Biological As(III) Oxidation Coupled with As(V) Interception by Fibrous Anion Exchange Material FFA-1
Abstract
:1. Introduction
2. Materials and Methods
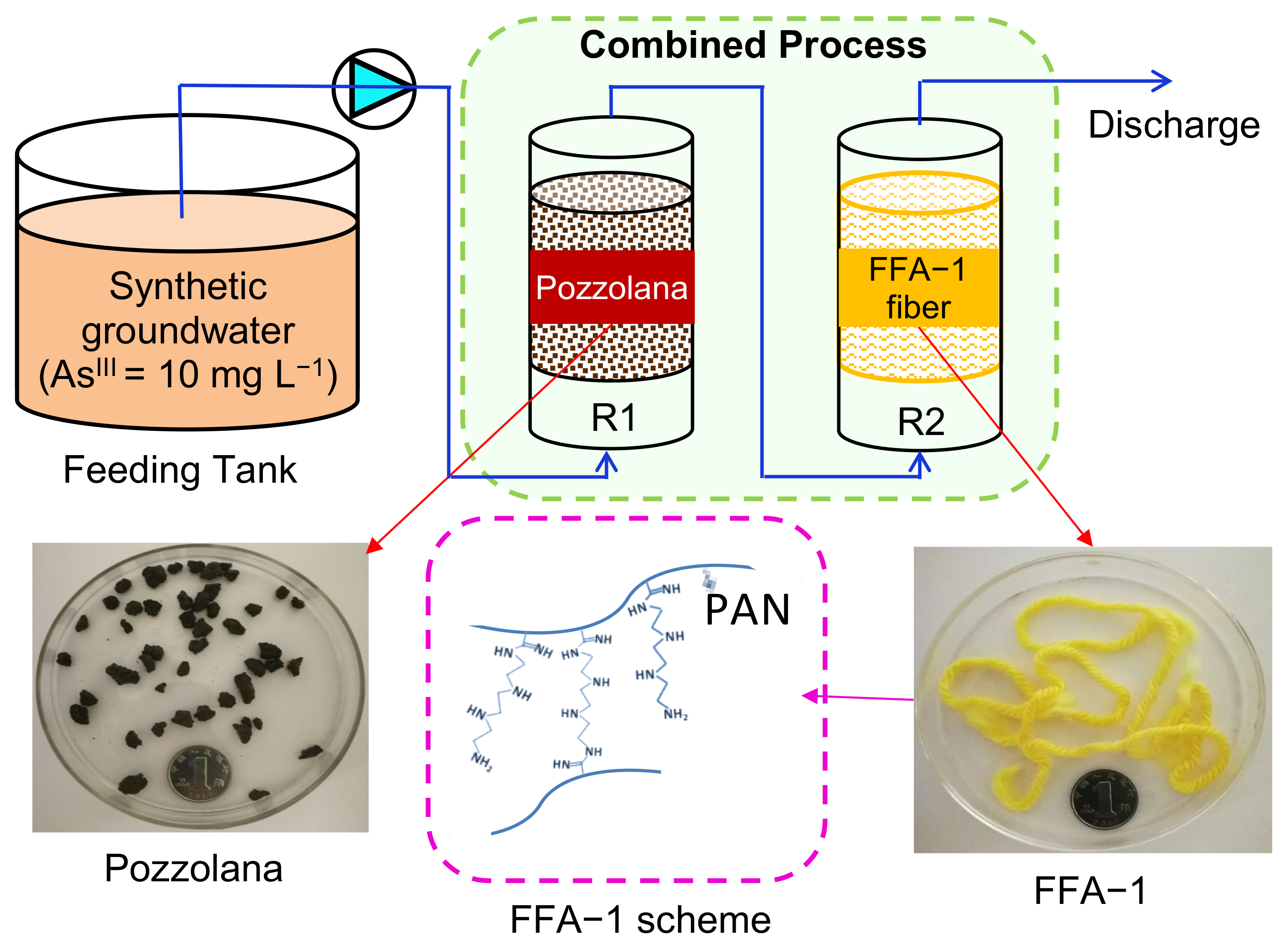
2.1. Experimental Set-Up
2.2. Inoculation and Substrate Composition
2.3. FFA-1 Characterization
2.3.1. Micromorphology Observation
2.3.2. Regeneration Test
2.3.3. Anion Interference
2.4. Microbial Diversity Analysis
2.5. Analysis Methods
2.6. Mass Balance of As
3. Results
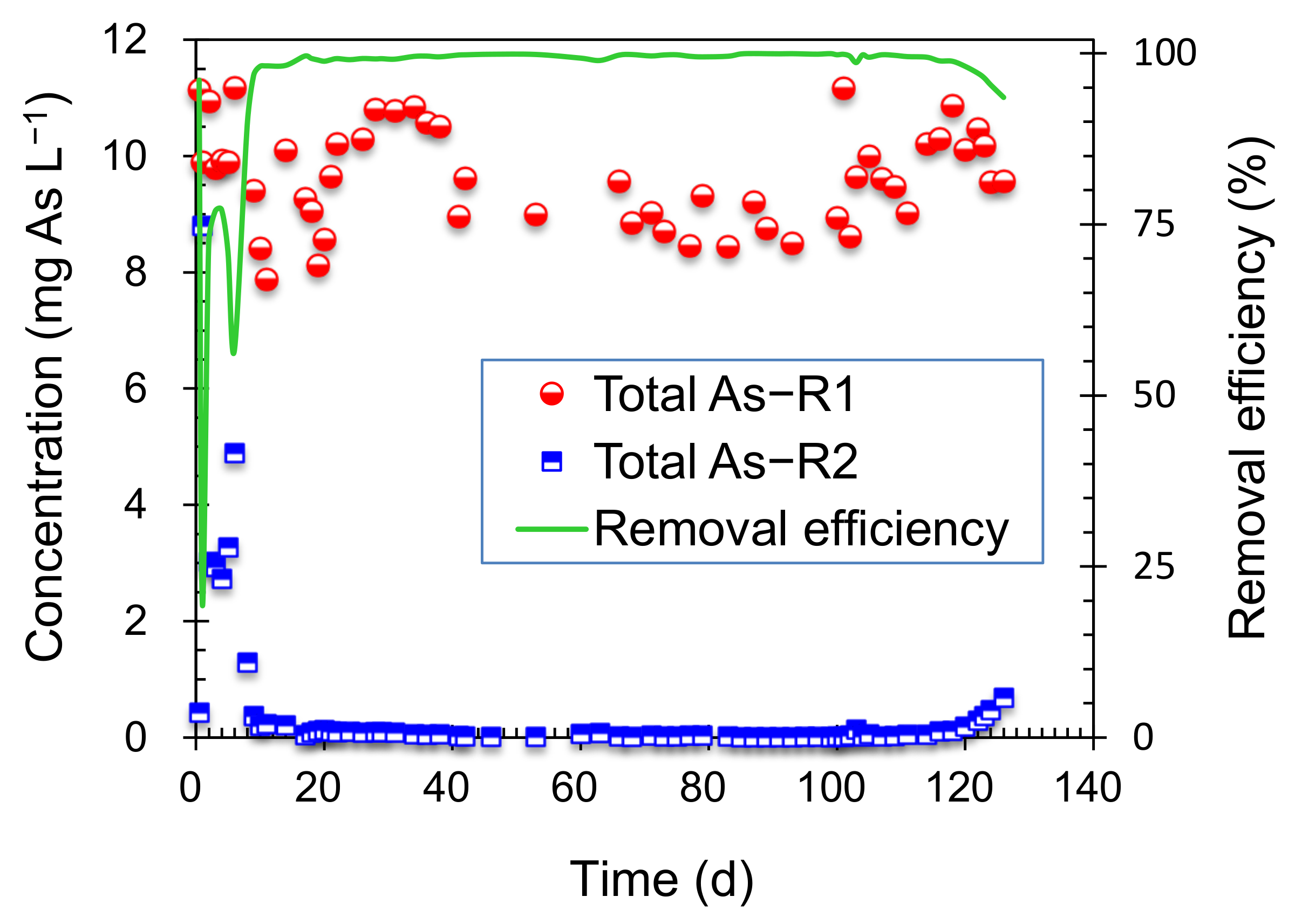
3.1. Performance of Combined System
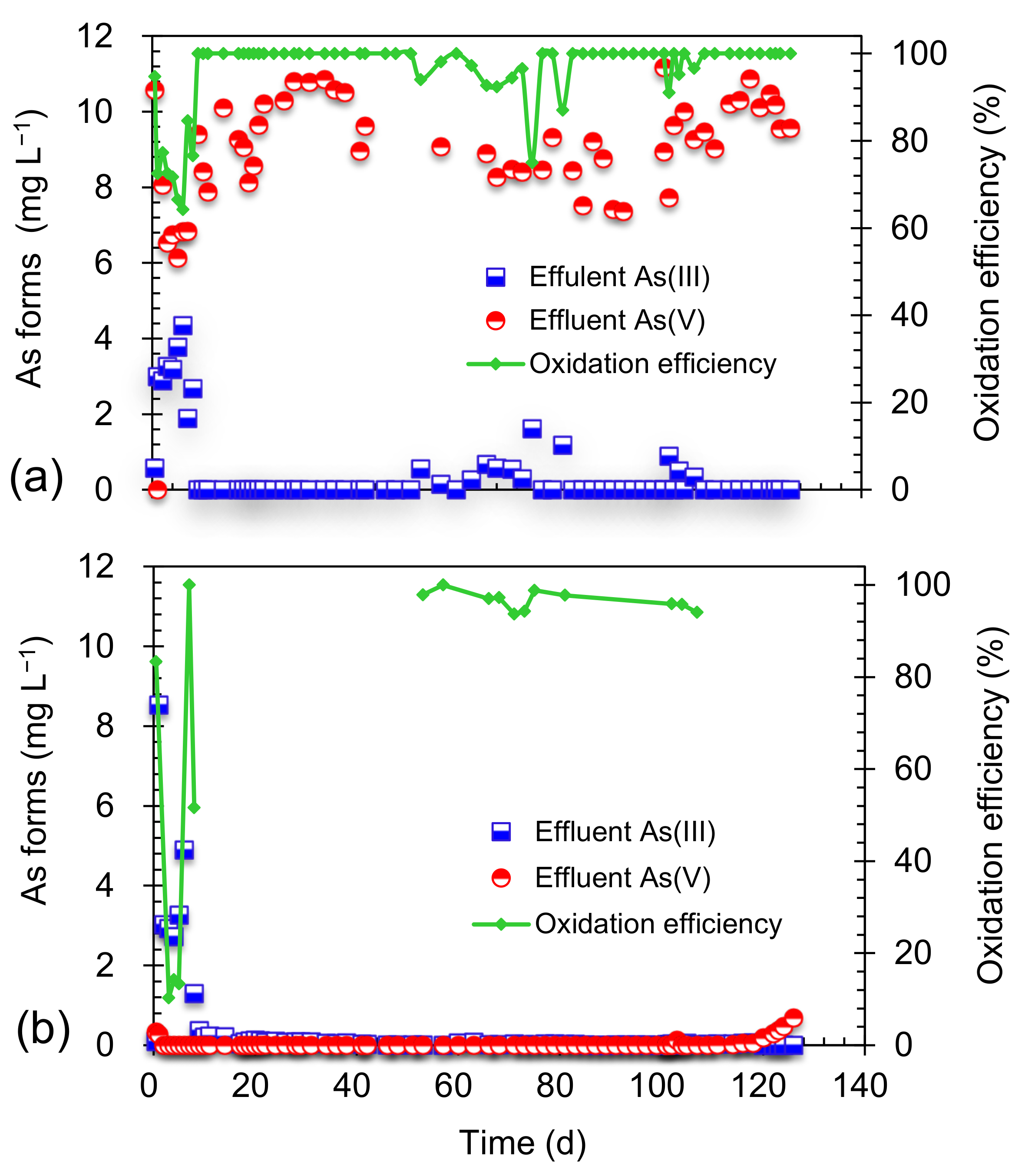
3.2. Arsenite Oxidation in the Combined System
3.3. Morphological Evolution of FFA-1
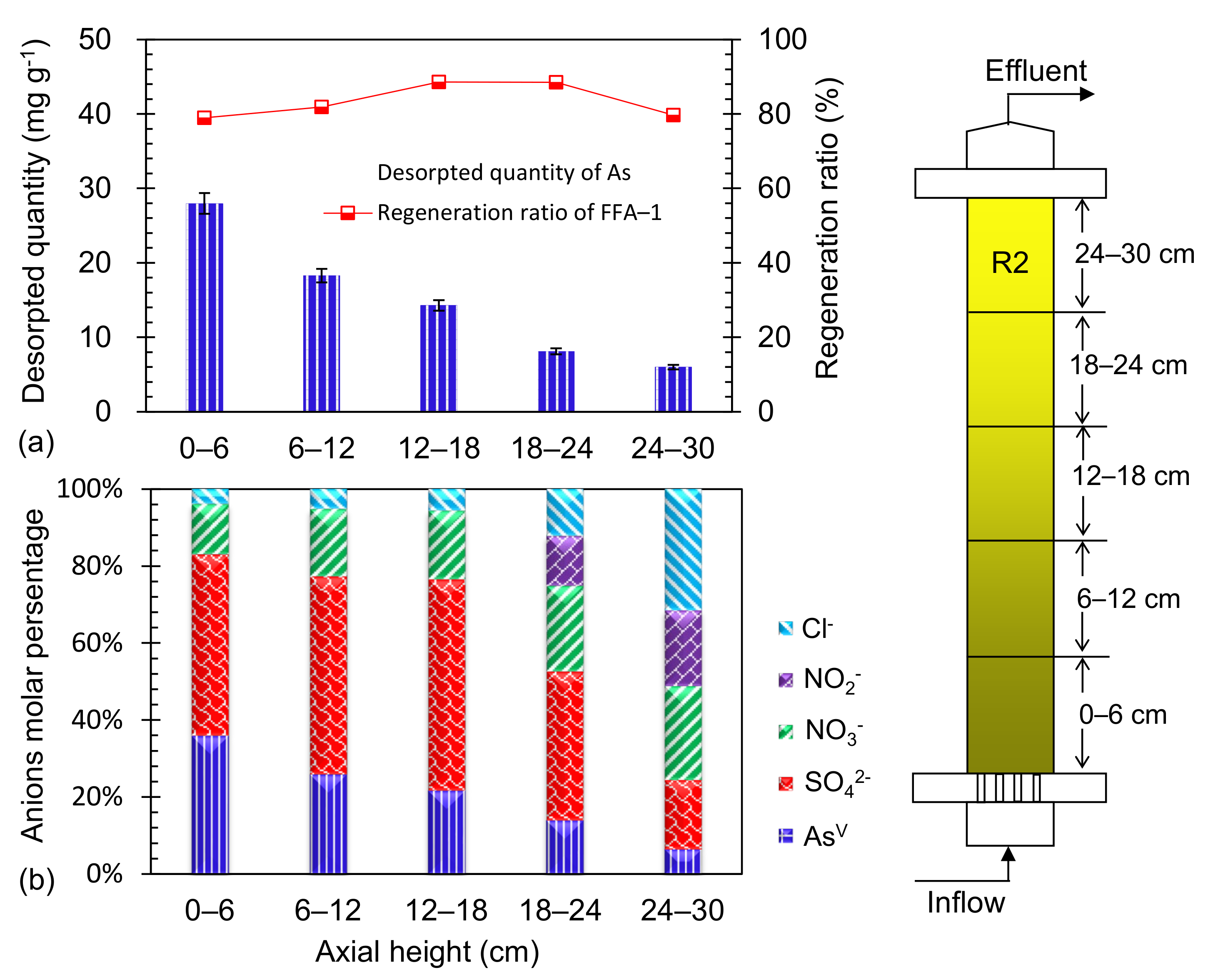
3.4. Distribution of As Trapped in R2
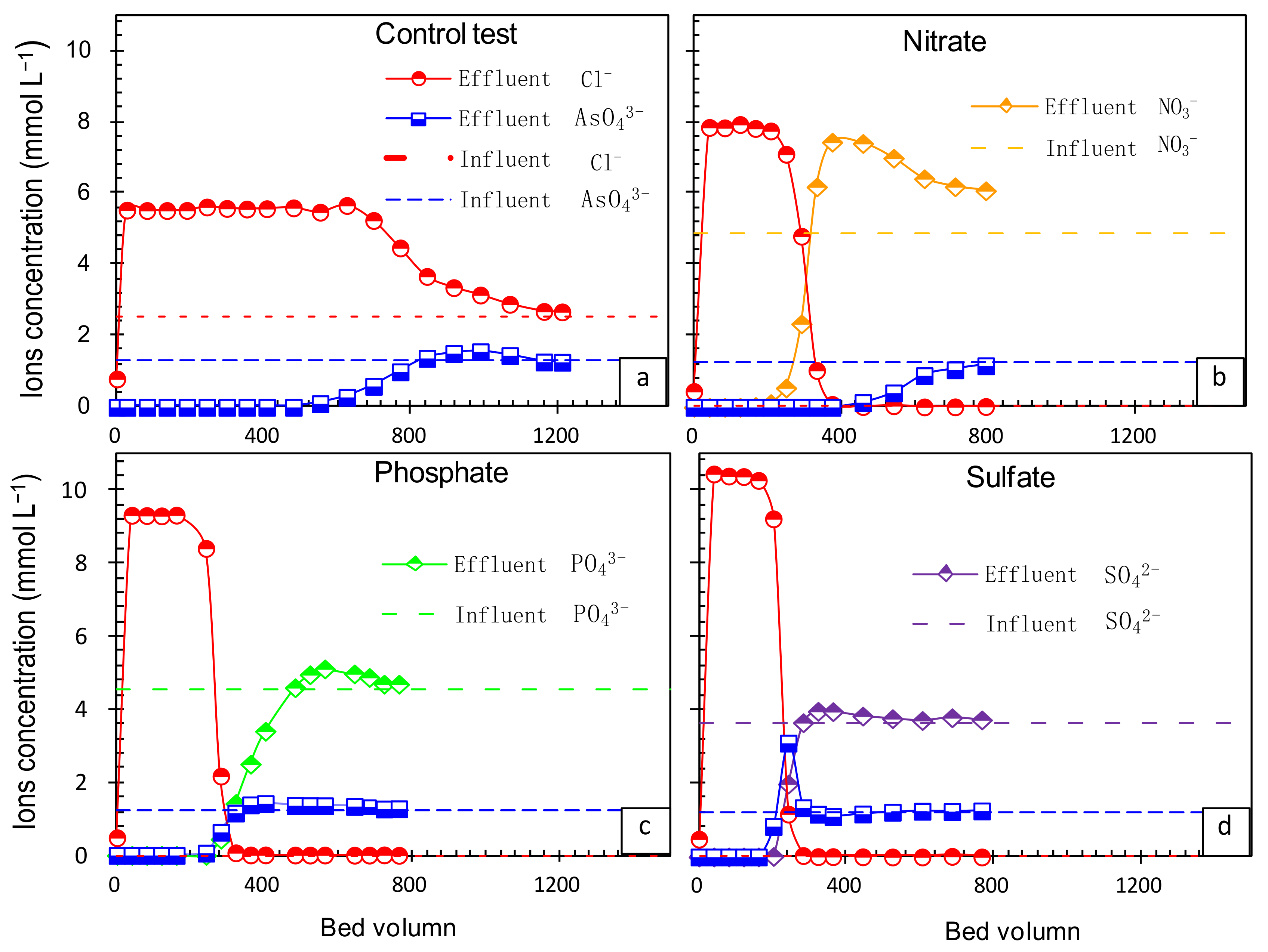
3.5. Effect of Co-Existing Anions on As(V) Removal by FFA-1
3.6. Microbial Diversity Analysis
4. Discussion
4.1. Feasibility of Ion Exchange Technology on As Removal
4.2. Role of Biological Arsenite Oxidation on the Combined Process
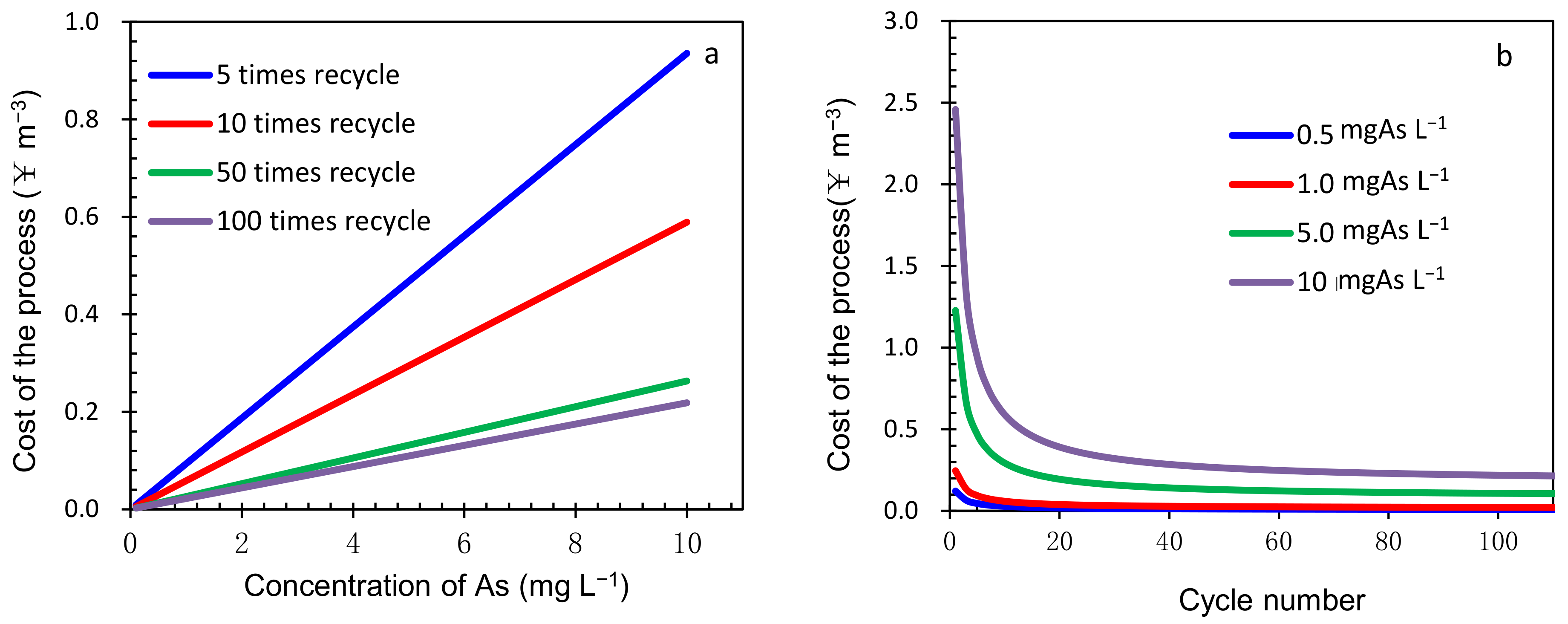
4.3. Operating Cost
5. Conclusions
- The combined process can efficiently and reliably remove As(III) from groundwater, achieving effluent total As concentration below 10 μg L−1 over 130 days;
- Attention should be paid to competing anions (e.g., SO42− and NO3−) present in the groundwater when the fibrous FFA-1 as an anion exchange material is utilized in the combined process for ultimate As(V) removal;
- This combined process would be economically feasible as an alternative for the remediation of As from polluted groundwater.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hughes, M.F. Arsenic toxicity and potential mechanisms of action. Toxicol. Lett. 2002, 133, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Ng, J.C.; Wang, J.; Shraim, A. A global health problem caused by arsenic from natural sources. Chemosphere 2003, 52, 1353–1359. [Google Scholar] [CrossRef]
- Li, C.; Li, F.; Wu, Z.; Cheng, J. Effects of landscape heterogeneity on the elevated trace metal concentrations in agricultural soils at multiple scales in the Pearl River Delta, South China. Environ. Pollut. 2015, 206, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Tjell, J.C.; Sloth, J.J.; Holm, P. Review of arsenic contamination, exposure through water and food and low cost mitigation options for rural areas. Appl. Geochem. 2014, 41, 11–33. [Google Scholar] [CrossRef]
- Yin, Y.; Wan, J.; Li, S.; Li, H.; Dagot, C.; Wang, Y. Transformation of roxarsone in the anoxic–oxic process when treating the livestock wastewater. Sci. Total Environ. 2018, 616–617, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Molinari, R.; Argurio, P. Arsenic removal from water by coupling photocatalysis and complexation-ultrafiltration processes: A preliminary study. Water Res. 2017, 109, 327–336. [Google Scholar] [CrossRef]
- Ficklin, W.H. Separation of arsenic(III) and arsenic(V) in ground waters by ion-exchange. Talanta 1983, 30, 371–373. [Google Scholar] [CrossRef]
- Liu, R.; Guo, J.; Tang, H. Adsorption of fluoride, phosphate, and arsenate ions on a new type of ion exchange fiber. J. Colloid Interf. Sci. 2002, 248, 268–274. [Google Scholar]
- Kim, J.; Benjamin, M.M.; Kwan, P.; Chang, Y. A novel ion exchange process for As removal. J. Am. Water Work. Assoc. 2003, 95, 77–85. [Google Scholar] [CrossRef]
- Awual, M.R.; El-Safty, S.A.; Jyo, A. Removal of trace arsenic(V) and phosphate from water by a highly selective ligand exchange adsorbent. J. Environ. Sci. 2011, 23, 1947–1954. [Google Scholar] [CrossRef]
- Awual, R.; Shenashen, M.; Yaita, T.; Shiwaku, H.; Jyo, A. Efficient arsenic(V) removal from water by ligand exchange fibrous adsorbent. Water Res. 2012, 46, 5541–5550. [Google Scholar] [CrossRef] [PubMed]
- Awual, R.; Shenashen, M.; Jyo, A.; Shiwaku, H.; Yaita, T. Preparing of novel fibrous ligand exchange adsorbent for rapid column-mode trace phosphate removal from water. J. Ind. Eng. Chem. 2013, 20, 2840–2847. [Google Scholar] [CrossRef]
- Fan, H.-T.; Sun, T.; Xu, H.-B.; Yang, Y.-J.; Tang, Q.; Sun, Y. Removal of arsenic(V) from aqueous solutions using 3-[2-(2-aminoethylamino)ethylamino]propyl-trimethoxysilane functionalized silica gel adsorbent. Desalination 2011, 278, 238–243. [Google Scholar] [CrossRef]
- Manning, B.A.; Fendorf, S.E.; Bostick, B.; Suarez, D.L. Arsenic(III) Oxidation and Arsenic(V) Adsorption Reactions on Synthetic Birnessite. Environ. Sci. Technol. 2002, 36, 976–981. [Google Scholar] [CrossRef] [Green Version]
- Nordstrom, D.K. Worldwide Occurrences of Arsenic in Ground Water. Science 2002, 296, 2143–2145. [Google Scholar] [CrossRef] [PubMed]
- Bissen, M.; Frimmel, F.H. Arsenic—A Review. Part II: Oxidation of Arsenic and its Removal in Water Treatment. Acta Hydrochim. Hydrobiol. 2003, 31, 97–107. [Google Scholar] [CrossRef]
- Cullen, W.R.; Reimer, K.J. Arsenic speciation in the environment. Chem. Rev. 1989, 89, 713–764. [Google Scholar] [CrossRef] [Green Version]
- Jain, C.K.; Ali, I. Arsenic: Occurrence, toxicity and speciation techniques. Water Res. 2000, 34, 4304–4312. [Google Scholar] [CrossRef]
- Li, H.; Zeng, X.C.; He, Z.; Chen, X.; Guoji, E.; Han, Y.; Wang, Y. Long-term performance of rapid oxidation of arsenite in simulated groundwater using a population of arsenite-oxidizing microorganisms in a bioreactor. Water Res. 2016, 101, 393–401. [Google Scholar] [CrossRef]
- Corsini, A.; Zaccheo, P.; Muyzer, G.; Andreoni, V.; Cavalca, L. Arsenic transforming abilities of groundwater bacteria and the combined use of Aliihoeflea sp. strain 2WW and goethite in metalloid removal. J. Hazard. Mater. 2013, 269, 89–97. [Google Scholar] [CrossRef]
- Dastidar, A.; Wang, Y.-T. Modeling arsenite oxidation by chemoautotrophic Thiomonas arsenivorans strain b6 in a packed-bed bioreactor. Sci. Total Environ. 2012, 432, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Miura, J.-I.; Ishikawa, N.; Umita, T. Biological oxidation of arsenite in synthetic groundwater using immobilised bacteria. Water Res. 2012, 46, 4825–4831. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Klein, J.; Simon, S.; Joulian, C.; Dictor, M.-C.; Deluchat, V.; Dagot, C. AsIII oxidation by Thiomonas arsenivorans in up-flow fixed-bed reactors coupled to As sequestration onto zero-valent iron-coated sand. Water Res. 2010, 44, 5098–5108. [Google Scholar] [CrossRef]
- Xiao, Y.; Roberts, D.J.; Zuo, G.; Badruzzaman, M.; Lehman, G.S. Characterization of microbial populations in pilot-scale fluidized-bed reactors treating perchlorate- and nitrate-laden brine. Water Res. 2010, 44, 4029–4036. [Google Scholar] [CrossRef]
- Weerasundara, L.; Ok, Y.-S.; Bundschuh, J. Selective removal of arsenic in water: A critical review. Environ. Pollut. 2020, 268, 115668. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Cui, L.; Zhou, D.; Huang, J.; Yuan, S. Resource recovery of Cr(VI) from electroplating wastewater: Laboratory and pilot-scale investigation using fibrous weak anion exchanger. J. Taiwan Inst. Chem. Eng. 2015, 54, 170–177. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, J.; Wang, Y. Removal of trivalent arsenic in water by ion exchange fiber FFA-1 loaded with AsOB. Acta Sci. Circumstantiae 2020, 40, 1667–1673. [Google Scholar]
- Wang, J.; Wan, J.; Li, H.; Li, H.; Dagot, C.; Wang, Y. Biological arsenite oxidation with nitrate as sole electron acceptor. Environ. Technol. 2016, 38, 2070–2076. [Google Scholar] [CrossRef] [PubMed]
- Battaglia-Brunet, F.; Dictor, M.-C.; Garrido, F.; Crouzet, C.; Morin, D.; DeKeyser, K.; Clarens, M.; Baranger, P. An arsenic(III)-oxidizing bacterial population: Selection, characterization, and performance in reactors. J. Appl. Microbiol. 2002, 93, 656–667. [Google Scholar] [CrossRef]
- Michon, J.; Dagot, C.; Deluchat, V.; Dictor, M.-C.; Battaglia-Brunet, F.; Baudu, M. As(III) biological oxidation by CAsO1 consortium in fixed-bed reactors. Process Biochem. 2010, 45, 171–178. [Google Scholar] [CrossRef]
- Hao, T.; Wei, L.; Lu, H.; Chui, H.; Mackey, H.R.; van Loosdrecht, M.C.; Chen, G. Characterization of sulfate-reducing granular sludge in the SANI((R)) process. Water Res. 2013, 47, 7042–7052. [Google Scholar] [CrossRef] [PubMed]
- Baalsrud, K. Studies on thiobacillus denitrificans. Arch. Microbiol. 1954, 20, 34–62. [Google Scholar] [CrossRef] [PubMed]
- Oremland, R.S.; Stolz, J.F. The Ecology of Arsenic. Science 2003, 300, 939–944. [Google Scholar] [CrossRef] [Green Version]
- Sultana, M.; Vogler, S.; Zargar, K.; Schmidt, A.C.; Saltikov, C.; Schlömann, S. New clusters of arsenite oxidase and unusual bacterial groups in enrichments from arsenic-contaminated soil. Arch. Microbiol. 2012, 194, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, H.; Yokoyama, T.; Eldridge, R.J.; Bolto, B.A. Adsorption characteristics of arsenic(III) and arsenic(V) on iron(III)-loaded chelating resin having lysine-N α,N α -diacetic acid moiety. React. Funct. Polym. 1996, 29, 167–174. [Google Scholar] [CrossRef]
- Yoshida, I.; Ueno, K.; Kobayashi, H. Selective Separation of Arsenic(III) and (V) Ions with Ferric Complex of Chelating Ion-Exchange Resin. Sep. Sci. Technol. 1978, 13, 173–184. [Google Scholar] [CrossRef]
- Dominguez, L.; Economy, J.; Benak, K.; Mangun, C.L. Anion exchange fibers for arsenate removal derived from a vinylbenzyl chloride precursor. Polym. Adv. Technol. 2003, 14, 632–637. [Google Scholar] [CrossRef]
- Ortega, A.; Oliva, I.; Contreras, K.E.; González, I.; Cruz-Díaz, M.R.; Rivero, E.P. Arsenic removal from water by hybrid electro-regenerated anion exchange resin/electrodialysis process. Sep. Purif. Technol. 2017, 184, 319–326. [Google Scholar] [CrossRef]
- Oehmen, A.; Valerio, R.; Llanos, J.; Fradinho, J.; Serra, S.; Reis, M.A.; Crespo, J.G.; Velizarov, S. Arsenic removal from drinking water through a hybrid ion exchange membrane–Coagulation process. Sep. Purif. Technol. 2011, 83, 137–143. [Google Scholar] [CrossRef]
- Mohanty, D. Conventional as well as Emerging Arsenic Removal Technologies—a Critical Review. Water, Air, Soil Pollut. 2017, 228, 381. [Google Scholar] [CrossRef]
- Du, Q.; Zhang, S.; Pan, B.; Lv, L.; Zhang, W.; Zhang, Q. Bifunctional resin-ZVI composites for effective removal of arsenite through simultaneous adsorption and oxidation. Water Res. 2013, 47, 6064–6074. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.S.; Korving, L.; van Loosdrecht, M.C.; Witkamp, G.-J. Adsorption as a technology to achieve ultra-low concentrations of phosphate: Research gaps and economic analysis. Water Res. X 2019, 4, 100029. [Google Scholar] [CrossRef] [PubMed]


| Element (%) | C | N | O | As | P | S | Cl | Fe |
|---|---|---|---|---|---|---|---|---|
| Raw FFA-1 | 75.4 | 11.9 | 12.7 | - | - | - | - | - |
| Pre-treatment FFA-1 | 63.4 | 14.6 | 11.8 | - | 0.5 | - | 9.7 | - |
| Reacted FFA-1 in R2 | 58.6 | 13.5 | 18.7 | 1.2 | 0.6 | 5.4 | 0.3 | 1.7 |
| References | Ion Exchanger | Form of Ion Exchanger | As Removal Capacity (mg g−1) | Regeneration Ratio (%) | Main Interfering Ions | |
|---|---|---|---|---|---|---|
| As(III) | As(V) | |||||
| This study | FFA-1 | Fiber | - | 82–89 | 90 | PO43−, SO42− |
| [10] | Zr-MPR | Bead | - | 0.35–0.42 * | - | PO43− |
| [11] | Zr-FCPS | Fiber | - | 8.17–9.52 | renewable | - |
| [37] | Vinylbenzyl chloride copolymer coated on a glass fiber | Fiber | - | >5.53 | - | - |
| [8] | Modified polyacrylonitrile fiber | Fiber | - | 96.0 | - | F−, PO43− |
| [13] | 3-[2-(2-Aminoethylamino)ethylamino]propyl-trimethoxysilanesilica gel | Bead | 2 | 13.2 | excellent | - |
| [36] | Uniselec UR-10 | Bead (100–200 mesh) | 35.2 | 39.7 | nonrenewable | - |
| [35] | IronIII-loaded chelating resin (Fe-LDA) | - | 62.9 | 55.4 | renewable | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, J.; Hai, R.; Zhang, Y.; Cui, L.; Guo, X.; Battaglia-Brunet, F.; Deluchat, V.; Yuan, S.; Huang, J.; Wang, Y. Biological As(III) Oxidation Coupled with As(V) Interception by Fibrous Anion Exchange Material FFA-1. Water 2022, 14, 856. https://doi.org/10.3390/w14060856
Wan J, Hai R, Zhang Y, Cui L, Guo X, Battaglia-Brunet F, Deluchat V, Yuan S, Huang J, Wang Y. Biological As(III) Oxidation Coupled with As(V) Interception by Fibrous Anion Exchange Material FFA-1. Water. 2022; 14(6):856. https://doi.org/10.3390/w14060856
Chicago/Turabian StyleWan, Junfeng, Rattanak Hai, Yucong Zhang, Lihui Cui, Xiaoying Guo, Fabienne Battaglia-Brunet, Véronique Deluchat, Siguo Yuan, Jiajia Huang, and Yan Wang. 2022. "Biological As(III) Oxidation Coupled with As(V) Interception by Fibrous Anion Exchange Material FFA-1" Water 14, no. 6: 856. https://doi.org/10.3390/w14060856
APA StyleWan, J., Hai, R., Zhang, Y., Cui, L., Guo, X., Battaglia-Brunet, F., Deluchat, V., Yuan, S., Huang, J., & Wang, Y. (2022). Biological As(III) Oxidation Coupled with As(V) Interception by Fibrous Anion Exchange Material FFA-1. Water, 14(6), 856. https://doi.org/10.3390/w14060856






