Heterogenization of Molecular Water Oxidation Catalysts in Electrodes for (Photo)Electrochemical Water Oxidation
Abstract
1. Introduction
1.1. Energy Challenge, Natural and Artificial Photosynthesis
1.2. Water Oxidation Reaction
1.3. (Photo)Electrocatalysts for WO: Homogeneous vs. Heterogeneous and Heterogenization of Molecular Complexes
2. Approaches for the Development of WO (Photo)Anodes
- −
- Covalent anchoring,
- −
- Supramolecular interactions,
- −
- Drop-casting,
- −
- Physical confinement into porous materials such as metal organic frameworks (MOFs) or covalent organic frameworks (COFs),
- −
- Electropolymerization,
- −
- Atomic layer deposition (ALD), and
- −
- Layer-by-layer deposition.
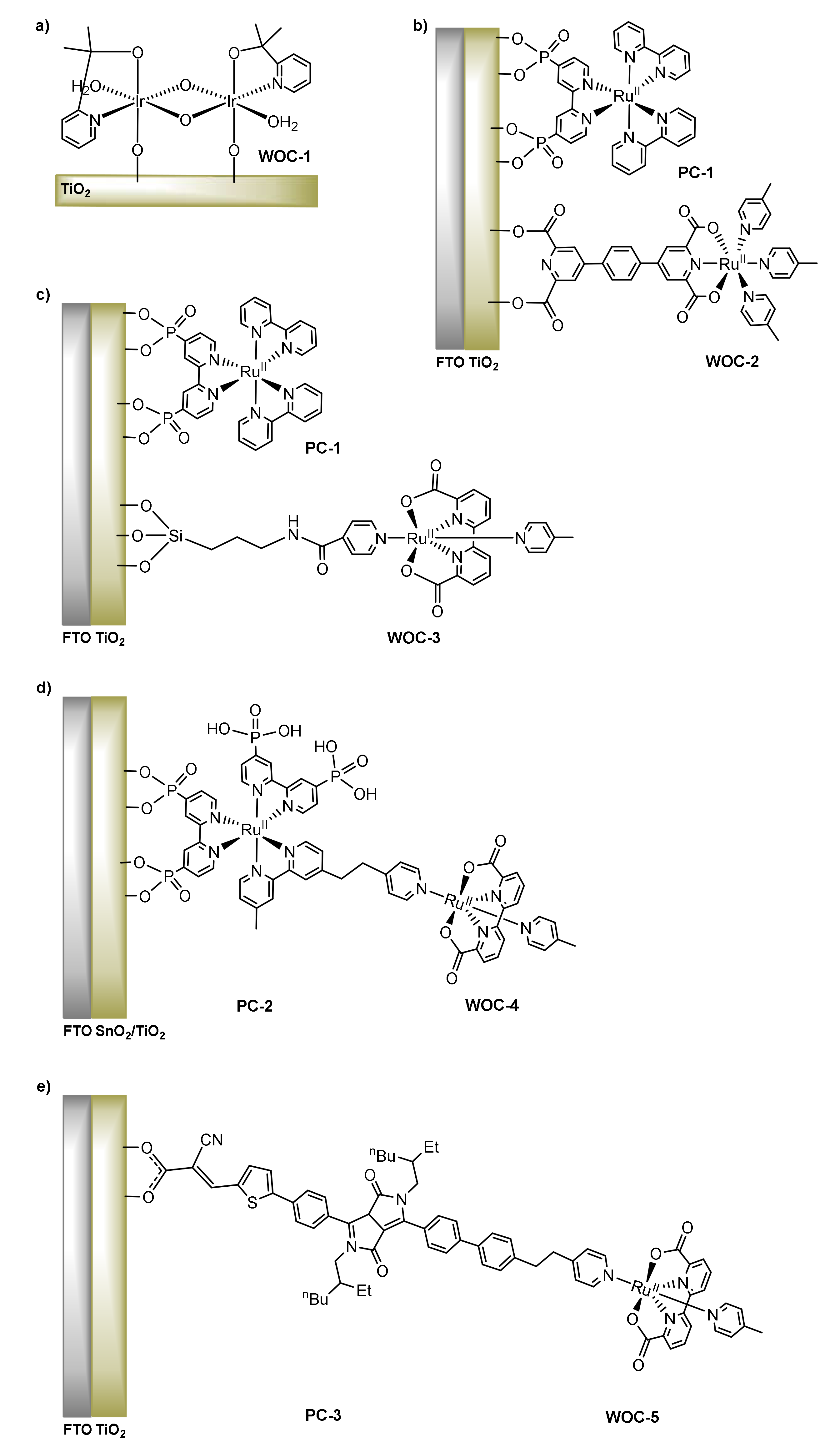
2.1. Covalent Immobilization
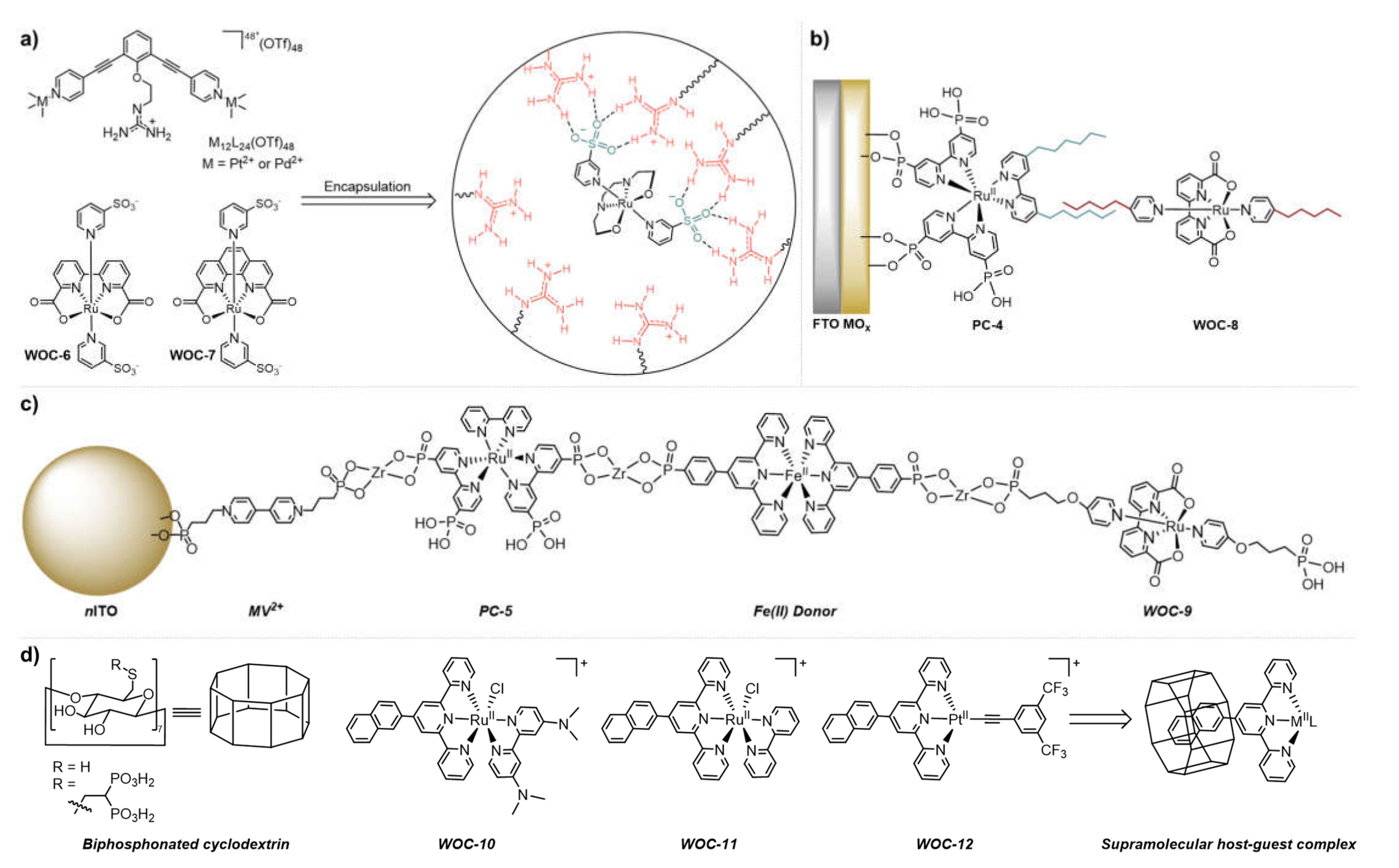
2.2. Supramolecular Host–Guest Interactions
2.3. Drop Casting
2.4. Immobilization via Physical Confinement
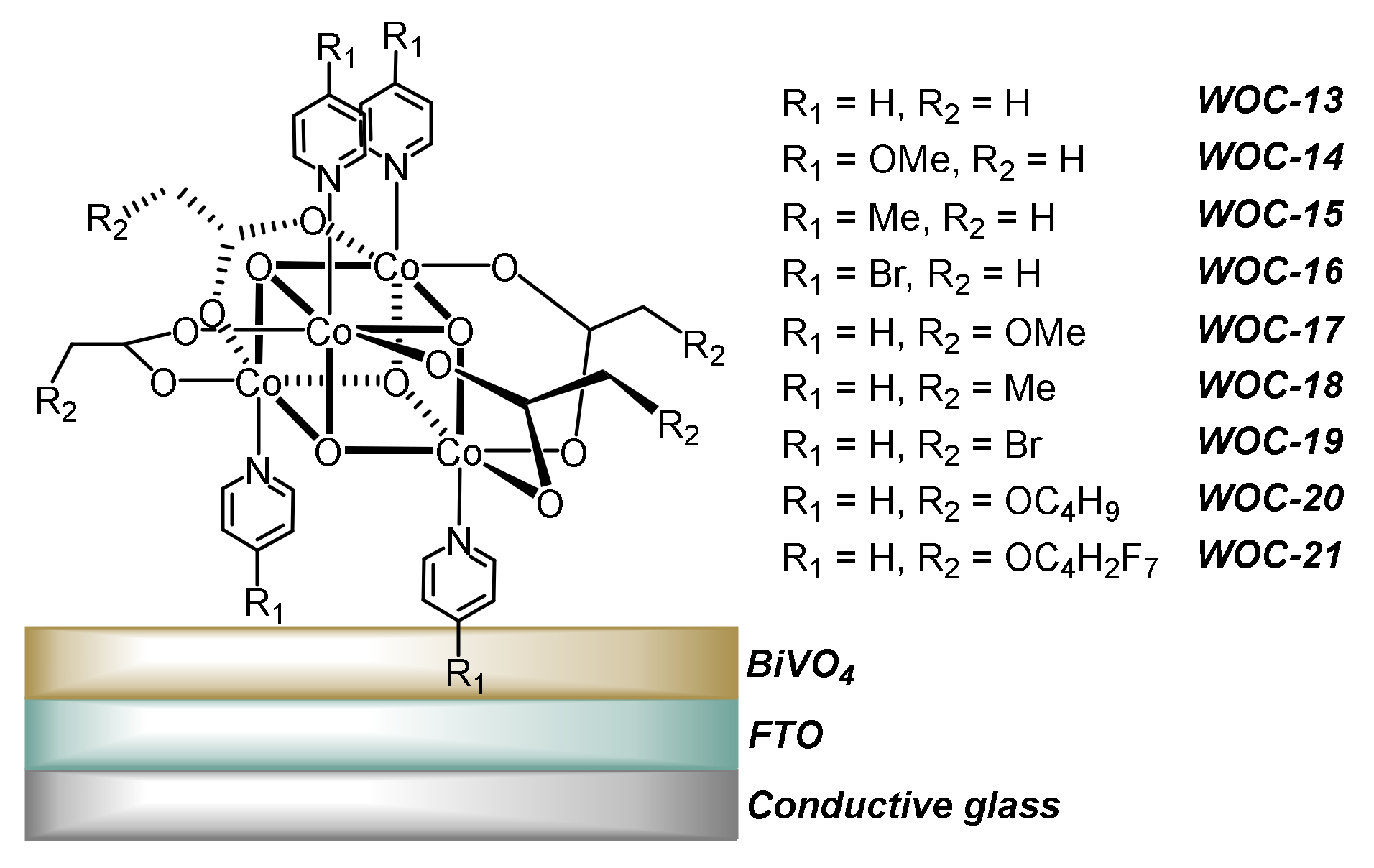
2.5. Electrografting/Electropolymerization
2.6. Atomic Layer Deposition
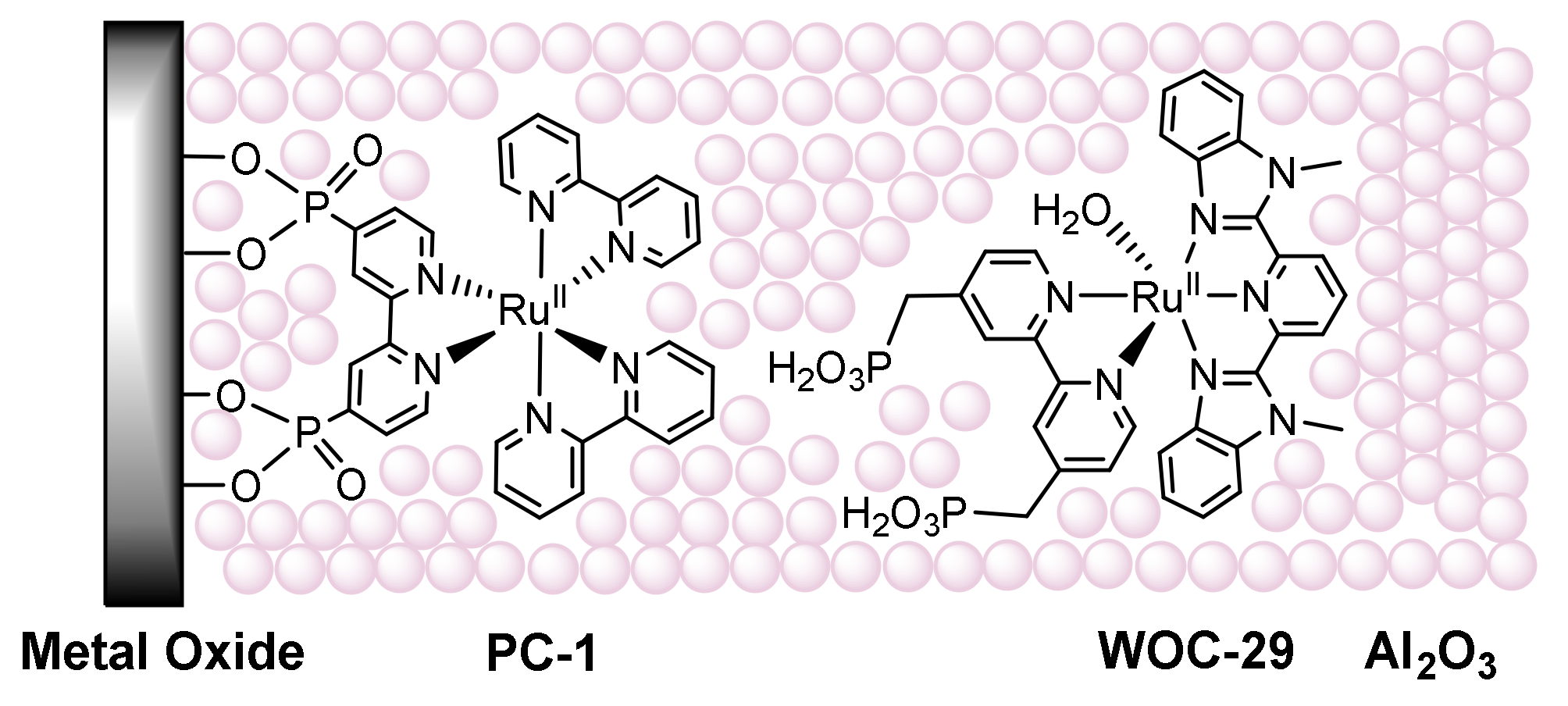
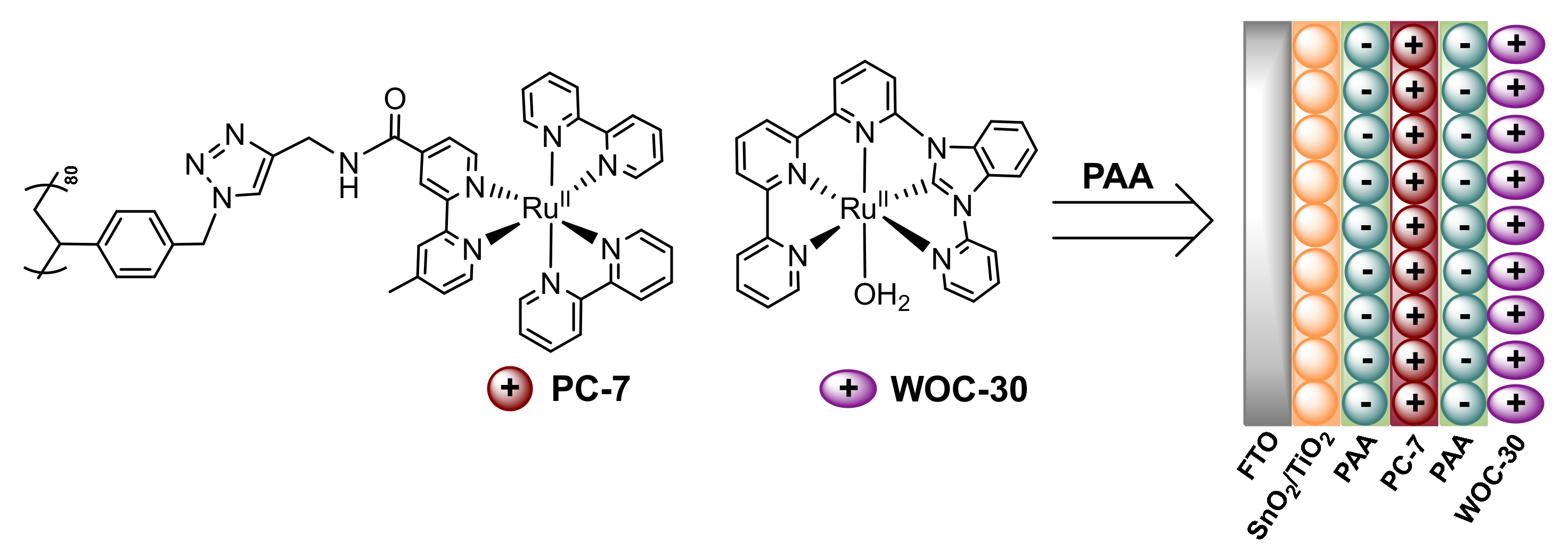
2.7. Layer-by-Layer Assembly: Electrostatic Self-Assembly Non-Covalent Immobilization
3. Selected Examples of WO (Photo)Anodes
3.1. Covalent Anchoring of Molecular WOCs onto Electrodes
3.2. Supramolecular Assembly of Molecular WOCs onto Electrodes
3.3. Integration of Molecular WOCs onto Electrode Surfaces by Drop-Casting
3.4. Utilization of Physical Confinement Strategies for the Generation of WO (Photo)Anodes
3.5. Heterogenization of WOCs by Electropolymerization
3.6. Use of Atomic Layer Deposition for the Fabrication of WO (Photo)Anodes
3.7. Use of Layer-by-Layer Deposition to Deposit WOCs onto Electrodes
4. Conclusions and Perspective
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masson-Delmotte, V.; Zhai, P.; Pörtner, H.-O.; Roberts, D.; Skea, J.; Shukla, P.R.; Pirani, A.; Moufouma-Okia, W.; Péan, C.; Pidcock, R.S.; et al. Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; IPCC special report on the impacts of global warming; IPCC: Geneva, Switzerland, 2018. [Google Scholar]
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P. Primary Production of the Biosphere: Integrating Terrestrial and Oceanic Components. Science 1998, 281, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Lewis, N.S. Research opportunities to advance solar energy utilization. Science 2016, 351, aad1920. [Google Scholar] [CrossRef]
- Schultz, D.M.; Yoon, T.P. Solar Synthesis: Prospects in Visible Light Photocatalysis. Science 2014, 343, 1239176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Sun, L. Artificial photosynthesis: Opportunities and challenges of molecular catalysts. Chem. Soc. Rev. 2019, 48, 2216–2264. [Google Scholar] [CrossRef] [PubMed]
- Cox, N.; Pantazis, D.A.; Lubitz, W. Current Understanding of the Mechanism of Water Oxidation in Photosystem II and Its Relation to XFEL Data. Annu. Rev. Biochem. 2020, 89, 795–820. [Google Scholar] [CrossRef] [PubMed]
- Crowe, S.A.; Døssing, L.N.; Beukes, N.J.; Bau, M.; Kruger, S.J.; Frei, R.; Canfield, D.E. Atmospheric oxygenation three billion years ago. Nature 2013, 501, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Amunts, A.; Drory, O.; Nelson, N. The structure of a plant photosystem I supercomplex at 3.4 Å resolution. Nature 2007, 447, 58–63. [Google Scholar] [CrossRef]
- Umena, Y.; Kawakami, K.; Shen, J.R.; Kamiya, N. Crystal structure of oxygen–evolving photosystem II at a resolution of 1.9 Å. Nature 2011, 473, 55–60. [Google Scholar] [CrossRef]
- Kornienko, N.; Zhang, J.Z.; Sakimoto, K.K.; Yang, P.; Reisner, E. Interfacing nature’s catalytic machinery with synthetic materials for semi–artificial photosynthesis. Nat. Nanotechnol. 2018, 13, 890–899. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Sokol, K.P.; Paul, N.; Romero, E.; van Grondelle, R.; Reisner, E. Competing charge transfer pathways at the photosystem II–electrode interface. Nat. Chem. Biol. 2016, 12, 1046. [Google Scholar] [CrossRef]
- Schwizer, F.; Okamoto, Y.; Heinisch, T.; Gu, Y.; Pellizzoni, M.M.; Lebrun, V.; Reuter, R.; Köhler, V.; Lewis, J.C.; Ward, T.R. Artificial Metalloenzymes: Reaction Scope and Optimization Strategies. Chem. Rev. 2018, 118, 142–231. [Google Scholar] [CrossRef]
- Bonke, S.A.; Wiechen, M.; MacFarlane, D.R.; Spiccia, L. Renewable fuels from concentrated solar power: Towards practical artificial photosynthesis. Energy Environ. Sci. 2015, 8, 2791–2796. [Google Scholar] [CrossRef]
- Michel, H. Editorial: The Nonsense of Biofuels. Angew. Chem. Int. Ed. 2012, 51, 2516–2518. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Du, M.; Ma, Y.; Zhu, Q.; Xing, M.; Zhang, J. Integration of redox cocatalysts for artificial photosynthesis. Energy Environ. Sci. 2021, 14, 5260–5288. [Google Scholar] [CrossRef]
- Li, F.; Yang, H.; Li, W.; Sun, L. Device Fabrication for Water Oxidation, Hydrogen Generation, and CO2 Reduction via Molecular Engineering. Joule 2018, 2, 36–60. [Google Scholar] [CrossRef]
- Villa, K.; Galán–Mascarós, J.R.; López, N.; Palomares, E. Photocatalytic water splitting: Advantages and challenges. Sustain. Energy Fuels 2021, 5, 4560–4569. [Google Scholar] [CrossRef]
- Li, Y.; Du, X.; Huang, J.; Wu, C.; Sun, Y.; Zou, G.; Yang, C.; Xiong, J. Recent Progress on Surface Reconstruction of Earth–Abundant Electrocatalysts for Water Oxidation. Small 2019, 15, 1901980. [Google Scholar] [CrossRef]
- Casadevall, C.; Bucci, A.; Costas, M.; Lloret–Fillol, J. Chapter Four—Water oxidation catalysis with well–defined molecular iron complexes. In Advances in Inorganic Chemistry; Eldik, R., Hubbard, C.D., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 74, pp. 151–196. [Google Scholar]
- Kato, N.; Mizuno, S.; Shiozawa, M.; Nojiri, N.; Kawai, Y.; Fukumoto, K.; Morikawa, T.; Takeda, Y. A large–sized cell for solar–driven CO2 conversion with a solar-to-formate conversion efficiency of 7.2%. Joule 2021, 5, 687–705. [Google Scholar] [CrossRef]
- Qiao, J.; Liu, Y.; Hong, F.; Zhang, J. A review of catalysts for the electroreduction of carbon dioxide to produce low–carbon fuels. Chem. Soc. Rev. 2014, 43, 631–675. [Google Scholar] [CrossRef]
- Franco, F.; Rettenmaier, C.; Jeon, H.S.; Roldan Cuenya, B. Transition metal–based catalysts for the electrochemical CO2 reduction: From atoms and molecules to nanostructured materials. Chem. Soc. Rev. 2020, 49, 6884–6946. [Google Scholar] [CrossRef]
- Franco, F.; Fernández, S.; Lloret–Fillol, J. Advances in the electrochemical catalytic reduction of CO2 with metal complexes. Curr. Opin. Electrochem. 2019, 15, 109–117. [Google Scholar] [CrossRef]
- Kim, J.H.; Hansora, D.; Sharma, P.; Jang, J.–W.; Lee, J.S. Toward practical solar hydrogen production—an artificial photosynthetic leaf–to–farm challenge. Chem. Soc. Rev. 2019, 48, 1908–1971. [Google Scholar] [CrossRef] [PubMed]
- Jessop, P.G.; Ikariya, T.; Noyori, R. Homogeneous Hydrogenation of Carbon Dioxide. Chem. Rev. 1995, 95, 259–272. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, L.; Jackstell, R.; Beller, M. Using carbon dioxide as a building block in organic synthesis. Nat. Commun. 2015, 6, 5933. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S. Opportunities and prospects in the chemical recycling of carbon dioxide to fuels. Catal. Today 2009, 148, 191–205. [Google Scholar] [CrossRef]
- Garrido-Barros, P.; Matheu, R.; Gimbert-Suriñach, C.; Llobet, A. Electronic, mechanistic, and structural factors that influence the performance of molecular water oxidation catalysts anchored on electrode surfaces. Curr. Opin. Electrochem. 2019, 15, 140–147. [Google Scholar] [CrossRef]
- Godwin, I.; Rovetta, A.; Lyons, M.; Coleman, J. Electrochemical water oxidation: The next five years. Curr. Opin. Electrochem. 2018, 7, 31–35. [Google Scholar] [CrossRef]
- Bairagya, M.D.; Bujol, R.J.; Elgrishi, N. Fighting Deactivation: Classical and Emerging Strategies for Efficient Stabilization of Molecular Electrocatalysts. Chem. A Eur. J. 2020, 26, 3991–4000. [Google Scholar] [CrossRef]
- Bullock, R.M.; Das, A.K.; Appel, A.M. Surface Immobilization of Molecular Electrocatalysts for Energy Conversion. Chem. A Eur. J. 2017, 23, 7626–7641. [Google Scholar] [CrossRef]
- Lubitz, W.; Chrysina, M.; Cox, N. Water oxidation in photosystem II. Photosynth. Res. 2019, 142, 105–125. [Google Scholar] [CrossRef]
- Cox, N.; Pantazis, D.A.; Neese, F.; Lubitz, W. Biological Water Oxidation. Acc. Chem. Res. 2013, 46, 1588–1596. [Google Scholar] [CrossRef]
- Blakemore, J.D.; Crabtree, R.H.; Brudvig, G.W. Molecular Catalysts for Water Oxidation. Chem. Rev. 2015, 115, 12974–13005. [Google Scholar] [CrossRef] [PubMed]
- Kärkäs, M.D.; Verho, O.; Johnston, E.V.; Åkermark, B. Artificial Photosynthesis: Molecular Systems for Catalytic Water Oxidation. Chem. Rev. 2014, 114, 11863–12001. [Google Scholar] [CrossRef] [PubMed]
- Fukuzumi, S.; Kojima, T.; Lee, Y.-M.; Nam, W. High-valent metal-oxo complexes generated in catalytic oxidation reactions using water as an oxygen source. Coord. Chem. Rev. 2017, 333, 44–56. [Google Scholar] [CrossRef]
- Casadevall, C.; Codolà, Z.; Costas, M.; Lloret-Fillol, J. Spectroscopic, Electrochemical and Computational Characterisation of Ru Species Involved in Catalytic Water Oxidation: Evidence for a [RuV(O)(Py2Metacn)] Intermediate. Chem. A Eur. J. 2016, 22, 10111–10126. [Google Scholar] [CrossRef]
- Lloret–Fillol, J.; Costas, M. Chapter One—Water oxidation at base metal molecular catalysts. In Advances in Organometallic Chemistry; Pérez, P.J., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 71, pp. 1–52. [Google Scholar]
- Meyer, T.J.; Sheridan, M.V.; Sherman, B.D. Mechanisms of molecular water oxidation in solution and on oxide surfaces. Chem. Soc. Rev. 2017, 46, 6148–6169. [Google Scholar] [CrossRef]
- Casadevall, C.; Martin-Diaconescu, V.; Browne, W.R.; Fernández, S.; Franco, F.; Cabello, N.; Benet-Buchholz, J.; Lassalle-Kaiser, B.; Lloret-Fillol, J. Isolation of a Ru(iv) side–on peroxo intermediate in the water oxidation reaction. Nat. Chem. 2021, 13, 800–804. [Google Scholar] [CrossRef]
- Codolà, Z.; Gamba, I.; Acuña-Parés, F.; Casadevall, C.; Clémancey, M.; Latour, J.-M.; Luis, J.M.; Lloret-Fillol, J.; Costas, M. Design of Iron Coordination Complexes as Highly Active Homogenous Water Oxidation Catalysts by Deuteration of Oxidation-Sensitive Sites. J. Am. Chem. Soc. 2019, 141, 323–333. [Google Scholar] [CrossRef]
- Rüdiger, O.; Levin, N.; Casadevall, C.; Cutsail, G.E.; Lloret-Fillol, J.; DeBeer, S. XAS and EPR in situ observation of Ru(V) oxo intermediate in a Ru water oxidation complex. ChemElectroChem 2021, e202101271. [Google Scholar] [CrossRef]
- Hessels, J.; Detz, R.J.; Koper, M.T.M.; Reek, J.N.H. Rational Design Rules for Molecular Water Oxidation Catalysts based on Scaling Relationships. Chem. A Eur. J. 2017, 23, 16413–16418. [Google Scholar] [CrossRef]
- Duan, L.; Bozoglian, F.; Mandal, S.; Stewart, B.; Privalov, T.; Llobet, A.; Sun, L. A molecular ruthenium catalyst with water–oxidation activity comparable to that of photosystem II. Nat. Chem. 2012, 4, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Wang, L.; Li, F.; Li, F.; Sun, L. Highly Efficient Bioinspired Molecular Ru Water Oxidation Catalysts with Negatively Charged Backbone Ligands. Acc. Chem. Res. 2015, 48, 2084–2096. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Duan, L.; Ambre, R.B.; Daniel, Q.; Chen, H.; Sun, J.; Das, B.; Thapper, A.; Uhlig, J.; Dinér, P.; et al. A nickel (II) PY5 complex as an electrocatalyst for water oxidation. J. Catal. 2016, 335, 72–78. [Google Scholar] [CrossRef]
- Geletii, Y.V.; Botar, B.; Kögerler, P.; Hillesheim, D.A.; Musaev, D.G.; Hill, C.L. An All-Inorganic, Stable, and Highly Active Tetraruthenium Homogeneous Catalyst for Water Oxidation. Angew. Chem. Int. Ed. 2008, 47, 3896–3899. [Google Scholar] [CrossRef] [PubMed]
- Azmani, K.; Besora, M.; Soriano-López, J.; Landolsi, M.; Teillout, A.-L.; de Oliveira, P.; Mbomekallé, I.-M.; Poblet, J.M.; Galán-Mascarós, J.-R. Understanding polyoxometalates as water oxidation catalysts through iron vs. cobalt reactivity. Chem. Sci. 2021, 12, 8755–8766. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Geletii, Y.V.; Zhao, C.; Vickers, J.W.; Zhu, G.; Luo, Z.; Song, J.; Lian, T.; Musaev, D.G.; Hill, C.L. Polyoxometalate water oxidation catalysts and the production of green fuel. Chem. Soc. Rev. 2012, 41, 7572–7589. [Google Scholar] [CrossRef]
- Li, P.; Zhao, R.; Chen, H.; Wang, H.; Wei, P.; Huang, H.; Liu, Q.; Li, T.; Shi, X.; Zhang, Y.; et al. Recent Advances in the Development of Water Oxidation Electrocatalysts at Mild pH. Small 2019, 15, 1805103. [Google Scholar] [CrossRef]
- Bo, X.; Dastafkan, K.; Zhao, C. Design of Multi-Metallic-Based Electrocatalysts for Enhanced Water Oxidation. Chemphyschem 2019, 20, 2936–2945. [Google Scholar] [CrossRef]
- Lei, Z.; Wang, T.; Zhao, B.; Cai, W.; Liu, Y.; Jiao, S.; Li, Q.; Cao, R.; Liu, M. Recent Progress in Electrocatalysts for Acidic Water Oxidation. Adv. Energy Mater. 2020, 10, 2000478. [Google Scholar] [CrossRef]
- Song, N.; Concepcion, J.J.; Binstead, R.A.; Rudd, J.; Vannucci, A.K.; Dares, C.J.; Coggins, M.K.; Meyer, T.J. Base-enhanced catalytic water oxidation by a carboxylate-bipyridine Ru(II) complex. Proc. Natl. Acad. Sci. USA 2015, 112, 4935–4940. [Google Scholar] [CrossRef]
- Matheu, R.; Garrido-Barros, P.; Gil-Sepulcre, M.; Ertem, M.Z.; Sala, X.; Gimbert-Suriñach, C.; Llobet, A. The development of molecular water oxidation catalysts. Nat. Rev. Chem. 2019, 3, 331–341. [Google Scholar] [CrossRef]
- Bae, S.; Jang, J.-E.; Lee, H.-W.; Ryu, J. Tailored Assembly of Molecular Water Oxidation Catalysts on Photoelectrodes for Artificial Photosynthesis. Eur. J. Inorg. Chem. 2019, 2019, 2040–2057. [Google Scholar] [CrossRef]
- Zhang, H.; Tian, W.; Duan, X.; Sun, H.; Liu, S.; Wang, S. Catalysis of a Single Transition Metal Site for Water Oxidation: From Mononuclear Molecules to Single Atoms. Adv. Mater. 2020, 32, 1904037. [Google Scholar] [CrossRef]
- Lyons, M.E.G.; Doyle, R.L.; Browne, M.P.; Godwin, I.J.; Rovetta, A.A.S. Recent developments in electrochemical water oxidation. Curr. Opin. Electrochem. 2017, 1, 40–45. [Google Scholar] [CrossRef]
- Lee, K.J.; McCarthy, B.D.; Dempsey, J.L. On decomposition, degradation, and voltammetric deviation: The electrochemist’s field guide to identifying precatalyst transformation. Chem. Soc. Rev. 2019, 48, 2927–2945. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Elgrishi, N.; Kandemir, B.; Dempsey, J.L. Electrochemical and spectroscopic methods for evaluating molecular electrocatalysts. Nat. Rev. Chem. 2017, 1, 0039. [Google Scholar] [CrossRef]
- Zahran, Z.N.; Tsubonouchi, Y.; Mohamed, E.A.; Yagi, M. Recent Advances in the Development of Molecular Catalyst-Based Anodes for Water Oxidation toward Artificial Photosynthesis. ChemSusChem 2019, 12, 1775–1793. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Wu, X.; Sun, L. Copper-based homogeneous and heterogeneous catalysts for electrochemical water oxidation. Nanoscale 2020, 12, 4187–4218. [Google Scholar] [CrossRef] [PubMed]
- Materna, K.L.; Crabtree, R.H.; Brudvig, G.W. Anchoring groups for photocatalytic water oxidation on metal oxide surfaces. Chem. Soc. Rev. 2017, 46, 6099–6110. [Google Scholar] [CrossRef]
- Zhang, L.; Cole, J.M. Anchoring Groups for Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 3427–3455. [Google Scholar] [CrossRef]
- Luitel, T.; Zamborini, F.P. Covalent Modification of Photoanodes for Stable Dye–Sensitized Solar Cells. Langmuir 2013, 29, 13582–13594. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Savini, A.; Bucci, A.; Nocchetti, M.; Vivani, R.; Idriss, H.; Macchioni, A. Activity and Recyclability of an Iridium-EDTA Water Oxidation Catalyst Immobilized onto Rutile TiO2. ACS Catal. 2015, 5, 264–271. [Google Scholar] [CrossRef]
- Hara, K.; Sugihara, H.; Tachibana, Y.; Islam, A.; Yanagida, M.; Sayama, K.; Arakawa, H.; Fujihashi, G.; Horiguchi, A.T.; Kinoshita, T. Dye-Sensitized Nanocrystalline TiO2 Solar Cells Based on Ruthenium(II) Phenanthroline Complex Photosensitizers. Langmuir 2001, 17, 5992–5999. [Google Scholar] [CrossRef]
- Hanson, K.; Brennaman, M.K.; Luo, H.; Glasson, C.R.K.; Concepcion, J.J.; Song, W.; Meyer, T.J. Photostability of Phosphonate–Derivatized, RuII Polypyridyl Complexes on Metal Oxide Surfaces. ACS Appl. Mater. Interfaces 2012, 4, 1462–1469. [Google Scholar] [CrossRef] [PubMed]
- Hanson, K.; Brennaman, M.K.; Ito, A.; Luo, H.; Song, W.; Parker, K.A.; Ghosh, R.; Norris, M.R.; Glasson, C.R.K.; Concepcion, J.J.; et al. Structure–Property Relationships in Phosphonate-Derivatized, RuII Polypyridyl Dyes on Metal Oxide Surfaces in an Aqueous Environment. J. Phys. Chem. C 2012, 116, 14837–14847. [Google Scholar] [CrossRef]
- Zhong, D.K.; Zhao, S.; Polyansky, D.E.; Fujita, E. Diminished photoisomerization of active ruthenium water oxidation catalyst by anchoring to metal oxide electrodes. J. Catal. 2013, 307, 140–147. [Google Scholar] [CrossRef]
- Zhang, L.; Cole, J.M.; Dai, C. Variation in Optoelectronic Properties of Azo Dye-Sensitized TiO2 Semiconductor Interfaces with Different Adsorption Anchors: Carboxylate, Sulfonate, Hydroxyl and Pyridyl Groups. ACS Appl. Mater. Interfaces 2014, 6, 7535–7546. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Li, C.; Zeng, Z.-H.; Wang, W.-B.; Wang, X.-S.; Zhang, B.-W. Efficient electron injection due to a special adsorbing group’s combination of carboxyl and hydroxyl: Dye-sensitized solar cells based on new hemicyanine dyes. J. Mater. Chem. 2005, 15, 1654–1661. [Google Scholar] [CrossRef]
- Wang, D.; Sampaio, R.N.; Troian-Gautier, L.; Marquard, S.L.; Farnum, B.H.; Sherman, B.D.; Sheridan, M.V.; Dares, C.J.; Meyer, G.J.; Meyer, T.J. Molecular Photoelectrode for Water Oxidation Inspired by Photosystem II. J. Am. Chem. Soc. 2019, 141, 7926–7933. [Google Scholar] [CrossRef]
- Antón-García, D.; Warnan, J.; Reisner, E. A diketopyrrolopyrrole dye-based dyad on a porous TiO2 photoanode for solar-driven water oxidation. Chem. Sci. 2020, 11, 12769–12776. [Google Scholar] [CrossRef]
- Wang, D.; Farnum, B.H.; Dares, C.J.; Meyer, T.J. Chemical approaches to artificial photosynthesis: A molecular, dye–sensitized photoanode for O2 production prepared by layer-by-layer self-assembly. J. Chem. Phys. 2020, 152, 244706. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Marquard, S.L.; Troian-Gautier, L.; Sheridan, M.V.; Sherman, B.D.; Wang, Y.; Eberhart, M.S.; Farnum, B.H.; Dares, C.J.; Meyer, T.J. Interfacial Deposition of Ru(II) Bipyridine-Dicarboxylate Complexes by Ligand Substitution for Applications in Water Oxidation Catalysis. J. Am. Chem. Soc. 2018, 140, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Li, F.; Wang, L.; Daniel, Q.; Gabrielsson, E.; Sun, L. Pt-free tandem molecular photoelectrochemical cells for water splitting driven by visible light. Phys. Chem. Chem. Phys. 2014, 16, 25234–25240. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.G.; Schauer, P.A.; Borau-Garcia, J.; Fancy, B.R.; Berlinguette, C.P. Stabilization of ruthenium sensitizers to TiO2 surfaces through cooperative anchoring groups. J. Am. Chem Soc. 2013, 135, 1692–1695. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Li, F.; Wang, L.; Daniel, Q.; Chen, H.; Gabrielsson, E.; Sun, J.; Sun, L. Immobilization of a Molecular Ruthenium Catalyst on Hematite Nanorod Arrays for Water Oxidation with Stable Photocurrent. ChemSusChem 2015, 8, 3242–3247. [Google Scholar] [CrossRef]
- Klepser, B.M.; Bartlett, B.M. Anchoring a Molecular Iron Catalyst to Solar-Responsive WO3 Improves the Rate and Selectivity of Photoelectrochemical Water Oxidation. J. Am. Chem. Soc. 2014, 136, 1694–1697. [Google Scholar] [CrossRef]
- Ohshita, J.; Adachi, Y.; Tanaka, D.; Nakashima, M.; Ooyama, Y. Synthesis of D-A polymers with a disilanobithiophene donor and a pyridine or pyrazine acceptor and their applications to dye-sensitized solar cells. RSC Adv. 2015, 5, 36673–36679. [Google Scholar] [CrossRef]
- Casarin, L.; Swords, W.B.; Caramori, S.; Bignozzi, C.A.; Meyer, G.J. Rapid Static Sensitizer Regeneration Enabled by Ion Pairing. Inorg. Chem. 2017, 56, 7324–7327. [Google Scholar] [CrossRef]
- Das, B.; Thapper, A.; Ott, S.; Colbran, S.B. Structural features of molecular electrocatalysts in multi-electron redox processes for renewable energy-recent advances. Sustain. Energy Fuels 2019, 3, 2159–2175. [Google Scholar] [CrossRef]
- Cho, I.; Koshika, M.; Wagner, P.; Koumura, N.; Innis, P.C.; Mori, S.; Mozer, A.J. Exploiting Intermolecular Interactions between Alkyl-Functionalized Redox-Active Molecule Pairs to Enhance Interfacial Electron Transfer. J. Am. Chem. Soc. 2018, 140, 13935–13944. [Google Scholar] [CrossRef]
- Chai, Q.; Li, W.; Wu, Y.; Pei, K.; Liu, J.; Geng, Z.; Tian, H.; Zhu, W. Effect of a Long Alkyl Group on Cyclopentadithiophene as a Conjugated Bridge for D-A-π-A Organic Sensitizers: IPCE, Electron Diffusion Length, and Charge Recombination. ACS Appl. Mater. Interfaces 2014, 6, 14621–14630. [Google Scholar] [CrossRef]
- Brennan, B.J.; Keirstead, A.E.; Liddell, P.A.; Vail, S.A.; Moore, T.A.; Moore, A.L.; Gust, D. 1-(3'-Amino)propylsilatrane derivatives as covalent surface linkers to nanoparticulate metal oxide films for use in photoelectrochemical cells. Nanotechnology 2009, 20, 505203. [Google Scholar] [CrossRef] [PubMed]
- Cecconi, B.; Mordini, A.; Reginato, G.; Zani, L.; Taddei, M.; de Biani, F.F.; De Angelis, F.; Marotta, G.; Salvatori, P.; Calamante, M. Pyridine-N-Oxide 2-Carboxylic Acid: An Acceptor Group for Organic Sensitizers with Enhanced Anchoring Stability in Dye–Sensitized Solar Cells. Asian J. Org. Chem. 2014, 3, 140–152. [Google Scholar] [CrossRef]
- Behar, D.; Frei, H.; Macnaughtan, M.; Rabani, J. Determination of the Redox Potential of Immobilized Oxo–Bridged Metals in Porous Supports. The Ti-O-Mn-SBA System. J. Phys. Chem. C 2012, 116, 23477–23484. [Google Scholar] [CrossRef]
- Materna, K.L.; Jiang, J.; Crabtree, R.H.; Brudvig, G.W. Silatrane Anchors for Metal Oxide Surfaces: Optimization for Potential Photocatalytic and Electrocatalytic Applications. ACS Appl. Mater. Interfaces 2019, 11, 5602–5609. [Google Scholar] [CrossRef] [PubMed]
- Troiano, J.L.; Hu, G.; Crabtree, R.H.; Brudvig, G.W. Diazo coupling for surface attachment of small molecules to TiO2 nanoparticles. Chem. Commun. 2020, 56, 9340–9343. [Google Scholar] [CrossRef]
- Pandit, B.; Luitel, T.; Cummins, D.R.; Thapa, A.K.; Druffel, T.; Zamborini, F.; Liu, J. Spectroscopic Investigation of Photoinduced Charge-Transfer Processes in FTO/TiO2/N719 Photoanodes with and without Covalent Attachment through Silane-Based Linkers. J. Phys. Chem. A 2013, 117, 13513–13523. [Google Scholar] [CrossRef]
- Gao, Y.; Ding, X.; Liu, J.; Wang, L.; Lu, Z.; Li, L.; Sun, L. Visible Light Driven Water Splitting in a Molecular Device with Unprecedentedly High Photocurrent Density. J. Am. Chem. Soc. 2013, 135, 4219–4222. [Google Scholar] [CrossRef]
- Lauinger, S.M.; Sumliner, J.M.; Yin, Q.; Xu, Z.; Liang, G.; Glass, E.N.; Lian, T.; Hill, C.L. High Stability of Immobilized Polyoxometalates on TiO2 Nanoparticles and Nanoporous Films for Robust, Light-Induced Water Oxidation. Chem. Mater. 2015, 27, 5886–5891. [Google Scholar] [CrossRef]
- Weng, B.; Yang, M.-Q.; Zhang, N.; Xu, Y.-J. Toward the enhanced photoactivity and photostability of ZnO nanospheres via intimate surface coating with reduced graphene oxide. J. Mater. Chem. A 2014, 2, 9380–9389. [Google Scholar] [CrossRef]
- Wang, F.; Zhou, Y.; Pan, X.; Lu, B.; Huang, J.; Ye, Z. Enhanced photocatalytic properties of ZnO nanorods by electrostatic self–assembly with reduced graphene oxide. Phys. Chem. Chem. Phys. 2018, 20, 6959–6969. [Google Scholar] [CrossRef] [PubMed]
- Singh, Z.; Donnarumma, P.R.; Majewski, M.B. Molecular Copper(I)-Copper(II) Photosensitizer-Catalyst Photoelectrode for Water Oxidation. Inorg. Chem. 2020, 59, 12994–12999. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Eberhart, M.; Nayak, A.; Brennaman, M.K.; Shan, B.; Meyer, T.J. A Molecular Silane-Derivatized Ru(II) Catalyst for Photoelectrochemical Water Oxidation. J. Am. Chem. Soc. 2018, 140, 15062–15069. [Google Scholar] [CrossRef] [PubMed]
- Keijer, T.; Bouwens, T.; Hessels, J.; Reek, J.N.H. Supramolecular strategies in artificial photosynthesis. Chem. Sci. 2021, 12, 50–70. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Poole, D.; Mathew, S.; Yan, N.; Hessels, J.; Orth, N.; Ivanović-Burmazović, I.; Reek, J.N.H. Control over Electrochemical Water Oxidation Catalysis by Preorganization of Molecular Ruthenium Catalysts in Self-Assembled Nanospheres. Angew. Chem. Int. Ed. 2018, 57, 11247–11251. [Google Scholar] [CrossRef]
- Richmond, C.J.; Matheu, R.; Poater, A.; Falivene, L.; Benet-Buchholz, J.; Sala, X.; Cavallo, L.; Llobet, A. Supramolecular Water Oxidation with Ru-bda-Based Catalysts. Chem. A Eur. J. 2014, 20, 17282–17286. [Google Scholar] [CrossRef]
- Yang, B.; Jiang, X.; Guo, Q.; Lei, T.; Zhang, L.; Chen, B.; Tung, C.; Wu, L. Self-Assembled Amphiphilic Water Oxidation Catalysts: Control of O-O Bond Formation Pathways by Different Aggregation Patterns. Angew. Chem. Int. Ed. 2016, 55, 6229–6234. [Google Scholar] [CrossRef]
- Li, B.; Li, F.; Bai, S.; Wang, Z.; Sun, L.; Yang, Q.; Li, C. Oxygen evolution from water oxidation on molecular catalysts confined in the nanocages of mesoporous silicas. Energy Environ. Sci. 2012, 5, 8229–8233. [Google Scholar] [CrossRef]
- Kunz, V.; Lindner, J.O.; Schulze, M.; Röhr, M.I.S.; Schmidt, D.; Mitrić, R.; Würthner, F. Cooperative water oxidation catalysis in a series of trinuclear metallosupramolecular ruthenium macrocycles. Energy Environ. Sci. 2017, 10, 2137–2153. [Google Scholar] [CrossRef]
- Wang, L.; Polyansky, D.E.; Concepcion, J.J. Self-Assembled Bilayers as an Anchoring Strategy: Catalysts, Chromophores, and Chromophore-Catalyst Assemblies. J. Am. Chem. Soc. 2019, 141, 8020–8024. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Lee, Y.-M.; Nam, W. Immobilization of Molecular Catalysts for Enhanced Redox Catalysis. ChemCatChem 2018, 10, 1686–1702. [Google Scholar] [CrossRef]
- Mantovani, K.M.; Molgero Westrup, K.C.; da Silva Junior, R.M.; Jaerger, S.; Wypych, F.; Nakagaki, S. Oxidation catalyst obtained by the immobilization of layered double hydroxide/Mn(iii) porphyrin on monodispersed silica spheres. Dalton Trans. 2018, 47, 3068–3073. [Google Scholar] [CrossRef] [PubMed]
- Kaliyaraj Selva Kumar, A.; Zhang, Y.; Li, D.; Compton, R.G. A mini-review: How reliable is the drop casting technique? Electrochem. Commun. 2020, 121, 106867. [Google Scholar] [CrossRef]
- Wang, Y.; Li, F.; Zhou, X.; Yu, F.; Du, J.; Bai, L.; Sun, L. Highly Efficient Photoelectrochemical Water Splitting with an Immobilized Molecular Co4O4 Cubane Catalyst. Angew. Chem. Int. Ed. 2017, 56, 6911–6915. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, J.; Ma, L.; Cai, W.; Cheng, H. Recent Developments on Alternative Proton Exchange Membranes: Strategies for Systematic Performance Improvement. Energy Technol. 2015, 3, 675–691. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Su, Y.-H.; Chang, C.-M.; Suryani; Wang, D.-M.; Lai, J.-Y. Preparation and applications of Nafion-functionalized multiwalled carbon nanotubes for proton exchange membrane fuel cells. J. Mater. Chem. 2010, 20, 4409–4416. [Google Scholar] [CrossRef]
- Murthy, A.P.; Theerthagiri, J.; Madhavan, J. Highly Water Dispersible Polymer Acid–Doped Polyanilines as Low-Cost, Nafion-Free Ionomers for Hydrogen Evolution Reaction. ACS Appl. Energy Mater. 2018, 1, 1512–1521. [Google Scholar] [CrossRef]
- Johnson, B.A.; Bhunia, A.; Ott, S. Electrocatalytic water oxidation by a molecular catalyst incorporated into a metal-organic framework thin film. Dalton Trans. 2017, 46, 1382–1388. [Google Scholar] [CrossRef]
- Buru, C.T.; Li, P.; Mehdi, B.L.; Dohnalkova, A.; Platero-Prats, A.E.; Browning, N.D.; Chapman, K.W.; Hupp, J.T.; Farha, O.K. Adsorption of a Catalytically Accessible Polyoxometalate in a Mesoporous Channel-type Metal-Organic Framework. Chem. Mater. 2017, 29, 5174–5181. [Google Scholar] [CrossRef]
- Yamada, Y.; Oyama, K.; Gates, R.; Fukuzumi, S. High Catalytic Activity of Heteropolynuclear Cyanide Complexes Containing Cobalt and Platinum Ions: Visible-Light Driven Water Oxidation. Angew. Chem. Int. Ed. 2015, 54, 5613–5617. [Google Scholar] [CrossRef]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Farha, O.K.; Roberts, J.; Scheidt, K.A.; Nguyen, S.T.; Hupp, J.T. Metal-organic framework materials as catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459. [Google Scholar] [CrossRef] [PubMed]
- Ashford, D.L.; Lapides, A.M.; Vannucci, A.K.; Hanson, K.; Torelli, D.; Harrison, D.; Templeton, J.L.; Meyer, T.J. Water Oxidation by an Electropolymerized Catalyst on Derivatized Mesoporous Metal Oxide Electrodes. J. Am. Chem. Soc. 2014, 136, 6578–6581. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Cheng, Y.; Hobson, T.; Liu, J. Design and Synthesis of Hierarchical MnO2 Nanospheres/Carbon Nanotubes/Conducting Polymer Ternary Composite for High Performance Electrochemical Electrodes. Nano Lett. 2010, 10, 2727–2733. [Google Scholar] [CrossRef] [PubMed]
- deKrafft, K.E.; Wang, C.; Xie, Z.; Su, X.; Hinds, B.J.; Lin, W. Electrochemical Water Oxidation with Carbon-Grafted Iridium Complexes. ACS Appl. Mater. Interfaces 2012, 4, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Lapides, A.M.; Sherman, B.D.; Brennaman, M.K.; Dares, C.J.; Skinner, K.R.; Templeton, J.L.; Meyer, T.J. Synthesis, characterization, and water oxidation by a molecular chromophore-catalyst assembly prepared by atomic layer deposition. The “mummy” strategy. Chem. Sci. 2015, 6, 6398–6406. [Google Scholar] [CrossRef]
- Kirkland, J.J. Porous Thin-Layer Modified Glass Bead Supports for Gas Liquid Chromatography. Anal. Chem. 1965, 37, 1458–1461. [Google Scholar] [CrossRef]
- Richardson, J.J.; Björnmalm, M.; Caruso, F. Technology-driven layer-by-ayer assembly of nanofilms. Science 2015, 348, aaa2491. [Google Scholar] [CrossRef]
- Richardson, J.J.; Cui, J.; Björnmalm, M.; Braunger, J.A.; Ejima, H.; Caruso, F. Innovation in Layer-by-Layer Assembly. Chem. Rev. 2016, 116, 14828–14867. [Google Scholar] [CrossRef]
- Borges, J.; Mano, J.F. Molecular Interactions Driving the Layer-by-Layer Assembly of Multilayers. Chem. Rev. 2014, 114, 8883–8942. [Google Scholar] [CrossRef]
- Choi, Y.; Jeon, D.; Choi, Y.; Kim, D.; Kim, N.; Gu, M.; Bae, S.; Lee, T.; Lee, H.-W.; Kim, B.-S.; et al. Interface Engineering of Hematite with Nacre-like Catalytic Multilayers for Solar Water Oxidation. ACS Nano 2019, 13, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Decher, G. Fuzzy Nanoassemblies: Toward Layered Polymeric Multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Li, J.; Triana, C.A.; Wan, W.; Saseendran, D.P.A.; Zhao, Y.; Balaghi, S.E.; Heidari, S.; Patzke, G.R. Molecular and heterogeneous water oxidation catalysts: Recent progress and joint perspectives. Chem. Soc. Rev. 2021, 50, 2444–2485. [Google Scholar] [CrossRef] [PubMed]
- Kunz, V.; Schmidt, D.; Röhr, M.I.S.; Mitrić, R.; Würthner, F. Supramolecular Approaches to Improve the Performance of Ruthenium–Based Water Oxidation Catalysts. Adv. Energy Mater. 2017, 7, 1602939. [Google Scholar] [CrossRef]
- Li, W.; Sheehan, S.W.; He, D.; He, Y.; Yao, X.; Grimm, R.L.; Brudvig, G.W.; Wang, D. Hematite-Based Solar Water Splitting in Acidic Solutions: Functionalization by Mono-and Multilayers of Iridium Oxygen-Evolution Catalysts. Angew. Chem. Int. Ed. 2015, 54, 11428–11432. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, S.W.; Thomsen, J.M.; Hintermair, U.; Crabtree, R.H.; Brudvig, G.W.; Schmuttenmaer, C.A. A molecular catalyst for water oxidation that binds to metal oxide surfaces. Nat. Commun. 2015, 6, 6469. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, J.; Wu, H.-L.; Liu, W.-Q.; Jiang, X.; Li, Z.-J.; Chen, B.; Tung, C.-H.; Wu, L.-Z. Improved Photoelectrocatalytic Performance for Water Oxidation by Earth-Abundant Cobalt Molecular Porphyrin Complex-Integrated BiVO4 Photoanode. ACS Appl. Mater. Interfaces 2016, 8, 18577–18583. [Google Scholar] [CrossRef]
- Daniel, Q.; Duan, L.; Timmer, B.; Chen, H.; Luo, X.; Ambre, R.; Wang, Y.; Zhang, B.; Zhang, P.; Wang, L.; et al. Water Oxidation Initiated by In Situ Dimerization of the Molecular Ru(pdc) Catalyst. ACS Catal. 2018, 8, 4375–4382. [Google Scholar] [CrossRef]
- Duan, L.; Fischer, A.; Xu, Y.; Sun, L. Isolated Seven-Coordinate Ru(IV) Dimer Complex with [HOHOH]-Bridging Ligand as an Intermediate for Catalytic Water Oxidation. J. Am. Chem. Soc. 2009, 131, 10397–10399. [Google Scholar] [CrossRef]
- Sherman, B.D.; Xie, Y.; Sheridan, M.V.; Wang, D.; Shaffer, D.W.; Meyer, T.J.; Concepcion, J.J. Light-Driven Water Splitting by a Covalently Linked Ruthenium-Based Chromophore-Catalyst Assembly. ACS Energy Lett. 2017, 2, 124–128. [Google Scholar] [CrossRef]
- Sato, S.; Iida, J.; Suzuki, K.; Kawano, M.; Ozeki, T.; Fujita, M. Fluorous Nanodroplets Structurally Confined in an Organopalladium Sphere. Science 2006, 313, 1273–1276. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Duan, L.; Xu, Y.; Privalov, T.; Sun , L. Structural Modifications of Mononuclear Ruthenium Complexes: A Combined Experimental and Theoretical Study on the Kinetics of Ruthenium-Catalyzed Water Oxidation. Angew. Chem. Int. Ed. 2011, 50, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Habisreutinger, S.N.; Leijtens, T.; Eperon, G.E.; Stranks, S.D.; Nicholas, R.J.; Snaith, H.J. Carbon Nanotube/Polymer Composites as a Highly Stable Hole Collection Layer in Perovskite Solar Cells. Nano Lett. 2014, 14, 5561–5568. [Google Scholar] [CrossRef]
- Wee, K.-R.; Brennaman, M.K.; Alibabaei, L.; Farnum, B.H.; Sherman, B.; Lapides, A.M.; Meyer, T.J. Stabilization of Ruthenium(II) Polypyridyl Chromophores on Nanoparticle Metal-Oxide Electrodes in Water by Hydrophobic PMMA Overlayers. J. Am. Chem. Soc. 2014, 136, 13514–13517. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Gao, Y.; Ye, L.; Zhang, L.; Sun, L. Assembling Supramolecular Dye-Sensitized Photoelectrochemical Cells for Water Splitting. ChemSusChem 2015, 8, 3992–3995. [Google Scholar] [CrossRef]
- Wang, D.; Wang, L.; Brady, M.D.; Dares, C.J.; Meyer, G.J.; Meyer, T.J.; Concepcion, J.J. Self-Assembled Chromophore-Catalyst Bilayer for Water Oxidation in a Dye-Sensitized Photoelectrosynthesis Cell. J. Phys. Chem. C 2019, 123, 30039–30045. [Google Scholar] [CrossRef]
- Sévery, L.; Szczerbiński, J.; Taskin, M.; Tuncay, I.; Nunes, F.B.; Cignarella, C.; Tocci, G.; Blacque, O.; Osterwalder, J.; Zenobi, R.; et al. Immobilization of molecular catalysts on electrode surfaces using host-guest interactions. Nat. Chem. 2021, 13, 523–529. [Google Scholar] [CrossRef]
- Cao, G.; Hong, H.G.; Mallouk, T.E. Layered metal phosphates and phosphonates: From crystals to monolayers. Acc. Chem. Res. 1992, 25, 420–427. [Google Scholar] [CrossRef]
- Wang, D.; Xu, Z.; Sheridan, M.V.; Concepcion, J.J.; Li, F.; Lian, T.; Meyer, T.J. Photodriven water oxidation initiated by a surface bound chromophore-donor-catalyst assembly. Chem. Sci. 2021, 12, 14441–14450. [Google Scholar] [CrossRef]
- Hoque, M.A.; Gil-Sepulcre, M.; de Aguirre, A.; Elemans, J.A.A.W.; Moonshiram, D.; Matheu, R.; Shi, Y.; Benet-Buchholz, J.; Sala, X.; Malfois, M.; et al. Water oxidation electrocatalysis using ruthenium coordination oligomers adsorbed on multiwalled carbon nanotubes. Nat. Chem. 2020, 12, 1060–1066. [Google Scholar] [CrossRef]
- Garrido–Barros, P.; Gimbert–Suriñach, C.; Moonshiram, D.; Picón, A.; Monge, P.; Batista, V.S.; Llobet, A. Electronic π-Delocalization Boosts Catalytic Water Oxidation by Cu(II) Molecular Catalysts Heterogenized on Graphene Sheets. J. Am. Chem. Soc. 2017, 139, 12907–12910. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lei, H.; Liu, J.; Zhao, X.; Ding, S.; Zhang, Z.; Tao, X.; Zhang, W.; Wang, W.; Zheng, X.; et al. Carbon Nanotubes with Cobalt Corroles for Hydrogen and Oxygen Evolution in pH 0–14 Solutions. Angew. Chem. Int. Ed. Engl. 2018, 57, 15070–15075. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xie, Z.; deKrafft, K.E.; Lin, W. Doping Metal-Organic Frameworks for Water Oxidation, Carbon Dioxide Reduction, and Organic Photocatalysis. J. Am. Chem. Soc. 2011, 133, 13445–13454. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.-F.; Liao, P.-Q.; Wang, J.-W.; Wu, J.-X.; Chen, X.-W.; He, C.-T.; Zhang, J.-P.; Li, G.-R.; Chen, X.-M. An Alkaline-Stable, Metal Hydroxide Mimicking Metal-Organic Framework for Efficient Electrocatalytic Oxygen Evolution. J. Am. Chem. Soc. 2016, 138, 8336–8339. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Debgupta, J.; Bose, S.; Das, S.K. A Mononuclear CoII Coordination Complex Locked in a Confined Space and Acting as an Electrochemical Water-Oxidation Catalyst: A “Ship-in-a-Bottle” Approach. Angew. Chem. Int. Ed. 2016, 55, 2425–2430. [Google Scholar] [CrossRef]
- Wang, S.; Hou, Y.; Lin, S.; Wang, X. Water oxidation electrocatalysis by a zeolitic imidazolate framework. Nanoscale 2014, 6, 9930–9934. [Google Scholar] [CrossRef]
- Wurster, B.; Grumelli, D.; Hötger, D.; Gutzler, R.; Kern, K. Driving the Oxygen Evolution Reaction by Nonlinear Cooperativity in Bimetallic Coordination Catalysts. J. Am. Chem. Soc. 2016, 138, 3623–3626. [Google Scholar] [CrossRef]
- Dogutan, D.K.; McGuire, R.; Nocera, D.G. Electocatalytic Water Oxidation by Cobalt(III) Hangman β-Octafluoro Corroles. J. Am. Chem. Soc. 2011, 133, 9178–9180. [Google Scholar] [CrossRef]
- Moss, J.A.; Yang, J.C.; Stipkala, J.M.; Wen, X.; Bignozzi, C.A.; Meyer, G.J.; Meyer, T.J. Sensitization and Stabilization of TiO2 Photoanodes with Electropolymerized Overlayer Films of Ruthenium and Zinc Polypyridyl Complexes: A Stable Aqueous Photoelectrochemical Cell. Inorg. Chem. 2004, 43, 1784–1792. [Google Scholar] [CrossRef]
- Lapides, A.M.; Ashford, D.L.; Hanson, K.; Torelli, D.A.; Templeton, J.L.; Meyer, T.J. Stabilization of a Ruthenium(II) Polypyridyl Dye on Nanocrystalline TiO2 by an Electropolymerized Overlayer. J. Am. Chem. Soc. 2013, 135, 15450–15458. [Google Scholar] [CrossRef]
- Ashford, D.L.; Sherman, B.D.; Binstead, R.A.; Templeton, J.L.; Meyer, T.J. Electro-assembly of a Chromophore-Catalyst Bilayer for Water Oxidation and Photocatalytic Water Splitting. Angew. Chem. Int. Ed. 2015, 54, 4778–4781. [Google Scholar] [CrossRef] [PubMed]
- Leem, G.; Sherman, B.D.; Burnett, A.J.; Morseth, Z.A.; Wee, K.-R.; Papanikolas, J.M.; Meyer, T.J.; Schanze, K.S. Light-Driven Water Oxidation Using Polyelectrolyte Layer-by-Layer Chromophore-Catalyst Assemblies. ACS Energy Lett. 2016, 1, 339–343. [Google Scholar] [CrossRef]





| Property | Homogeneous | Heterogenous | Heterogenized |
|---|---|---|---|
| Activity | High | Moderate | High |
| Selectivity | High | Low-Medium | High |
| Synthesis | Complex | Simple | Complex |
| Tuneability | High | Low | High |
| Efficiency | Moderate | High | High |
| Stability | Short term | Long-term | Elongated |
| Robustness | Low | High | High |
| Study | Established protocols, valuable information | Less explored | Established protocols, valuable information |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casadevall, C. Heterogenization of Molecular Water Oxidation Catalysts in Electrodes for (Photo)Electrochemical Water Oxidation. Water 2022, 14, 371. https://doi.org/10.3390/w14030371
Casadevall C. Heterogenization of Molecular Water Oxidation Catalysts in Electrodes for (Photo)Electrochemical Water Oxidation. Water. 2022; 14(3):371. https://doi.org/10.3390/w14030371
Chicago/Turabian StyleCasadevall, Carla. 2022. "Heterogenization of Molecular Water Oxidation Catalysts in Electrodes for (Photo)Electrochemical Water Oxidation" Water 14, no. 3: 371. https://doi.org/10.3390/w14030371
APA StyleCasadevall, C. (2022). Heterogenization of Molecular Water Oxidation Catalysts in Electrodes for (Photo)Electrochemical Water Oxidation. Water, 14(3), 371. https://doi.org/10.3390/w14030371






