Removal of Nutrients and Pesticides from Agricultural Runoff Using Microalgae and Cyanobacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Agricultural Runoff
2.2. Strains
2.3. Experimental Design
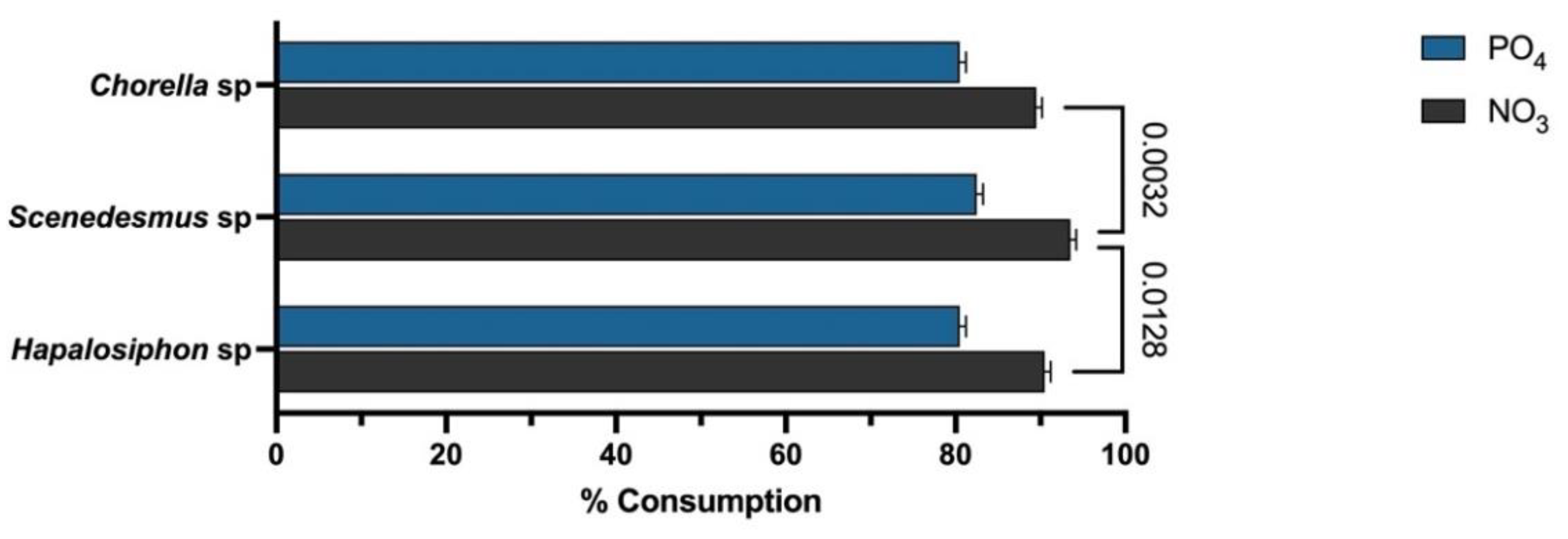
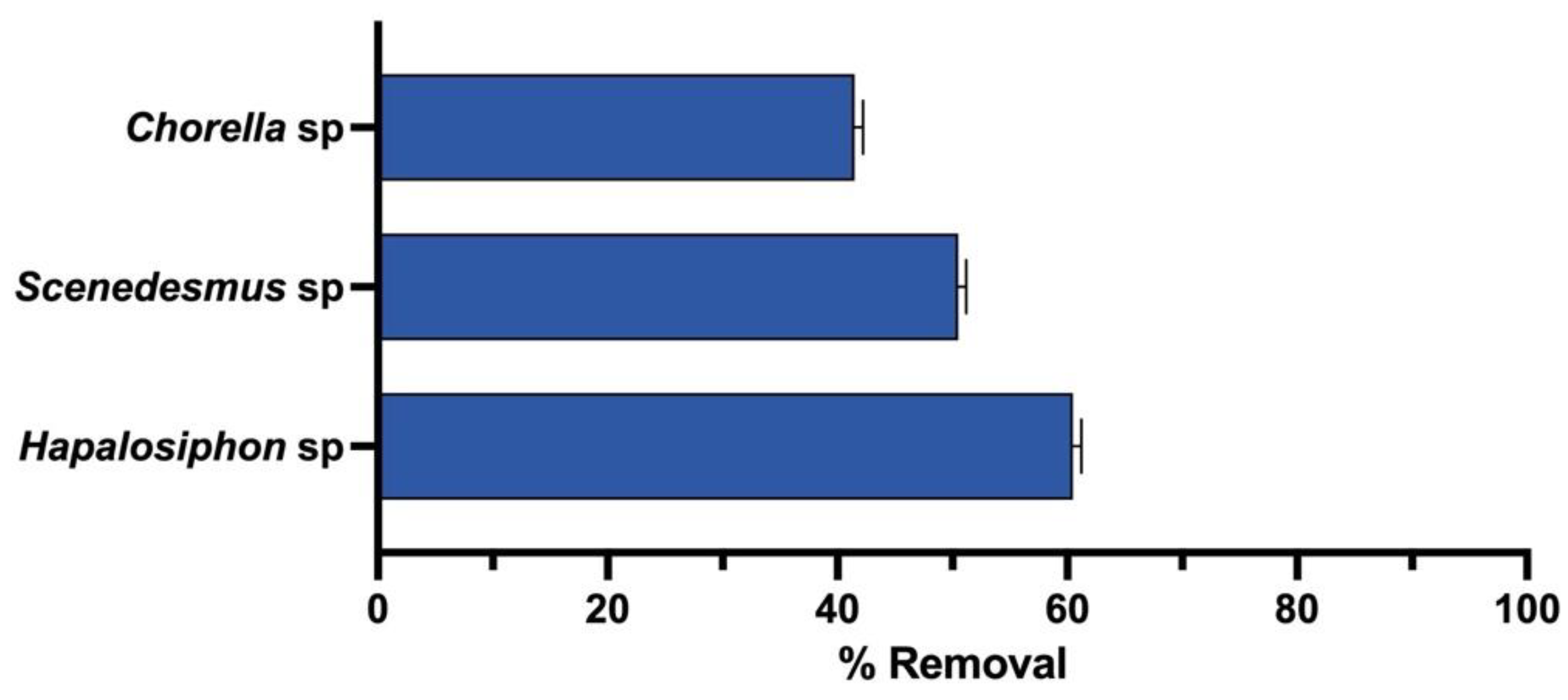
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Setty, K.; Jiménez, A.; Willetts, J.; Leifels, M.; Bartram, J. Global Water, Sanitation and Hygiene Research Priorities and Learning Challenges under Sustainable Development Goal 6. Dev. Policy Rev. 2020, 38, 64–84. [Google Scholar] [CrossRef] [PubMed]
- de Souza, R.M.; Seibert, D.; Quesada, H.B.; de Jesus Bassetti, F.; Fagundes-Klen, M.R.; Bergamasco, R. Occurrence, Impacts and General Aspects of Pesticides in Surface Water: A Review. Process Saf. Environ. Prot. 2020, 135, 22–37. [Google Scholar] [CrossRef]
- Peña, A.; Delgado-Moreno, L.; Rodríguez-Liébana, J.A. A Review of the Impact of Wastewater on the Fate of Pesticides in Soils: Effect of Some Soil and Solution Properties. Sci. Total Environ. 2020, 718, 134468. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.M.; Pires, J.C.M. Microalgae Cultivation in Wastewater to Recycle Nutrients as Biofertilizer BT—Environmental Biotechnology; Gothandam, K.M., Ranjan, S., Dasgupta, N., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2020; Volume 1, pp. 71–86. [Google Scholar] [CrossRef]
- Mehariya, S.; Goswami, R.K.; Verma, P.; Lavecchia, R.; Zuorro, A. Integrated Approach for Wastewater Treatment and Biofuel Production in Microalgae Biorefineries. Energies 2021, 14, 2282. [Google Scholar] [CrossRef]
- Li, K.; Liu, Q.; Fang, F.; Luo, R.; Lu, Q.; Zhou, W.; Huo, S.; Cheng, P.; Liu, J.; Addy, M.; et al. Microalgae-Based Wastewater Treatment for Nutrients Recovery: A Review. Bioresour. Technol. 2019, 291, 121934. [Google Scholar] [CrossRef]
- Kotoula, D.; Iliopoulou, A.; Irakleous-Palaiologou, E.; Gatidou, G.; Aloupi, M.; Antonopoulou, P.; Fountoulakis, M.S.; Stasinakis, A.S. Municipal Wastewater Treatment by Combining in Series Microalgae Chlorella Sorokiniana and Macrophyte Lemna Minor: Preliminary Results. J. Clean. Prod. 2020, 271, 122704. [Google Scholar] [CrossRef]
- Hariz, H.B.; Takriff, M.S.; Ba-Abbad, M.M.; Mohd Yasin, N.H.; Mohd Hakim, N.I.N. CO2 Fixation Capability of Chlorella Sp. and Its Use in Treating Agricultural Wastewater. J. Appl. Phycol. 2018, 30, 3017–3027. [Google Scholar] [CrossRef]
- Shahid, A.; Malik, S.; Zhu, H.; Xu, J.; Nawaz, M.Z.; Nawaz, S.; Asraful Alam, M.; Mehmood, M.A. Cultivating Microalgae in Wastewater for Biomass Production, Pollutant Removal, and Atmospheric Carbon Mitigation; a Review. Sci. Total Environ. 2020, 704, 135303. [Google Scholar] [CrossRef]
- Mehariya, S.; Fratini, F.; Lavecchia, R.; Zuorro, A. Green Extraction of Value-Added Compounds Form Microalgae: A Short Review on Natural Deep Eutectic Solvents (NaDES) and Related Pre-Treatments. J. Environ. Chem. Eng. 2021, 9, 105989. [Google Scholar] [CrossRef]
- Rani, A.; Saini, K.C.; Bast, F.; Mehariya, S.; Bhatia, S.K.; Lavecchia, R.; Zuorro, A. Microorganisms: A Potential Source of Bioactive Molecules for Antioxidant Applications. Molecules 2021, 26, 1142. [Google Scholar] [CrossRef]
- Quintero-Dallos, V.; García-Martínez, J.B.; Contreras-Ropero, J.E.; Barajas-Solano, A.F.; Barajas-Ferrerira, C.; Lavecchia, R.; Zuorro, A. Vinasse as a Sustainable Medium for the Production of Chlorella vulgaris UTEX 1803. Water 2019, 11, 1526. [Google Scholar] [CrossRef] [Green Version]
- Zuorro, A.; Maffei, G.; Lavecchia, R. Kinetic Modeling of Azo Dye Adsorption on Non-Living Cells of Nannochloropsis Oceanica. J. Environ. Chem. Eng. 2017, 5, 4121–4127. [Google Scholar] [CrossRef]
- Mohsenpour, S.F.; Hennige, S.; Willoughby, N.; Adeloye, A.; Gutierrez, T. Integrating Micro-Algae into Wastewater Treatment: A Review. Sci. Total Environ. 2021, 752, 142168. [Google Scholar] [CrossRef] [PubMed]
- Dębowski, M.; Zieliński, M.; Kazimierowicz, J.; Kujawska, N.; Talbierz, S. Microalgae Cultivation Technologies as an Opportunity for Bioenergetic System Development—Advantages and Limitations. Sustainability 2020, 12, 9980. [Google Scholar] [CrossRef]
- Ammar, S.H.; Khadim, H.J.; Mohamed, A.I. Cultivation of Nannochloropsis Oculata and Isochrysis Galbana Microalgae in Produced Water for Bioremediation and Biomass Production. Environ. Technol. Innov. 2018, 10, 132–142. [Google Scholar] [CrossRef]
- Rueda, E.; García-Galán, M.J.; Ortiz, A.; Uggetti, E.; Carretero, J.; García, J.; Díez-Montero, R. Bioremediation of Agricultural Runoff and Biopolymers Production from Cyanobacteria Cultured in Demonstrative Full-Scale Photobioreactors. Process Saf. Environ. Prot. 2020, 139, 241–250. [Google Scholar] [CrossRef]
- García-Galán, M.J.; Monllor-Alcaraz, L.S.; Postigo, C.; Uggetti, E.; López de Alda, M.; Díez-Montero, R.; García, J. Microalgae-Based Bioremediation of Water Contaminated by Pesticides in Peri-Urban Agricultural Areas. Environ. Pollut. 2020, 265, 114579. [Google Scholar] [CrossRef]
- Li, X.; Yang, C.; Zeng, G.; Wu, S.; Lin, Y.; Zhou, Q.; Lou, W.; Du, C.; Nie, L.; Zhong, Y. Nutrient Removal from Swine Wastewater with Growing Microalgae at Various Zinc Concentrations. Algal Res. 2020, 46, 101804. [Google Scholar] [CrossRef]
- Pacheco, D.; Rocha, A.C.; Pereira, L.; Verdelhos, T. Microalgae Water Bioremediation: Trends and Hot Topics. Appl. Sci. 2020, 10, 1886. [Google Scholar] [CrossRef] [Green Version]
- Baird, R.; Bridgewater, L. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Zalat, O.A.; Elsayed, M.A.; Fayed, M.S.; Abd El Megid, M.K. Validation of UV Spectrophotometric and HPLC Methods for Quantitative Determination of Chlorpyrifos. Int. Lett. Chem. Phys. Astron. 2013, 21, 58–63. [Google Scholar] [CrossRef] [Green Version]
- Andersen, R.A.; Berges, J.A.; Harrison, P.J.; Watanabe, M.M. Appendix A—Recipes for Freshwater and Seawater Media. In Algal Culturing Techniques; Andersen, R.A., Ed.; Elsevier Academic Press: Burlington, MA, USA, 2005; pp. 429–538. [Google Scholar]
- Garcia-Martinez, J.B.; Urbina-Suarez, N.A.; Zuorro, A.; Barajas-Solano, A.F.; Kafarov, V. Fisheries Wastewater as a Sustainable Media for the Production of Algae-Based Products. Chem. Eng. Trans. 2019, 76, 1339–1344. [Google Scholar] [CrossRef]
- Sanchez-Galvis, E.M.; Cardenas-Gutierrez, I.Y.; Contreras-Ropero, J.E.; García-Martínez, J.B.; Barajas-Solano, A.F.; Zuorro, A. An Innovative Low-Cost Equipment for Electro-Concentration of Microalgal Biomass. Appl. Sci. 2020, 10, 4841. [Google Scholar] [CrossRef]
- García-Martínez, J.B.; Ayala-Torres, E.; Reyes-Gómez, O.; Zuorro, A.; Andrés, F.; Barajas-Solano, B.; Crisóstomo, C.; Barajas-Ferreira, B. Evaluation of a Two-Phase Extraction System of Carbohydrates and Proteins from Chlorella Vulgaris Utex 1803. Chem. Eng. Trans. 2016, 49, 355–360. [Google Scholar] [CrossRef]
- Mishra, S.K.; Suh, W.I.; Farooq, W.; Moon, M.; Shrivastav, A.; Park, M.S.; Yang, J.W. Rapid Quantification of Microalgal Lipids in Aqueous Medium by a Simple Colorimetric Method. Bioresour. Technol. 2014, 155, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.F.S.; Souza, M.F.; Bon, E.P.S.; Rodrigues, M.A.; Freitas, S.P. Colorimetric Protein Determination in Microalgae (Chlorophyta): Association of Milling and SDS Treatment for Total Protein Extraction. J. Phycol. 2018, 54, 577–580. [Google Scholar] [CrossRef]
- Hynstova, V.; Sterbova, D.; Klejdus, B.; Hedbavny, J.; Huska, D.; Adam, V. Separation, Identification and Quantification of Carotenoids and Chlorophylls in Dietary Supplements Containing Chlorella Vulgaris and Spirulina Platensis Using High Performance Thin Layer Chromatography. J. Pharm. Biomed. Anal. 2018, 148, 108–118. [Google Scholar] [CrossRef]
- Zuorro, A.; Leal-Jerez, A.G.; Morales-Rivas, L.K.; Mogollón-Londoño, S.O.; Sanchez-Galvis, E.M.; García-Martínez, J.B.; Barajas-Solano, A.F. Enhancement of Phycobiliprotein Accumulation in Thermotolerant Oscillatoria Sp. through Media Optimization. ACS Omega 2021, 6, 10527–10536. [Google Scholar] [CrossRef]
- Rasoul-Amini, S.; Montazeri-Najafabady, N.; Shaker, S.; Safari, A.; Kazemi, A.; Mousavi, P.; Mobasher, M.A.; Ghasemi, Y. Removal of Nitrogen and Phosphorus from Wastewater Using Microalgae Free Cells in Bath Culture System. Biocatal. Agric. Biotechnol. 2014, 3, 126–131. [Google Scholar] [CrossRef]
- Ahmad, A.; Banat, F.; Alsafar, H.; Hasan, S.W. Algae Biotechnology for Industrial Wastewater Treatment, Bioenergy Production, and High-Value Bioproducts. Sci. Total Environ. 2022, 806, 150585. [Google Scholar] [CrossRef]
- Zuorro, A.; García-Martínez, J.B.; Barajas-Solano, A.F. The Application of Catalytic Processes on the Production of Algae-Based Biofuels: A Review. Catalysts 2021, 11, 22. [Google Scholar] [CrossRef]
- Guiza-Franco, L.; Orozco-Rojas, L.G.; Sanchez-Galvis, M.; Garcia-Martinez, J.B.; Barajas-Ferreira, C.; Zuorro, A.; Barajas-Solano, A.F. Production of Chlorella Vulgaris Biomass on UV-Treated Wastewater as an Alternative for Environmental Sustainability on High-Mountain Fisheries. Chem. Eng. Trans. 2018, 64, 517–522. [Google Scholar] [CrossRef]
- Sivaramakrishnan, R.; Incharoensakdi, A. Enhancement of Total Lipid Yield by Nitrogen, Carbon, and Iron Supplementation in Isolated Microalgae. J. Phycol. 2017, 53, 855–868. [Google Scholar] [CrossRef] [PubMed]
- Shuyu, L.; Jingling, X.; Hongyan, Y.; Cen, Z.; Wenli, C.; Fang, M. Comparing the Effect of C, N, and P Factors on Photosynthesis, Biomass, and Lipid Production in Chlorella Sp. J. Environ. Eng. 2018, 144, 4018116. [Google Scholar] [CrossRef]
- El Shenawy, E.A.; Elkelawy, M.; Bastawissi, H.A.-E.; Taha, M.; Panchal, H.; Sadasivuni, K.K.; Thakar, N. Effect of Cultivation Parameters and Heat Management on the Algae Species Growth Conditions and Biomass Production in a Continuous Feedstock Photobioreactor. Renew. Energy 2020, 148, 807–815. [Google Scholar] [CrossRef]
- Tu, Z.; Liu, L.; Lin, W.; Xie, Z.; Luo, J. Potential of Using Sodium Bicarbonate as External Carbon Source to Cultivate Microalga in Non-Sterile Condition. Bioresour. Technol. 2018, 266, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Lohman, E.J.; Gardner, R.D.; Pedersen, T.; Peyton, B.M.; Cooksey, K.E.; Gerlach, R. Optimized Inorganic Carbon Regime for Enhanced Growth and Lipid Accumulation in Chlorella Vulgaris. Biotechnol. Biofuels 2015, 8, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, G.-Y.; Heo, J.; Kim, H.-S.; Han, J.-I. Bicarbonate-Based Cultivation of Dunaliella Salina for Enhancing Carbon Utilization Efficiency. Bioresour. Technol. 2017, 237, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Pancha, I.; Chokshi, K.; Ghosh, T.; Paliwal, C.; Maurya, R.; Mishra, S. Bicarbonate Supplementation Enhanced Biofuel Production Potential as Well as Nutritional Stress Mitigation in the Microalgae Scenedesmus Sp. CCNM 1077. Bioresour. Technol. 2015, 193, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Umetani, I.; Janka, E.; Sposób, M.; Hulatt, C.J.; Kleiven, S.; Bakke, R. Bicarbonate for Microalgae Cultivation: A Case Study in a Chlorophyte, Tetradesmus Wisconsinensis Isolated from a Norwegian Lake. J. Appl. Phycol. 2021, 33, 1341–1352. [Google Scholar] [CrossRef]
- Khalid, A.A.H.; Yaakob, Z.; Abdullah, S.R.S.; Takriff, M.S. Analysis of the Elemental Composition and Uptake Mechanism of Chlorella Sorokiniana for Nutrient Removal in Agricultural Wastewater under Optimized Response Surface Methodology (RSM) Conditions. J. Clean. Prod. 2019, 210, 673–686. [Google Scholar] [CrossRef]
- Matamoros, V.; Rodríguez, Y. Batch vs Continuous-Feeding Operational Mode for the Removal of Pesticides from Agricultural Run-off by Microalgae Systems: A Laboratory Scale Study. J. Hazard. Mater. 2016, 309, 126–132. [Google Scholar] [CrossRef]
- Vazirzadeh, A.; Jafarifard, K.; Ajdari, A.; Chisti, Y. Removal of Nitrate and Phosphate from Simulated Agricultural Runoff Water by Chlorella Vulgaris. Sci. Total Environ. 2022, 802, 149988. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Park, S.Y.; Li, Y. Nutrient Recovery from Wastewater Streams by Microalgae: Status and Prospects. Renew. Sustain. Energy Rev. 2013, 19, 360–369. [Google Scholar] [CrossRef]
- Kumar, P.K.; Vijaya Krishna, S.; Verma, K.; Pooja, K.; Bhagawan, D.; Himabindu, V. Phycoremediation of Sewage Wastewater and Industrial Flue Gases for Biomass Generation from Microalgae. South African J. Chem. Eng. 2018, 25, 133–146. [Google Scholar] [CrossRef]
- Liu, J.; Danneels, B.; Vanormelingen, P.; Vyverman, W. Nutrient Removal from Horticultural Wastewater by Benthic Filamentous Algae Klebsormidium Sp., Stigeoclonium Spp. and Their Communities: From Laboratory Flask to Outdoor Algal Turf Scrubber (ATS). Water Res. 2016, 92, 61–68. [Google Scholar] [CrossRef] [PubMed]
- García-Galán, M.J.; Gutiérrez, R.; Uggetti, E.; Matamoros, V.; García, J.; Ferrer, I. Use of Full-Scale Hybrid Horizontal Tubular Photobioreactors to Process Agricultural Runoff. Biosyst. Eng. 2018, 166, 138–149. [Google Scholar] [CrossRef] [Green Version]
- Díez-Montero, R.; Belohlav, V.; Ortiz, A.; Uggetti, E.; García-Galán, M.J.; García, J. Evaluation of Daily and Seasonal Variations in a Semi-Closed Photobioreactor for Microalgae-Based Bioremediation of Agricultural Runoff at Full-Scale. Algal Res. 2020, 47, 101859. [Google Scholar] [CrossRef]
- Marella, T.K.; Saxena, A.; Tiwari, A.; Datta, A.; Dixit, S. Treating Agricultural Non-Point Source Pollutants Using Periphyton Biofilms and Biomass Volarization. J. Environ. Manage. 2022, 301, 113869. [Google Scholar] [CrossRef]
- Bohutskyi, P.; Chow, S.; Ketter, B.; Fung Shek, C.; Yacar, D.; Tang, Y.; Zivojnovich, M.; Betenbaugh, M.J.; Bouwer, E.J. Phytoremediation of Agriculture Runoff by Filamentous Algae Poly-Culture for Biomethane Production, and Nutrient Recovery for Secondary Cultivation of Lipid Generating Microalgae. Bioresour. Technol. 2016, 222, 294–308. [Google Scholar] [CrossRef] [PubMed]
- de-Bashan, L.E.; Bashan, Y. Recent Advances in Removing Phosphorus from Wastewater and Its Future Use as Fertilizer (1997–2003). Water Res. 2004, 38, 4222–4246. [Google Scholar] [CrossRef]
- Larsdotter, K.; la Cour Jansen, J.; Dalhammar, G. Phosphorus Removal from Wastewater by Microalgae in Sweden—A Year-round Perspective. Environ. Technol. 2010, 31, 117–123. [Google Scholar] [CrossRef] [PubMed]



| Parameters | Units | Results | Max Limit (Res 0631 2015) |
|---|---|---|---|
| Nitrates (NO3) | mg/L NO3 | 35.23 | analysis and report |
| Phosphates (PO4) | mg/L PO4 | 4.74 | analysis and report |
| pH | pH units | 7.08 | 6.00 to 9.00 |
| Turbidity | FAU | 20 | N/A |
| Conductivity | µS | 164.5 | N/A |
| Temperature | °C | 25 | N/A |
| Salinity | ppm | 102 | N/A |
| Total Dissolved Solids | ppm | 117 | N/A |
| Chemical Oxygen Demand (COD) | mg/L | 20.01 | 150.00 |
| Biochemical Oxygen Demand (BOD5) | mg/L | 2 | 50.00 |
| Total solids (TS) | mg/L | 160 | N/A |
| Total Suspended Solids (TSS) | mg/L | 25 | 50 |
| Volatile Suspended Solids (VSS) | mg/L | 12 | N/A |
| Sedimentable Solids (SS) | mL/L*h | 4 | 1 |
| Chlorpyrifos | mg/L | 1.5 | 0.05 |
| Strain | Carbon Source | Culture Media | Biomass (g/L) | Reference | |
|---|---|---|---|---|---|
| Name | Concentration (g/L) | ||||
| Chlamydomonas sp. | Na2CO3 | 0.03 | BG11 | 1.7 | [35] |
| Chlorella sp. | 1.6 | ||||
| Scenedesmus sp. | 1.7 | ||||
| Chlorella sp. (FACHB-1298) | 0.005 | 1.89 | [36] | ||
| S. Obliquus | 5 | n/a | 0.02 | [37] | |
| Chlorella sp. LPF | NaHCO3 | 80 | F/2 | n/a | [38] |
| C. vulgaris UTEX 395 | 4.2 | Bold Basal | 0.6 | [39] | |
| Dunaliella salina JDS 001 | 5.0 | MJ | 3.17 | [40] | |
| Scenedesmus sp. CCNM 1077 | 1.5 | BG11 | 0.55 | [41] | |
| Tetradesmus wisconsinensis | 1.68 | Bold Basal | 0.7 | [42] | |
| Strain | Wastewater | Pesticide | NO3 Removal | PO4 Removal | Biomass Produced | Reference |
|---|---|---|---|---|---|---|
| Naturally occurring algal mixture | horticultural wastewater | n/a | 86% | 52% | 0.51 g/L | [48] |
| C. vulgaris | simulated agricultural runoff | n/a | 85% | 91% | 4.2 g/L | [17] |
| Naturally occurring algal mixture | agricultural runoff | n/a | 0.72 g m−2 d−1 b | 0.37 g m−2 d−1 c | 11.45 g m−2 d−1 | [51] |
| Naturally occurring algal mixture | peri-urban agricultural runoff | Multiple pesticides including Chlorpyrifos | 54% | 100% | 6.9 gVSS m−2 d−1 a | [18] |
| microalgae consortium | agricultural drainage water | n/a | n/a | 0.64 g/L | [44] | |
| filamentous green algae | agricultural stormwater | n/a | 6 | 22 | 22 g m−2 d−1 | [52] |
| Mixture of Pediastrum sp. Chlorella sp. Scenedesmus sp. and Gloeothece sp. | agricultural runoff | n/a | 80% | 70% | 0.8 g/L | [49] |
| Mixture of Chlorella sp. Stigeoclonium sp. Nitzschia sp. and Navicula sp. | agricultural runoff and partially treated domestic wastewater | n/a | 85% | 99% | 0.6 g/L | [50] |
| Chlorella sp. | agricultural runoff from rice production fields | Chlorpyrifos | 85% | 82% | 1.0 g/L | This study |
| Scenedesmus sp. | 88% | 82% | 0.71 g/L | |||
| Hapalosiphon sp. | 85% | 82% | 0.83 g/L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castellanos-Estupiñan, M.A.; Carrillo-Botello, A.M.; Rozo-Granados, L.S.; Becerra-Moreno, D.; García-Martínez, J.B.; Urbina-Suarez, N.A.; López-Barrera, G.L.; Barajas-Solano, A.F.; Bryan, S.J.; Zuorro, A. Removal of Nutrients and Pesticides from Agricultural Runoff Using Microalgae and Cyanobacteria. Water 2022, 14, 558. https://doi.org/10.3390/w14040558
Castellanos-Estupiñan MA, Carrillo-Botello AM, Rozo-Granados LS, Becerra-Moreno D, García-Martínez JB, Urbina-Suarez NA, López-Barrera GL, Barajas-Solano AF, Bryan SJ, Zuorro A. Removal of Nutrients and Pesticides from Agricultural Runoff Using Microalgae and Cyanobacteria. Water. 2022; 14(4):558. https://doi.org/10.3390/w14040558
Chicago/Turabian StyleCastellanos-Estupiñan, Miguel A., Astrid M. Carrillo-Botello, Linell S. Rozo-Granados, Dorance Becerra-Moreno, Janet B. García-Martínez, Néstor A. Urbina-Suarez, Germán L. López-Barrera, Andrés F. Barajas-Solano, Samantha J. Bryan, and Antonio Zuorro. 2022. "Removal of Nutrients and Pesticides from Agricultural Runoff Using Microalgae and Cyanobacteria" Water 14, no. 4: 558. https://doi.org/10.3390/w14040558
APA StyleCastellanos-Estupiñan, M. A., Carrillo-Botello, A. M., Rozo-Granados, L. S., Becerra-Moreno, D., García-Martínez, J. B., Urbina-Suarez, N. A., López-Barrera, G. L., Barajas-Solano, A. F., Bryan, S. J., & Zuorro, A. (2022). Removal of Nutrients and Pesticides from Agricultural Runoff Using Microalgae and Cyanobacteria. Water, 14(4), 558. https://doi.org/10.3390/w14040558











