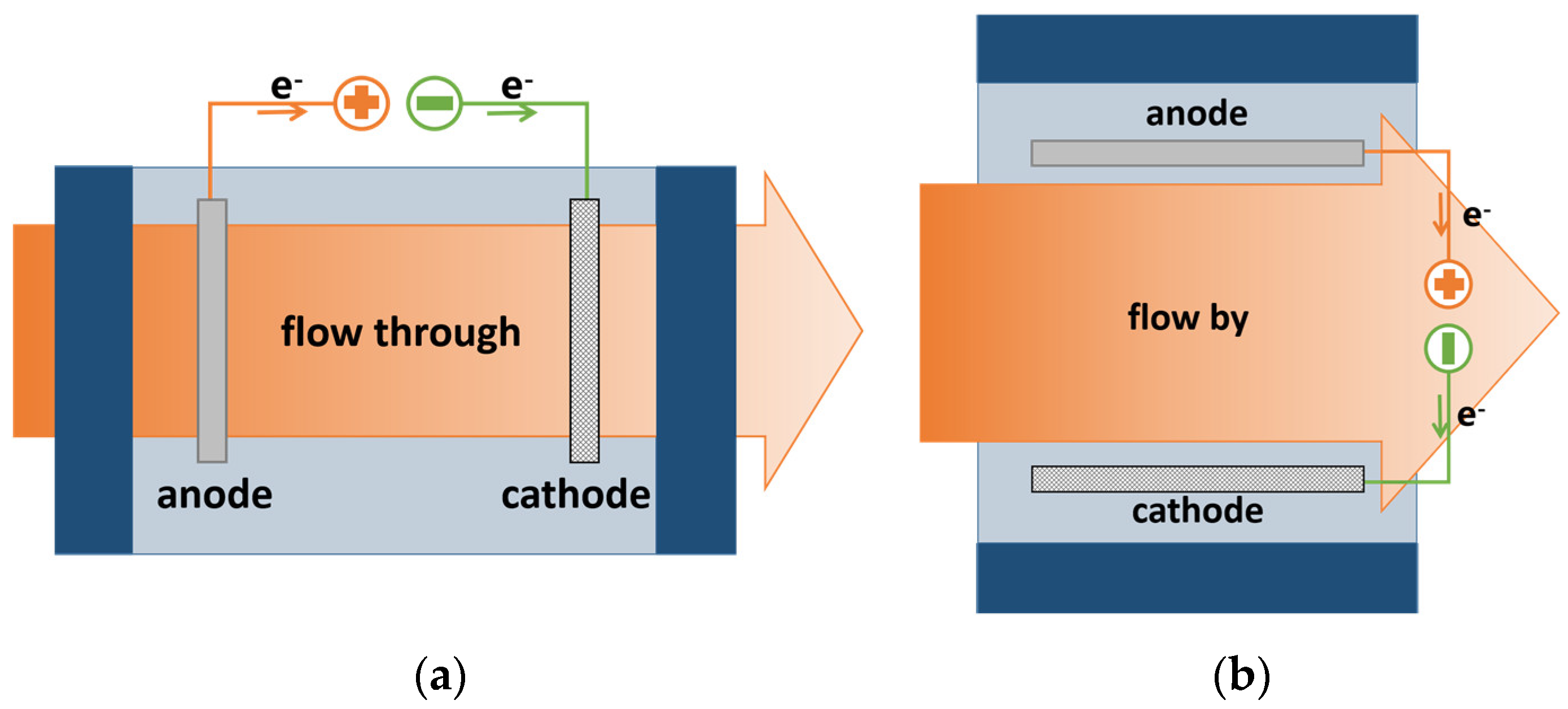
5. The Flow Mode and Electrode Configuration of ECRs
5.1. Flow-By Mode
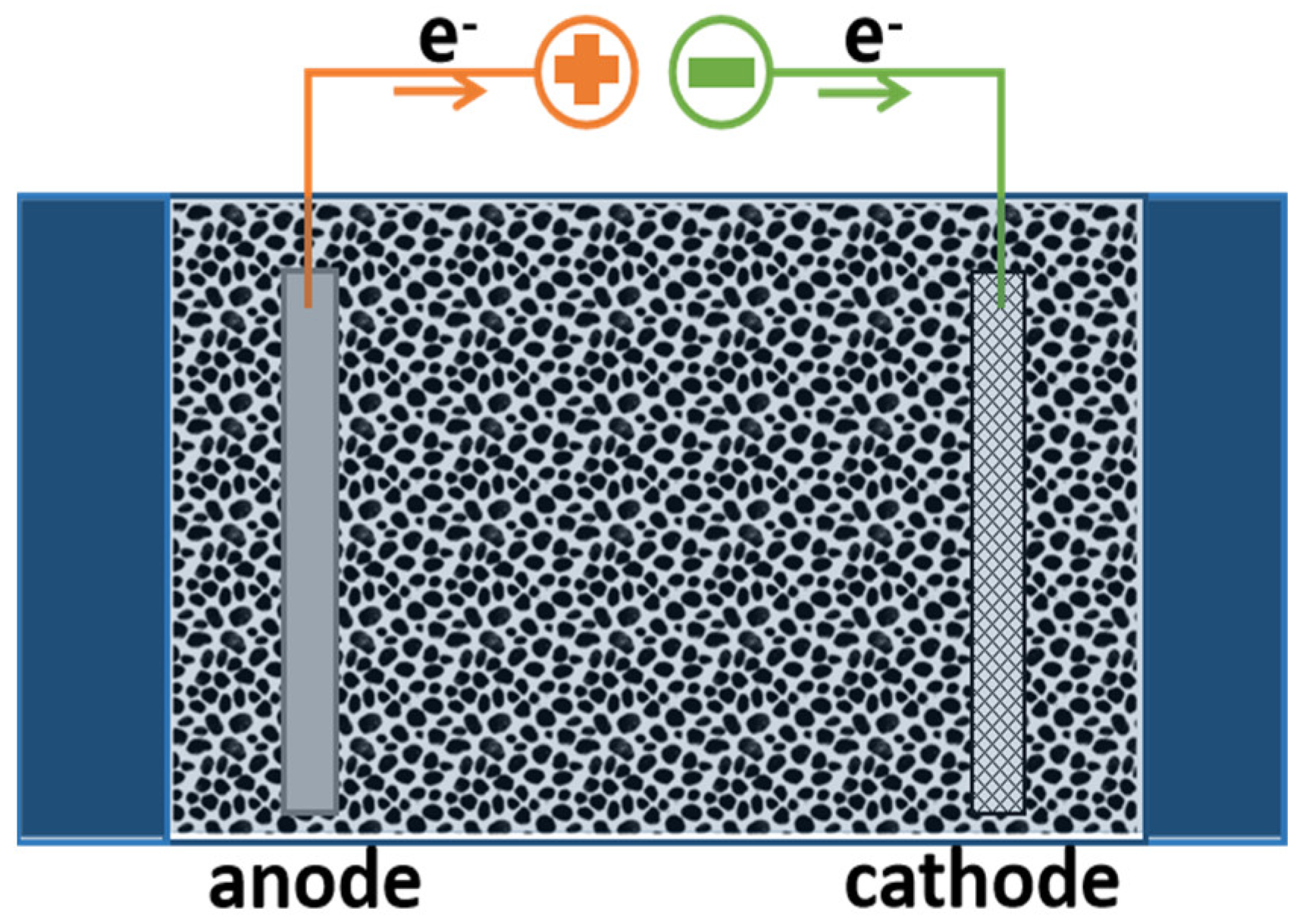
5.2. Flow-Through Mode
6. ECR Classification according to the Electrode Configuration
6.1. Fixed Bed 3D-ECR
6.2. Fluidized Bed 3D-ECR
7. Conclusions and Future Perspectives
- (1)
- Compared with the plate-frame ECR, the tubular ECR has less dead zone and a higher mass transfer efficiency due to the larger specific surface areas of electrodes. The mass transfer efficiency is the one of most important factors for degradation efficiency of wastewater in ECRs. The optimization reactors enhance degradation efficiency of wastewater mainly by changing the shape of electrodes or increasing fluid flow rate.
- (2)
- The limited mass transfer hindered by boundary layer in flow-by ECRs. Additionally, flow-through ECRs could enhance the mass transfer of reactant by suppressing the boundary layer.
- (3)
- From the engineering point of view, continuous mode operation is more preferred because of faster reactions, cleaner products and safer and easier scaling up than batch mode for large-scale wastewater treatment in EO system.
- (4)
- Particle electrodes in the 3D-ECR could improve the degradation efficiency by improving the concentration of pollutants at the particle electrode interface. The 3D-ECR system deserves comprehensive development due to the particle electrodes. However, the mechanism of reactions is more complicated in a 3D-ECR. Optimizing the operating parameters and enhancing electrode materials performance of adsorption and catalysis to maintain the stable operation are significant for future development of 3D-ECRs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luan, M.; Jing, G.; Piao, Y.; Liu, D.; Jin, L. Treatment of refractory organic pollutants in industrial wastewater by wet air oxidation. Arab. J. Chem. 2017, 10, S769–S776. [Google Scholar] [CrossRef] [Green Version]
- Deng, M.; Wu, K.; Yang, T.; Yu, D.; Liu, G.; Gong, S.; Sun, D.; Petru, M. Construction of Novel Electro-Fenton Systems by Magnetically Decorating Zero-Valent Iron onto RuO2-IrO2/Ti Electrode for Highly Efficient Pharmaceutical Wastewater Treatment. Water 2022, 14, 1044. [Google Scholar] [CrossRef]
- Zwane, B.N.; Orimolade, B.O.; Koiki, B.A.; Mabuba, N.; Gomri, C.; Petit, E.; Bonniol, V.; Lesage, G.; Rivallin, M.; Cretin, M.; et al. Combined Electro-Fenton and Anodic Oxidation Processes at a Sub-Stoichiometric Titanium Oxide (Ti4O7) Ceramic Electrode for the Degradation of Tetracycline in Water. Water 2021, 13, 2772. [Google Scholar] [CrossRef]
- Wu, D.; Li, Y.; Lu, G.; Lin, Q.; Wei, L.; Zhang, P. Removal of Aqueous Para-Aminobenzoic Acid Using a Compartmental Electro-Peroxone Process. Water 2021, 13, 2961. [Google Scholar] [CrossRef]
- Zangeneh, H.; Zinatizadeh, A.A.L.; Habibi, M.; Akia, M.; Hasnain Isa, M. Photocatalytic oxidation of organic dyes and pollutants in wastewater using different modified titanium dioxides: A comparative review. J. Ind. Eng. Chem. 2015, 26, 1–36. [Google Scholar] [CrossRef]
- Lin, R.; Li, Y.; Yong, T.; Cao, W.; Wu, J.; Shen, Y. Synergistic effects of oxidation, coagulation and adsorption in the integrated fenton-based process for wastewater treatment: A review. J. Environ. Manag. 2022, 306, 114460. [Google Scholar] [CrossRef]
- Anil, G.; Scaria, J.; Nidheesh, P.V. Removal of Synthetic Dye from Aqueous Solution Using MnFe2O4-GO Catalyzed Heterogeneous Electro-Fenton Process. Water 2022, 14, 3350. [Google Scholar] [CrossRef]
- Du, C.; Zhang, Y.; Zhang, Z.; Zhou, L.; Yu, G.; Wen, X.; Chi, T.; Wang, G.; Su, Y.; Deng, F.; et al. Fe-based metal organic frameworks (Fe-MOFs) for organic pollutants removal via photo-Fenton: A review. Chem. Eng. J. 2022, 431, 133932. [Google Scholar] [CrossRef]
- Tian, K.; Hu, L.; Li, L.; Zheng, Q.; Xin, Y.; Zhang, G. Recent advances in persulfate-based advanced oxidation processes for organic wastewater treatment. Chinese Chem. Lett. 2022, 33, 4461–4477. [Google Scholar] [CrossRef]
- Soomro, G.S.; Qu, C.; Ren, N.; Meng, S.; Li, X.; Liang, D.; Zhang, S.; Li, Y. Efficient removal of refractory organics in landfill leachate concentrates by electrocoagulation in tandem with simultaneous electro-oxidation and in-situ peroxone. Environ. Res. 2020, 183, 109249. [Google Scholar] [CrossRef]
- Lauzurique, Y.; Espinoza, L.C.; Huiliñir, C.; García, V.; Salazar, R. Anodic Oxidation of Industrial Winery Wastewater Using Different Anodes. Water 2022, 14, 95. [Google Scholar] [CrossRef]
- Qu, C.; Li, Y.-g.; Meng, S.-j.; Li, X.-h.; Zhang, S.-j.; Liang, D.-w. Enhanced refractory organics removal by •OH and 1O2 generated in an electro-oxidation system with cathodic Fenton-like reaction. J. Hazard. Mater. 2022, 434, 128923. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Soomro, G.S.; Ren, N.; Liang, D.-w.; Lu, S.-f.; Xiang, Y.; Zhang, S.-j. Enhanced electro-oxidation/peroxone (in situ) process with a Ti-based nickel-antimony doped tin oxide anode for phenol degradation. J. Hazard. Mater. 2020, 384, 121398. [Google Scholar] [CrossRef] [PubMed]
- Pulkka, S.; Martikainen, M.; Bhatnagar, A.; Sillanpää, M. Electrochemical methods for the removal of anionic contaminants from water—A review. Sepa. Purif. Technol. 2014, 132, 252–271. [Google Scholar] [CrossRef]
- Łuba, M.; Mikołajczyk, T.; Pierożyński, B.; Smoczyński, L.; Wojtacha, P.; Kuczyński, M. Electrochemical Degradation of Industrial Dyes in Wastewater through the Dissolution of Aluminum Sacrificial Anode of Cu/Al Macro-Corrosion Galvanic Cell. Molecules 2020, 25, 4108. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, G.; Pierozynski, B. Electrooxidation of phenol on carbon fibre-based anodes through continuous electrolysis of synthetic wastewater. Pol. J. Chem. Technol. 2018, 20, 96–102. [Google Scholar] [CrossRef] [Green Version]
- Qu, C.; Ren, N.; Zhang, S.-J.; Li, Y.-G.; Meng, S.-J.; Li, X.-H.; Wang, S.-Q.; Liang, D.-W.; Li, A.-R. Degradation of triclosan by anodic oxidation/in-situ peroxone process: Kinetics, pathway and reaction mechanism. Chemosphere 2021, 272, 129453. [Google Scholar] [CrossRef]
- Zhou, B.; Yu, Z.; Wei, Q.; Long, H.; Xie, Y.; Wang, Y. Electrochemical oxidation of biological pretreated and membrane separated landfill leachate concentrates on boron doped diamond anode. Appl. Surf. Sci. 2016, 377, 406–415. [Google Scholar] [CrossRef]
- Subba Rao, A.N.; Venkatarangaiah, V.T. Metal oxide-coated anodes in wastewater treatment. Environ. Sci. Pollut. R. 2014, 21, 3197–3217. [Google Scholar] [CrossRef]
- Coster, J.D.; Vanherck, W.; Appels, L.; Dewil, R. Selective electrochemical degradation of 4-chlorophenol at a Ti/RuO2-IrO2 anode in chloride rich wastewater. J. Environ. Manag. 2017, 190, 61–71. [Google Scholar] [CrossRef]
- Ornelas Dávila, O.; Lacalle Bergeron, L.; Dávila Jiménez, M.M.; Sirés, I.; Brillas, E.; Navarro, A.F.R.; Arandes, J.B.; Sancho Llopis, J.V. Study of the electrochemical oxidation of 4,6-dimethyldibenzothiophene on a BDD electrode employing different techniques. J. Electroanal. Chem. 2021, 894, 115364. [Google Scholar] [CrossRef]
- Alfonso-Muniozguren, P.; Cotillas, S.; Boaventura, R.A.R.; Moreira, F.C.; Lee, J.; Vilar, V.J.P. Single and combined electrochemical oxidation driven processes for the treatment of slaughterhouse wastewater. J. Clean. Prod. 2020, 270, 121858. [Google Scholar] [CrossRef]
- Lei, Y.; Zhan, Z.; Saakes, M.; van der Weijden, R.D.; Buisman, C.J.N. Electrochemical recovery of phosphorus from wastewater using tubular stainless-steel cathode for a scalable long-term operation. Water Res. 2021, 199, 117199. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ai, Z.; Zhang, L. Design of a neutral three-dimensional electro-Fenton system with foam nickel as particle electrodes for wastewater treatment. J. Hazard. Mater. 2012, 243, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Dai, Q.; Hu, C.; Tong, Y.; Wang, Y.; Song, S.; Dai, L. Earth-abundant metal-free carbon-based electrocatalysts for Zn-air batteries to power electrochemical generation of H2O2 for in-situ wastewater treatment. Chem. Eng. J. 2021, 416, 128338. [Google Scholar] [CrossRef]
- Qu, C.; Liang, D.-w. Novel electrochemical advanced oxidation processes with H2O2 generation cathode for water treatment: A review. J. Environ. Chem. Eng. 2022, 10, 107896. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, F.; Gao, M.; Fang, X.; Liu, L. Electro-Fenton degradation of azo dye using polypyrrole/anthraquinonedisulphonate composite film modified graphite cathode in acidic aqueous solutions. Electrochimi. Acta 2008, 53, 5155–5161. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, M.; Zhang, C.; Jiang, Y.; Bi, Z.; Yang, J. Electro-Fenton degradation of p-nitrophenol using the anodized graphite felts. Chem. Eng. J. 2013, 233, 185–192. [Google Scholar] [CrossRef]
- Khataee, A.R.; Safarpour, M.; Zarei, M.; Aber, S. Electrochemical generation of H2O2 using immobilized carbon nanotubes on graphite electrode fed with air: Investigation of operational parameters. J. Electroanal. Chem. 2011, 659, 63–68. [Google Scholar] [CrossRef]
- Hou, J.; Xu, Z.; Ji, J.; Zhao, Y.; Gao, M.; Jin, C. Enhanced in-situ electro-generation of H2O2 using PTFE and NH4HCO3 modified C/PTFE electrode for treatment of landfill leachate. J. Environ. Manage. 2021, 295, 112933. [Google Scholar] [CrossRef]
- Garcia-Rodriguez, O.; Lee, Y.Y.; Olvera-Vargas, H.; Deng, F.; Wang, Z.; Lefebvre, O. Mineralization of electronic wastewater by electro-Fenton with an enhanced graphene-based gas diffusion cathode. Electrochim. Acta 2018, 276, 12–20. [Google Scholar] [CrossRef]
- Geraldino, H.C.L.; Freitas, T.K.F.S.; Manholer, D.D.; França, F.; Oliveira, J.H.; Volnistem, E.A.; Lima, A.R.F.; Bertotti, M.; Girotto, E.M.; Garcia, J.C. Electrochemical generation of H2O2 using gas diffusion electrode improved with rGO intensified with the Fe3O4/GO catalyst for degradation of textile wastewater. J. Water Process Eng. 2020, 36, 101377. [Google Scholar] [CrossRef]
- Perry, S.C.; Ponce de León, C.; Walsh, F.C. Review—The Design, Performance and Continuing Development of Electrochemical Reactors for Clean Electrosynthesis. J. Electrochem. Soc. 2020, 167, 155525. [Google Scholar] [CrossRef]
- Szpyrkowicz, L.; Kaul, S.N.; Neti, R.N. Tannery wastewater treatment by electro-oxidation coupled with a biological process. J. Appl. Electrochem. 2005, 35, 381–390. [Google Scholar] [CrossRef]
- Monteil, H.; Pechaud, Y.; Oturan, N.; Trellu, C.; Oturan, M.A. Pilot scale continuous reactor for water treatment by electrochemical advanced oxidation processes: Development of a new hydrodynamic/reactive combined model. Chem. Eng. J. 2021, 404, 127048. [Google Scholar] [CrossRef]
- Regalado-Méndez, A.; Mentado-Morales, J.; Vázquez, C.E.; Martínez-Villa, G.; Cordero, M.E.; Zárate, L.G.; Skogestad, S.; Peralta-Reyes, E. Modeling and Hydraulic Characterization of a Filter-Press-Type Electrochemical Reactor by Using Residence Time Distribution Analysis and Hydraulic Indices. Int. J. Chem. React. Eng. 2018, 16, 1–15. [Google Scholar] [CrossRef]
- Frías-Ferrer, Á.; Tudela, I.; Louisnard, O.; Sáez, V.; Esclapez, M.D.; Díez-García, M.I.; Bonete, P.; González-García, J. Optimized design of an electrochemical filter-press reactor using CFD methods. Chem. Eng. J. 2011, 169, 270–281. [Google Scholar] [CrossRef]
- Arenas, L.F.; Ponce de León, C.; Walsh, F.C. Critical Review—The Versatile Plane Parallel Electrode Geometry: An Illustrated Review. J. Electrochemi. Soc. 2020, 167, 023504. [Google Scholar] [CrossRef]
- Pinto, C.; Fernandes, A.; Lopes, A.; Nunes, M.J.; Baía, A.; Ciríaco, L.; Pacheco, M.J. Reuse of Textile Dyeing Wastewater Treated by Electrooxidation. Water 2022, 14, 1084. [Google Scholar] [CrossRef]
- Barbosa, A.D.; da Silva, L.F.; de Paula, H.M.; Romualdo, L.L.; Sadoyama, G.; Andrade, L.S. Combined use of coagulation (M. oleifera) and electrochemical techniques in the treatment of industrial paint wastewater for reuse and/or disposal. Water Res. 2018, 145, 153–161. [Google Scholar] [CrossRef]
- Qu, C.; Lu, S.; Liang, D.; Chen, S.; Xiang, Y.; Zhang, S. Simultaneous electro-oxidation and in situ electro-peroxone process for the degradation of refractory organics in wastewater. J. Hazard. Mater. 2019, 364, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Quan, X.; Cheng, Z.; Chen, B.; Zhu, X. Electrochemical oxidation of recalcitrant organic compounds in biologically treated municipal solid waste leachate in a flow reactor. J. Environ. Sci. 2013, 25, 2023–2030. [Google Scholar] [CrossRef]
- Nippatla, N.; Philip, L. Performance evaluation of a novel electrolytic reactor with rotating and non rotating bipolar disc electrodes for synthetic textile wastewater treatment. J. Environ. Chem. Eng. 2020, 8, 103462. [Google Scholar] [CrossRef]
- Wachter, N.; Bocchi, N.; Rocha-Filho, R.C. Use of a turbulence promoter in an electrochemical filter-press reactor: Consolidated evidence of significant enhancement of organics mass transport and degradation rates. Sep. Purif. Technol. 2021, 276, 119292. [Google Scholar] [CrossRef]
- Palma-Goyes, R.E.; Vazquez-Arenas, J.; Torres-Palma, R.A.; Ostos, C.; Ferraro, F.; González, I. The abatement of indigo carmine using active chlorine electrogenerated on ternary Sb2O5-doped Ti/RuO2-ZrO2 anodes in a filter-press FM01-LC reactor. Electrochim. Acta 2015, 174, 735–744. [Google Scholar] [CrossRef]
- Brocenschi, R.F.; Rocha-Filho, R.C.; Bocchi, N.; Biaggio, S.R. Electrochemical degradation of estrone using a boron-doped diamond anode in a filter-press reactor. Electrochim. Acta 2016, 197, 186–193. [Google Scholar] [CrossRef]
- Dória, A.R.; Pupo, M.; Santos, G.d.O.S.; Vilar, D.d.S.; Torres, N.H.; Romanholo Ferreira, L.F.; Cavalcanti, E.B.; Eguiluz, K.I.B.; Salazar-Banda, G.R. Electrochemical oxidation of indanthrene blue dye in a filter-press flow reactor and toxicity analyses with Raphidocelis subcapitata and Lactuca sativa. Ecotox. Environ. Safe. 2020, 198, 110659. [Google Scholar] [CrossRef]
- Körbahti, B.K.; Tanyolaç, A. Electrochemical treatment of simulated industrial paint wastewater in a continuous tubular reactor. Chem. Eng. J. 2009, 148, 444–451. [Google Scholar] [CrossRef]
- Skban Ibrahim, D.; Seethala Devi, P.; Veerababhu, C.; Balasubramanian, N. Treatment of Petroleum Effluent Using a Tubular Electrochemical Reactor. Petrol. Sci. Technol. 2014, 32, 1932–1939. [Google Scholar] [CrossRef]
- Li, T.; Li, X.; Chen, F.; Wang, J. Three-Dimensional CFD Simulation of the Tubular Electrochemical Reactor with Meshed Plate Electrodes. J. Electrochem. Soc. 2014, 161, E81–E86. [Google Scholar] [CrossRef]
- Ibrahim, D.S.; Veerabahu, C.; Palani, R.; Devi, S.; Balasubramanian, N. Flow dynamics and mass transfer studies in a tubular electrochemical reactor with a mesh electrode. Comput. Fluids 2013, 73, 97–103. [Google Scholar] [CrossRef]
- Wang, J.; Li, T.; Zhou, M.; Li, X.; Yu, J. Characterization of hydrodynamics and mass transfer in two types of tubular electrochemical reactors. Electrochim. Acta 2015, 173, 698–704. [Google Scholar] [CrossRef]
- Fan, T.-X.; Cai, Y.; Chu, G.-W.; Luo, Y.; Zhang, L.-L.; Chen, J.-F. A Novel Rotating Multielectrodes Reactor for Electrochemical Oxidation Process Intensification. Ind. Eng. Chem. Res. 2019, 58, 2396–2404. [Google Scholar] [CrossRef]
- Guo, X.; You, S. Characterization of hydrodynamics and electrochemical treatment of dye wastewater in two types of tubular electrochemical reactors. IOP Conf. Ser. Earth Environ. Sci. 2017, 81, 012008. [Google Scholar] [CrossRef] [Green Version]
- Mythilishri, R.; Kamalakannan, V.P.; Saravanathamizhan, R.; Balasubramanian, N. Kinetic and residence time distribution modeling of tubular electrochemical reactor: Analysis of results using Taguchi method. Water Pract. Technol. 2020, 16, 108–116. [Google Scholar] [CrossRef]
- Vijayakumar, V.; Saravanathamizhan, R.; Balasubramanian, N. Electro oxidation of dye effluent in a tubular electrochemical reactor using TiO2/RuO2 anode. J. Water Process Eng. 2016, 9, 155–160. [Google Scholar] [CrossRef]
- Li, D.; Tang, J.; Zhou, X.; Li, J.; Sun, X.; Shen, J.; Wang, L.; Han, W. Electrochemical degradation of pyridine by Ti/SnO2-Sb tubular porous electrode. Chemosphere 2016, 149, 49–56. [Google Scholar] [CrossRef]
- Xu, A.; Han, W.; Li, J.; Sun, X.; Shen, J.; Wang, L. Electrogeneration of hydrogen peroxide using Ti/IrO2-Ta2O5 anode in dual tubular membranes Electro-Fenton reactor for the degradation of tricyclazole without aeration. Chem. Eng. J. 2016, 295, 152–159. [Google Scholar] [CrossRef]
- Chaplin, B.P. Critical review of electrochemical advanced oxidation processes for water treatment applications. Environ. Sci.-Proc. Imp. 2014, 16, 1182–1203. [Google Scholar] [CrossRef]
- Reis, R.M.; Baio, J.A.F.; Migliorini, F.L.; Rocha, R.d.S.; Baldan, M.R.; Ferreira, N.G.; Lanza, M.R.d.V. Degradation of dipyrone in an electrochemical flow-by reactor using anodes of boron-doped diamond (BDD) supported on titanium. J. Electroanal. Chem. 2013, 690, 89–95. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Student Solutions Manual to Accompany Electrochemical Methods: Fundamentals and Applications, 2nd ed; Wiley: New York, NY, USA, 2002. [Google Scholar]
- Chen, M.; Zhao, X.; Wang, C.; Pan, S.; Zhang, C.; Wang, Y. Electrochemical oxidation of reverse osmosis concentrates using macroporous Ti-ENTA/SnO2-Sb flow-through anode: Degradation performance, energy efficiency and toxicity assessment. J. Hazard. Mater. 2021, 401, 123295. [Google Scholar] [CrossRef]
- Yang, Q.; Li, M.; Wang, J.; Huang, H. Synergistic electro-catalytic oxidation of ibuprofen in electro-peroxone system with flow-through carbon nanotube membrane cathode. Chem. Eng. J. 2022, 435, 135180. [Google Scholar] [CrossRef]
- Chen, M.; Wang, C.; Zhao, X.; Wang, Y.; Zhang, W.; Chen, Z.; Meng, X.; Luo, J.; Crittenden, J. Development of a highly efficient electrochemical flow-through anode based on inner in-site enhanced TiO2-nanotubes array. Environ. Int. 2020, 140, 105813. [Google Scholar] [CrossRef] [PubMed]
- Pérez, J.F.; Llanos, J.; Sáez, C.; López, C.; Cañizares, P.; Rodrigo, M.A. Development of an innovative approach for low-impact wastewater treatment: A microfluidic flow-through electrochemical reactor. Chem. Eng. J. 2018, 351, 766–772. [Google Scholar] [CrossRef]
- Zeng, W.; Liang, H.; Zhang, H.; Luo, X.; Lin, D.; Li, G. Efficient electrochemical oxidation of sulfamethoxazole by a novel reduced TiO2 nanotube arrays-based flow-through electrocatalytic membrane. Sep. Purif. Technol. 2022, 289, 120720. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, J.; Gao, Q.; Jiang, B.; Li, C.; Zhao, Q. Synthesis of low-cost Ti4O7 membrane electrode for electrooxidation of tetracycline under flow-through conditions: Performance, kinetics and mechanism. Process Saf. Environ. 2022, 159, 931–943. [Google Scholar] [CrossRef]
- Maldonado, V.Y.; Landis, G.M.; Ensch, M.; Becker, M.F.; Witt, S.E.; Rusinek, C.A. A flow-through cell for the electrochemical oxidation of perfluoroalkyl substances in landfill leachates. J. Water Process Eng. 2021, 43, 102210. [Google Scholar] [CrossRef]
- Ormeno-Cano, N.; Radjenovic, J. Electrochemical degradation of antibiotics using flow-through graphene sponge electrodes. J. Hazard. Mater. 2022, 431, 128462. [Google Scholar] [CrossRef]
- Kothari, M.S.; Aly Hassan, A.; Shah, K.A. Three-Dimensional Electrochemical Oxidation of Recalcitrant Dye Using Green Iron Microparticles. Water 2021, 13, 1925. [Google Scholar] [CrossRef]
- Ghanbarlou, H.; Pedersen, N.L.; Simonsen, M.E.; Muff, J. Nitrogen-Doped Graphene Iron-Based Particle Electrode Outperforms Activated Carbon in Three-Dimensional Electrochemical Water Treatment Systems. Water 2020, 12, 3121. [Google Scholar] [CrossRef]
- Yu, D.; Pei, Y.; Ji, Z.; He, X.; Yao, Z. A review on the landfill leachate treatment technologies and application prospects of three-dimensional electrode technology. Chemosphere 2022, 291, 132895. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Gao, M.; Shi, H.; Ni, J.; Xu, Y.; Wang, Q. Progress in research and development of particle electrodes for three-dimensional electrochemical treatment of wastewater: A review. Environ. Sci. Pollut. Res. 2021, 28, 47800–47824. [Google Scholar] [CrossRef]
- Yu, D.; Cui, J.; Li, X.; Zhang, H.; Pei, Y. Electrochemical treatment of organic pollutants in landfill leachate using a three-dimensional electrode system. Chemosphere 2020, 243, 125438. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, H.; Cheng, J.; Hu, C.; Yang, Z.; Wu, C. Three-dimensional particle electrode system treatment of organic wastewater: A general review based on patents. J. Clean. Prod. 2021, 308, 127324. [Google Scholar] [CrossRef]
- Kitaev, A.; Rubanovich, M. Apparatus and Method for Treatment of Wastewater. U.S. Patent 12/958,098 WO2009147666, 10 December 2009. [Google Scholar]
- Ni, J.; Shi, H.; Xu, Y.; Wang, Q. A Comparison of the Mechanism of TOC and COD Degradation in Rhodamine B Wastewater by a Recycling-Flow Two- and Three-dimensional Electro-Reactor System. Water 2020, 12, 1853. [Google Scholar] [CrossRef]
- Wei, L.; Guo, S.; Yan, G.; Chen, C.; Jiang, X. Electrochemical pretreatment of heavy oil refinery wastewater using a three-dimensional electrode reactor. Electrochim. Acta 2010, 55, 8615–8620. [Google Scholar] [CrossRef]
- Zhan, J.; Li, Z.; Yu, G.; Pan, X.; Wang, J.; Zhu, W.; Han, X.; Wang, Y. Enhanced treatment of pharmaceutical wastewater by combining three-dimensional electrochemical process with ozonation to in situ regenerate granular activated carbon particle electrodes. Sep. Purif. Technol. 2019, 208, 12–18. [Google Scholar] [CrossRef]
- Li, X.-Y.; Xu, J.; Cheng, J.-P.; Feng, L.; Shi, Y.-F.; Ji, J. TiO2-SiO2/GAC particles for enhanced electrocatalytic removal of acid orange 7 (AO7) dyeing wastewater in a three-dimensional electrochemical reactor. Sep. Purif. Technol. 2017, 187, 303–310. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, Q.; Xie, S.; Nong, C.; Hu, J.; Li, M. Optimization of the Electrochemical Treatment of 4-chlorophenol Wastewater Using Response Surface Method. Electrochemistry 2020, 88, 282–289. [Google Scholar] [CrossRef]
- Xiong, Y.; He, C.; Karlsson, H.T.; Zhu, X. Performance of three-phase three-dimensional electrode reactor for the reduction of COD in simulated wastewater-containing phenol. Chemosphere 2003, 50, 131–136. [Google Scholar] [CrossRef]
- Wu, X.; Yang, X.; Wu, D.; Fu, R. Feasibility study of using carbon aerogel as particle electrodes for decoloration of RBRX dye solution in a three-dimensional electrode reactor. Chem. Eng. J. 2008, 138, 47–54. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Z.-y.; Peng, P.; Xie, H.-b.; Li, X.-y.; Xu, J.; Li, W.-h. A pilot-scale three-dimensional electrochemical reactor combined with anaerobic-anoxic-oxic system for advanced treatment of coking wastewater. J. Environ. Manag. 2020, 258, 110021. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Ma, H.; Wang, B.; Wang, Y.; Chen, Y. Electrochemical treatment of petroleum refinery wastewater with three-dimensional multi-phase electrode. Desalination 2011, 276, 397–402. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Z.; Hou, Y.; Peng, Z.; Wang, L.; Gong, Z.; Zhu, J.; Su, D. Highly efficient Pd-Fe/Ni foam as heterogeneous Fenton catalysts for the three-dimensional electrode system. Catal. Commun. 2016, 86, 63–66. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.-T.; Chou, W.-L.; Kuo, Y.-M.; Chang, F.-L. Paired removal of color and COD from textile dyeing wastewater by simultaneous anodic and indirect cathodic oxidation. J. Hazard. Mater. 2009, 169, 16–22. [Google Scholar] [CrossRef]
- Mohammadi, H.; Bina, B.; Ebrahimi, A. A novel three-dimensional electro-Fenton system and its application for degradation of anti-inflammatory pharmaceuticals: Modeling and degradation pathways. Process Saf. Environ. 2018, 117, 200–213. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, V.; Singh, J.; Kumar, P. Electrokinetic assisted anaerobic digestion of spent mushroom substrate supplemented with sugar mill wastewater for enhanced biogas production. Renew. Energy 2021, 179, 418–426. [Google Scholar] [CrossRef]
- Otter, P.; Mette, K.; Wesch, R.; Gerhardt, T.; Krüger, F.-M.; Goldmaier, A.; Benz, F.; Malakar, P.; Grischek, T. Oxidation of Selected Trace Organic Compounds through the Combination of Inline Electro-Chlorination with UV Radiation (UV/ECl2) as Alternative AOP for Decentralized Drinking Water Treatment. Water 2020, 12, 3275. [Google Scholar] [CrossRef]
- Nunes, R.R.; Ribeiro, R.; Morão, G.M.; Rezende, M.O.O.; Moreira-Santos, M. Treatment of Wastewaters Containing Sulfonylurea Herbicides by Electroflotation: Chemical and Ecotoxicological Efficacy. Water 2022, 14, 2723. [Google Scholar] [CrossRef]




| Reactor Type | Anode | Cathode | Wastewater | Removal Rate | Ref. |
|---|---|---|---|---|---|
| filter-press reactor | Sb2O5-doped Ti/RuO2-ZrO2 | stainless steel | indigo carmine | 90% COD in 120 min | [45] |
| filter-press reactor | Ti–Pt/β-PbO2 BDD | stainless steel | Estrone | 35% COD in 60 min 98% COD in 30 min | [46] |
| filter-press reactor | BDD | stainless steel | indanthrene blue dye | 91% color in 180 min | [47] |
| plate-frame reactor | Ti/SnO2-Sb2O5 | GDE | landfill leachate | 80% TOC in 240 min | [41] |
| single-compartment filter-press reactor | BDD | stainless steel | paint wastewater | 90% COD in 90 min | [40] |
| plate-frame reactor with perforated liquid distributors | Ti/RuO2-IrO2 | stainless steel | solid waste leachate | 98% COD in 60 min | [42] |
| rotating bipolar disc plate-frame reactor | Fe | Fe | textile wastewater | 90% COD in 12 min | [43] |
| filter-press reactor with turbulence promoter | BDD | stainless-steel | BPS | 84% TOC in 265 min | [39] |
| Reactor Type | Anode | Cathode | Wastewater | Removal Rate | Ref. |
|---|---|---|---|---|---|
| traditional tubular reactor | Pt/Ti | carbon fibre | dyeing wastewater | 44.3% COD in 6 h | [48] |
| traditional tubular reactor | Ti/RuO2 | stainless steel | petroleum refinery wastewater | 85% COD in 24 min | [49] |
| tubular reactor in batch with recirculation mode | Ti/Ti0.7Ru0.3O2 | stainless steel | dye waste water | 75% of Acid red 87 | [55] |
| tubular reactor with mesh plate electrodes | TiO2/RuO2 | Ti | Evans blue dye | 80% COD in 100 min | [56] |
| tubular reactor with porous electrodes | Ti/SnO2-Sb | stainless steel | pyridine wastewater | 86% TOC in 180 min | [57] |
| dual tubular membranes reactor | Ti/IrO2–Ta2O5 | CB-PTFE modified graphite | tricyclazole | 79% COD in 20 min | [58] |
| tubular reactor based on spiral flow | Ti/Ti4O7 | stainless steel | methylene blue simulation wastewater | 50% TOC in 105 min | [54] |
| Reactor Type | Electrode Materials | Wastewater | Treatment Results | Ref. |
|---|---|---|---|---|
| 3D fluidized bed ECR in batch | Anode: stainless steel Cathode: stainless steel Particle electrode:GCA | Phenol | COD removal of 60% at an airflow of 5 min−1 and voltage of 30 V | [82] |
| 3D fixed bed ECR | Anode: graphite Cathode: stainless steel Particle electrode: CAs | dye wastewater | decolorization ratio was 98% (pH = 2.8, voltage = 20 V, treatment time = 30 min, air flow rate = 0.4 L min−1) | [83] |
| recycling- flow 3D-ECR | Anode: Ti/RuO2/TiO2 Cathode: Ti/RuO2/TiO2 Particle electrode: Columnar GAC | Rhodamine B wastewater | COD removal of 86.9% at HRT = 20 min, initial concentration = 100 min, volume = 500 mL, pH = 7, NaSO4 = 2 g/L and voltage = 5 V. | [77] |
| pilot-scale plate-frame fluidized bed ER | Anode: Ti/RuO2-IrO2 Cathode: Ti/RuO2-IrO2 Particle electrode: GAC | coking wastewater | removal 94.4% of COD and 76.2% of TN at a low EC of 0.22 kWh/kg COD and 4.69 kWh/kg TN. | [84] |
| 3D multi-phase ECR | Anode: graphite Cathode: graphite Particle electrode: Fe | petroleum refinery wastewater | COD removal of 92.8% at pH = 6.5, cell voltage = 12 V and fine Fe particle = 4 g and air flow rate = 1.5 L/min | [85] |
| 3D-ECR | Anode: DSA Cathode: Ti Particle electrode: Sn/Sb-Mn-GAC | 4-chlorophenol | 4-chlorophenol removal of 96.13% in 60 min at Na2SO4 concentration of 2 g·L−1, electrode distance of 2 cm, current intensity of 2 A, and particle dosage of 14 g. | [81] |
| 3D-ECR | Anode: Ti/RuO2-IrO2 Cathode: stainless steel Particle electrode: TiO2-SiO2/GAC | dye wastewater | decolorization rate and COD removal efficiency are 83.20% and 48.95% at electric field intensity = 1 V/cm, TiO2-SiO2/GAC = 200 g/L, Na2SO4 = 0.07 mol/L, pH = 3 in 120 min. | [80] |
| 3D-ECR | Anode: Pt Cathode: Pt Particle electrode:Pd-Fe/Ni | Dimetridazole | The dimetridazole removal of 96.5 at current density = 31 mA/cm2, C0 = 50 mg/L, pH = 3, air flow rate = 1.0 L/min | [86] |
| 3D batch type ECR | Anode: Ti/Sb0.1Sn0.9O2 Cathode: stainless steel Particle electrode: GAC and ceramsite particle (PCP) | oil refinery wastewater | removal of COD, TOC and toxicity units were 45.5%, 43.3% and 67.2% (GAC percentage = 75%, current density = 30 mA/cm2, pH not adjusted and treatment time = 100 min) | [78] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Ren, N.; Qu, C.; Lu, S.; Xiang, Y.; Liang, D. Recent Advances in the Reactor Design for Industrial Wastewater Treatment by Electro-Oxidation Process. Water 2022, 14, 3711. https://doi.org/10.3390/w14223711
Liu J, Ren N, Qu C, Lu S, Xiang Y, Liang D. Recent Advances in the Reactor Design for Industrial Wastewater Treatment by Electro-Oxidation Process. Water. 2022; 14(22):3711. https://doi.org/10.3390/w14223711
Chicago/Turabian StyleLiu, Jun, Na Ren, Chao Qu, Shanfu Lu, Yan Xiang, and Dawei Liang. 2022. "Recent Advances in the Reactor Design for Industrial Wastewater Treatment by Electro-Oxidation Process" Water 14, no. 22: 3711. https://doi.org/10.3390/w14223711
APA StyleLiu, J., Ren, N., Qu, C., Lu, S., Xiang, Y., & Liang, D. (2022). Recent Advances in the Reactor Design for Industrial Wastewater Treatment by Electro-Oxidation Process. Water, 14(22), 3711. https://doi.org/10.3390/w14223711






