Mechanism and Kinetic Analysis of the Degradation of Atrazine by O3/H2O2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
2.2. Experimental Scheme
2.2.1. Solution Preparation
2.2.2. Experimental Scheme of ATZ Degradation by O3/H2O2
2.3. Analytical Method
2.3.1. Liquid Chromatography Analysis
2.3.2. Kinetic Analysis of ATZ Degradation by O3/H2O2
- qe: Adsorption capacity value at equilibrium, mg/g;
- qt: Adsorption capacity value at time t, mg/g;
- K1: Pseudo-first-order rate constant, 1/min;
- K2: Pseudo-second-order rate constant, L/(μmol·min);
- β: Represents the elimination rate of ATZ;
- m: Mass of the adsorbent, g;
- C0: Initial concentration of ATZ;
- Ce: Reaction ATZ concentration at equilibrium;
- Ct: ATZ concentration at any time, mg/L.
3. Results and Discussion
3.1. Effect of Temperature on ATZ Degradation by O3/H2O2
3.2. The Effect of H2O2 Concentration on ATZ Degradation by O3/H2O2
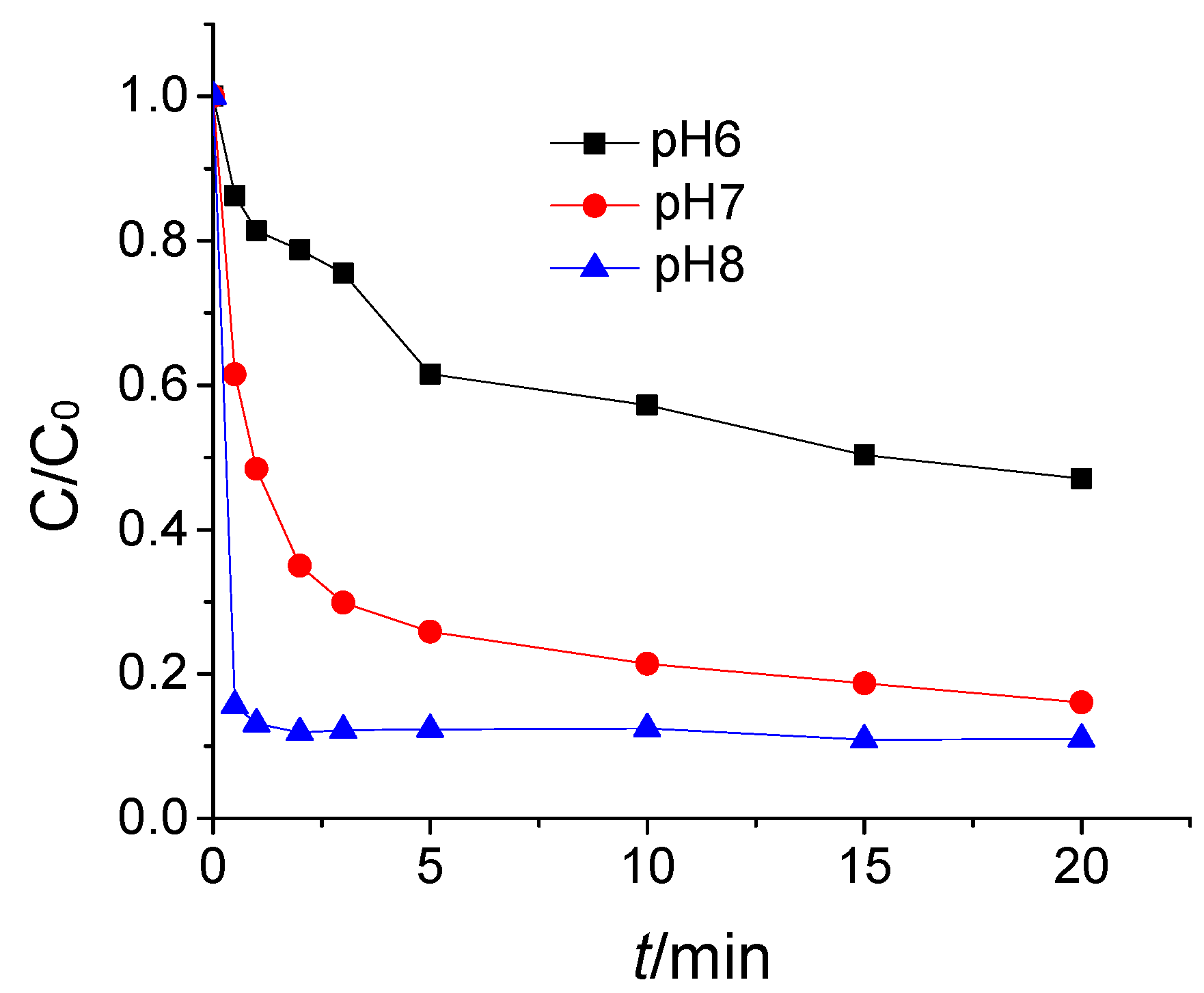
3.3. The Effect of pH Value on ATZ Degradation by O3/H2O2
3.4. Mechanism Analysis of ATZ Degradation by O3/H2O2
3.4.1. Mechanism Analysis of ATZ Degradation by O3/H2O2 in Phosphate Buffer at pH6
3.4.2. Mechanism Analysis of ATZ Degradation by O3/H2O2 in Phosphate Buffer at pH7
3.4.3. Mechanism Analysis of ATZ Degradation by O3/H2O2 in Phosphate Buffer at pH8
3.4.4. Intermediate Products and Degradation Mechanism of ATZ Degradation
3.5. Kinetic Analysis of ATZ Degradation by O3/H2O2
3.5.1. Kinetic Analysis of ATZ Degradation by O3/H2O2 under Different Temperatures
3.5.2. Kinetic Analysis of ATZ Degradation by O3/H2O2 under Different H2O2 Concentrations
3.5.3. Kinetic Analysis of ATZ Degradation by O3/H2O2 at Different pH Values
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Britt, A.; Bernini, M.; McSweeney, B.; Dalapati, S.; Duchin, S.; Cavanna, K.; Santos, N.; Donovan, G.; O’Byrne, K.; Noyes, S.; et al. The effects of atrazine on the microbiome of the eastern oyster: Crassostrea virginica. Sci. Rep. 2020, 10, 11088. [Google Scholar] [CrossRef] [PubMed]
- Recker, R.A.; Mitchell, P.D.; Stoltenberg, D.E.; Lauer, J.G.; Davis, V.M. Late-Season Weed Escape Survey Reveals Discontinued Atrazine Use Associated with Greater Abundance of Broadleaf Weeds. Weed Technol. 2015, 29, 451–463. [Google Scholar] [CrossRef]
- Ye, X.; Lu, Y.; Zhang, H. Use and harm of the herbicide atrazine. Environ. Sci. Manag. 2006, 8, 95–97. [Google Scholar]
- Khatoon, H.; Rai, J.P.N. Optimization studies on biodegradation of atrazine by Bacillus badius ABP6 strain using response surface methodology. Biotechnol. Rep. 2020, 26, e00459. [Google Scholar] [CrossRef]
- Sun, X.; Liu, F.; Shan, R.; Fan, Y. Spatiotemporal distributions of Cu, Zn, metribuzin, atrazine, and their transformation products in the surface water of a small plain stream in eastern China. Environ. Monit. Assess. 2019, 191, 433. [Google Scholar] [CrossRef]
- Eugenia Taverna, M.; Alberto Busatto, C.; Raquel Lescano, M.; Nicolau, V.V.; Susana Zalazar, C.; Raul Meira, G.; Alejandra Estenoz, D. Microparticles based on ionic and organosolv lignins for the controlled release of atrazine. J. Hazard. Mater. 2018, 359, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Hu, G.; Zhu, J.; Wu, W.; Chen, J. Research progress on atrazine residues in aquatic environment and their toxicological effects. Environ. Pollut. Prev. 2009, 31, 64–68+83. [Google Scholar]
- Buser, H.R. Atrazine and other s-triazine herbicides in lakes and in Switzerland. Environ. Sci. Technol. 1990, 24, 1049–1058. [Google Scholar] [CrossRef]
- He, H.; Liu, Y.; You, S.; Liu, J.; Xiao, H.; Tu, Z. A Review on Recent Treatment Technology for Herbicide Atrazine in Contaminated Environment. Int. J. Environ. Res. Public Health 2019, 16, 5129. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Li, C.; Sun, J.; Wang, H.; Wang, D.; Song, H.; Wang, Z. Residue Characteristics and Ecological Risk Assessment of Twenty-nine Pesticides in Surface Water of Major River-Basin in China. Asian J. Ecotoxicol. 2016, 11, 347–354. [Google Scholar]
- Sun, D.; Gao, G. Effects of atrazine and its degrading bacteria on the germination rate of rice seeds. Heilongjiang Agric. Sci. 2017, 4, 48–50. [Google Scholar]
- Fu, L.; Ni, J.; Ruan, Y.; Dong, R.; Shi, H. Effects of Atrazine on Embryonic Development and Histological Structure of Liver and Kidney in Red-eared Turtle (Trachemys scripta elegans). Fish. Sci. 2017, 36, 104–108. [Google Scholar]
- Belloni, V.; Dessi-Fulgheri, F.; Zaccaroni, M.; Di Consiglio, E.; De Angelis, G.; Testai, E.; Santochirico, M.; Alleva, E.; Santucci, D. Early exposure to low doses of atrazine affects behavior in juvenile and adult CD1 mice. Toxicology 2011, 279, 19–26. [Google Scholar] [CrossRef]
- Stradtman, S.C.; Freeman, J.L. Mechanisms of Neurotoxicity Associated with Exposure to the Herbicide Atrazine. Toxics 2021, 9, 207. [Google Scholar] [CrossRef] [PubMed]
- Sagarkar, S.; Gandhi, D.; Devi, S.S.; Sakharkar, A.; Kapley, A. Atrazine exposure causes mitochondrial toxicity in liver and muscle cell lines. Indian J. Pharmacol. 2016, 48, 200–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcus, S.R.; Fiumera, A.C. Atrazine exposure affects longevity, development time and body size in Drosophila melanogaster. J. Insect Physiol. 2016, 91–92, 18–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barchanska, H.; Sajdak, M.; Szczypka, K.; Swientek, A.; Tworek, M.; Kurek, M. Atrazine, triketone herbicides, and their degradation products in sediment, soil and surface water samples in Poland. Environ. Sci. Pollut. Res. 2017, 24, 644–658. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.T.; Pan, L.L.; Zhan, Y.; Tsang, D.C.W.; Zhu, L.Z.; Li, X.D. Atrazine contamination in agricultural soils from the Yangtze River Delta of China and associated health risks. Environ. Geochem. Health 2017, 39, 369–378. [Google Scholar] [CrossRef]
- Ding, Z.; Zhang, J.; He, Y.; Lu, Y. Experimental study on the degradation of atrazine by ozone/peroxymonosulfate in phosphate buffer. Water Treat. Technol. 2018, 44, 43–47+52. [Google Scholar] [CrossRef]
- Wardenier, N.; Liu, Z.; Nikiforov, A.; Van Hulle, S.W.H.; Leys, C. Micropollutant elimination by O3, UV and plasma-based AOPs: An evaluation of treatment and energy costs. Chemosphere 2019, 234, 715–724. [Google Scholar] [CrossRef]
- Hou, B.; Shen, J.; Li, T.; Xu, P. Ferric silicate catalyzed ozone removal of atrazine and nitrobenzene in water. J. Nat. Sci. Heilongjiang Univ. 2015, 32, 223–228. [Google Scholar] [CrossRef]
- Jing, L.; Chen, B.; Wen, D.Y.; Zheng, J.S.; Zhang, B.Y. Pilot-scale treatment of atrazine production wastewater by UV/O3/ultrasound: Factor effects and system optimization. J. Environ. Manag. 2017, 203, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Li, J.; Fu, C.X.; Wang, Z.Y.; Zhou, Y.X.; Zhang, X.L.; Yang, J.X.; Wang, K.; Liu, Y.H.; Song, Q. Establishment of sulfate radical advanced oxidation process based on Fe2+/O-2/dithionite for organic contaminants degradation. Chem. Eng. J. 2021, 410, 128204. [Google Scholar] [CrossRef]
- Khandarkhaeva, M.; Batoeva, A.; Aseev, D.; Sizykh, M.; Tsydenova, O. Oxidation of atrazine in aqueous media by solar- enhanced Fenton-like process involving persulfate and ferrous ion. Ecotoxicol. Environ. Saf. 2017, 137, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.Z.; Zhang, K.F.; Toe, C.Y.; Amal, R.; Zhang, X.W.; McCarthy, D.T.; Deletic, A. Stormwater herbicides removal with a solar-driven advanced oxidation process: A feasibility investigation. Water Res. 2021, 190, 116783. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.Y.; Chen, B.; Liu, B. An ultrasound/O3 and UV/O3 process for atrazine manufacturing wastewater treatment: A multiple scale experimental study. Water Sci. Technol. 2022, 85, 229–243. [Google Scholar] [CrossRef]
- Cruz-Alcalde, A.; Esplugas, S.; Sans, C. Continuous versus single H2O2 addition in peroxone process: Performance improvement and modelling in wastewater effluents. J. Hazard. Mater. 2020, 387, 121993. [Google Scholar] [CrossRef]
- Kordkandi, S.A.; Motlagh, A.M. Optimization of peroxone reaction rate using metaheuristic approach in the dearomatization and discoloration process. Environ. Prog. Sustain. Energy 2018, 37, 695–702. [Google Scholar] [CrossRef]
- Xu, X.; Zhu, T.; Chen, J. Detection method of ozone concentration in water. J. Hohai Univ. Chang. 2007, 1, 48–52. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Ho, Y.-S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Ma, J.; Sun, Z.Z.; Liu, H.L. Influencing mechanism of temperature on the degradation of nitrobenzene in aqueous solution by ceramic honeycomb catalytic ozonation. J. Hazard. Mater. 2009, 167, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.; Burch, R. An investigation of catalytic ozonation for the oxidation of halocarbons in drinking water preparation. Water Res. 1999, 33, 3695–3700. [Google Scholar] [CrossRef]
- Qiang, Z.; Liu, C.; Dong, B.; Zhang, Y. Degradation mechanism of alachlor during direct ozonation and O3/H2O2 advanced oxidation process. Chemosphere 2010, 78, 517–526. [Google Scholar] [CrossRef]
- Cuerda-Correa, E.M.; Alexandre-Franco, M.F.; Fernandez-Gonzalez, C. Advanced Oxidation Processes for the Removal of Antibiotics from Water. An Overview. Water 2020, 12, 102. [Google Scholar] [CrossRef] [Green Version]
- Balci, B.; Oturan, N.; Cherrier, R.; Oturan, M.A. Degradation of atrazine in aqueous medium by electrocatalytically generated hydroxyl radicals. A kinetic and mechanistic study. Water Res. 2009, 43, 1924–1934. [Google Scholar] [CrossRef]
- Kim, H.C.; Park, S.H.; Noh, J.H.; Choi, J.; Lee, S.; Maeng, S.K. Comparison of pre-oxidation between O3 and O3/H2O2 for subsequent managed aquifer recharge using laboratory-scale columns. J. Hazard. Mater. 2019, 377, 290–298. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.L. Degradation and mineralization of ofloxacin by ozonation and peroxone (O3/H2O2) process. Chemosphere 2021, 269. [Google Scholar] [CrossRef]
- Gounden, A.N.; Singh, S.; Jonnalagadda, S.B. Simultaneous removal of 2,4,6-tribromophenol from water and bromate ion minimization by ozonation. J. Hazard. Mater. 2018, 357, 415–423. [Google Scholar] [CrossRef]
- Piras, F.; Santoro, O.; Pastore, T.; Pio, I.; De Dominicis, E.; Gritti, E.; Caricato, R.; Lionetto, M.G.; Mele, G.; Santoro, D. Controlling micropollutants in tertiary municipal wastewater by O3/H2O2, granular biofiltration and UV254/H2O2 for potable reuse applications. Chemosphere 2020, 239, 124635. [Google Scholar] [CrossRef]
- Biard, P.F.; Dang, T.T.; Bocanegra, J.; Couvert, A. Intensification of the O3/H2O2 advanced oxidation process using a continuous tubular reactor filled with static mixers: Proof of concept. Chem. Eng. J. 2018, 344, 574–582. [Google Scholar] [CrossRef]
- Muhammad, A.; Shafeeq, A.; Butt, M.A.; Rizvi, Z.H.; Chughtai, M.A.; Rehman, S. Decolorization and removal of cod and bod from raw and biotreated textile dye bath effluent through advanced oxidation processes (AOPS). Braz. J. Chem. Eng. 2008, 25, 453–459. [Google Scholar] [CrossRef]
- Gu, Z.P.; Chen, W.M.; Wang, F.; Li, Q.B. Transformation and degradation of recalcitrant organic matter in membrane bioreactor leachate effluent by the O3/H2O2 process. Environ. Sci. -Water Res. Technol. 2019, 5, 1748–1757. [Google Scholar] [CrossRef]
- Qi, S.; Mao, Y.; Lv, M.; Sun, L.; Wang, X.; Yang, H.; Xie, Y.F. Pathway fraction of bromate formation during O3 and O3/H2O2 processes in drinking water treatment. Chemosphere 2016, 144, 2436–2442. [Google Scholar] [CrossRef]
- Yang, J.X.; Li, J.; Dong, W.Y.; Ma, J.; Cao, J.; Li, T.T.; Li, J.Y.; Gu, J.; Liu, P.X. Study on enhanced degradation of atrazine by ozonation in the presence of hydroxylamine. J. Hazard. Mater. 2016, 316, 110–121. [Google Scholar] [CrossRef]
- Bourgin, M.; Borowska, E.; Helbing, J.; Hollender, J.; Kaiser, H.P.; Kienle, C.; McArdell, C.S.; Simon, E.; von Gunten, U. Effect of operational and water quality parameters on conventional ozonation and the advanced oxidation process O3/H2O2: Kinetics of micropollutant abatement, transformation product and bromate formation in a surface water. Water Res. 2017, 122, 234–245. [Google Scholar] [CrossRef] [Green Version]
- Venta, M.B.; Castro, C.H.; Garcia, L.A.F.; Marzo, A.L.; Lorenzo, E.V.; Alvarez, C.A. Effect of O3/H2O2 molar concentration ratio at different pH values on cyclophosphamide degradation. J. Water Supply Res. Technol.-Aqua 2005, 54, 403–410. [Google Scholar] [CrossRef]
- Feng, H.R.; Liu, M.; Zeng, W.; Chen, Y. Optimization of the O3/H2O2 process with response surface methodology for pretreatment of mother liquor of gas field wastewater. Front. Environ. Sci. Eng. 2021, 15, 78. [Google Scholar] [CrossRef]
- Ochir, D.; Lee, Y.; Shin, J.; Kim, S.; Kwak, J.; Chon, K. Oxidative Treatments of Pesticides in Rainwater Runoff by HOCl, O3, and O3/H2O2: Effects of pH, Humic Acids and Inorganic Matters. Separations 2021, 8, 101. [Google Scholar] [CrossRef]
- Farzaneh, H.; Loganathan, K.; Saththasivam, J.; McKay, G. Selectivity and competition in the chemical oxidation processes for a binary pharmaceutical system in treated sewage effluent. Sci. Total Environ. 2021, 765, 142704. [Google Scholar] [CrossRef]
- Asaithambi, P.; Sajjadi, B.; Aziz, A.R.A. Integrated ozone-photo-Fenton process for the removal of pollutant from industrial wastewater. Chin. J. Chem. Eng. 2017, 25, 516–522. [Google Scholar] [CrossRef]
- Pillai, K.C.; Kwon, T.O.; Moon, I.S. Degradation of wastewater from terephthalic acid manufacturing process by ozonation catalyzed with Fe2+, H2O2 and UV light: Direct versus indirect ozonation reactions. Appl. Catal. B-Environ. 2009, 91, 319–328. [Google Scholar] [CrossRef]
- Li, S.; Liang, Y.; Zhang, R.; Li, P.; Ye, F. Study on Influencing Factors of O3/H2O2 Degradation of Atrazine. Chin. J. Environ. Eng. 2008, 2, 358–361. [Google Scholar]
- Wu, Z.; Chen, H. Research and Application Progress of O3/H2O2 Process in Water Treatment. Water Treat. Technol. 2013, 39, 1–5. [Google Scholar] [CrossRef]
- Chan, K.H.; Chu, W. Model applications and mechanism study on the degradation of atrazine by Fenton’s system. J. Hazard. Mater. 2005, 118, 227–237. [Google Scholar] [CrossRef]
- Liu, Y.C.; Ji, X.G.; Yang, J.J.; Tang, W.; Zhu, Y.L.; Wang, Y.; Zhang, Y.X.; Zhang, Y.; Duan, J.M.; Li, W. Degradation of the typical herbicide atrazine by UV/persulfate: Kinetics and mechanisms. Environ. Sci. Pollut. Res. 2015, 22, 7766–7775. [Google Scholar] [CrossRef]
- Diao, Z.H.; Chu, W. FeS2 assisted degradation of atrazine by bentonite-supported nZVI coupling with hydrogen peroxide process in water: Performance and mechanism. Sci. Total Environ. 2021, 754, 142155. [Google Scholar] [CrossRef]
- Shaban, M.; Abukhadra, M.R.; Ibrahim, S.S.; Shahien, M.G. Photocatalytic degradation and photo-Fenton oxidation of Congo red dye pollutants in water using natural chromite-response surface optimization. Appl. Water Sci. 2017, 7, 4743–4756. [Google Scholar] [CrossRef] [Green Version]












| pH | 0.2 mol/L NaH2PO4-NaOH (mL) | 0.2 mol/L NaOH (mL) |
|---|---|---|
| 6 | 250 | 28.50 |
| 7 | 250 | 148.15 |
| 8 | 250 | 244.00 |
| T(°C) | Fitted Equation | Reaction Order | K1 (1/min) | R2 |
|---|---|---|---|---|
| 10 | Y = −0.11198x − 0.37774 | First-order reaction | 0.11198 | 0.91718 |
| 15 | Y = −0.11688x − 0.63552 | First-order reaction | 0.11688 | 0.84758 |
| 20 | Y = −0.06995x − 0.65722 | First-order reaction | 0.06995 | 0.65698 |
| 25 | Y = −0.10641x − 1.42622 | First-order reaction | 0.10641 | 0.48264 |
| T(°C) | Fitted Equation | Reaction Order | K1 (1/min) | R2 |
|---|---|---|---|---|
| 10 | Y = 0.48111x + 1.00352 | Second-order reaction | 0.48111 | 0.99749 |
| 15 | Y = 0.6592x + 1.38581 | Second-order reaction | 0.65920 | 0.99585 |
| 20 | Y = 0.23151x + 1.08379 | Second-order reaction | 0.23151 | 0.87706 |
| 25 | Y = 0.9533x + 0.83282 | Second-order reaction | 0.95330 | 0.82373 |
| C(H2O2) (μmol/L) | Fitted Equation | Reaction Order | K1 (1/min) | R2 |
|---|---|---|---|---|
| 5 | Y = −0.08137x − 0.38598 | First-order reaction | 0.08137 | 0.84815 |
| 10 | Y = −0.11383x − 0.630929 | First-order reaction | 0.11383 | 0.85080 |
| 15 | Y = −0.07185x − 0.70582 | First-order reaction | 0.07185 | 0.68089 |
| 20 | Y = −0.06995x − 0.65722 | First-order reaction | 0.06995 | 0.65698 |
| C(H2O2) (μmol/L) | Fitted Equation | Reaction Order | K1 (1/min) | R2 |
|---|---|---|---|---|
| 5 | Y = 0.24548x + 1.36493 | Second-order reaction | 0.24548 | 0.97377 |
| 10 | Y = 0.62450x + 1.388 | Second-order reaction | 0.62450 | 0.99343 |
| 15 | Y = 0.25765x + 2.05714 | Second-order reaction | 0.25765 | 0.90920 |
| 20 | Y = 0.23151x + 1.99153 | Second-order reaction | 0.23151 | 0.87706 |
| pH | Fitted Equation | Reaction Order | K1 (1/min) | R2 |
|---|---|---|---|---|
| 6 | Y = −0.03397x – 0.15995 | First-order reaction | 0.03397 | 0.86677 |
| 7 | Y = −0.06995x – 0.65722 | First-order reaction | 0.06995 | 0.65698 |
| 8 | Y = −0.04215x – 1.59326 | First-order reaction | 0.04215 | 0.06647 |
| pH | Fitted Equation | Reaction Order | K1 (1/min) | R2 |
|---|---|---|---|---|
| 6 | Y = 0.05299x + 1.16329 | Second-order reaction | 0.05299 | 0.92574 |
| 7 | Y = 0.23151x + 1.99153 | Second-order reaction | 0.23151 | 0.87706 |
| 8 | Y = 0.19067x + 6.14035 | Second-order reaction | 0.19067 | 0.19382 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Tang, C.; Liu, Y.; Chen, J. Mechanism and Kinetic Analysis of the Degradation of Atrazine by O3/H2O2. Water 2022, 14, 1412. https://doi.org/10.3390/w14091412
Lu Y, Tang C, Liu Y, Chen J. Mechanism and Kinetic Analysis of the Degradation of Atrazine by O3/H2O2. Water. 2022; 14(9):1412. https://doi.org/10.3390/w14091412
Chicago/Turabian StyleLu, Yixin, Chenghan Tang, Yujie Liu, and Jiao Chen. 2022. "Mechanism and Kinetic Analysis of the Degradation of Atrazine by O3/H2O2" Water 14, no. 9: 1412. https://doi.org/10.3390/w14091412
APA StyleLu, Y., Tang, C., Liu, Y., & Chen, J. (2022). Mechanism and Kinetic Analysis of the Degradation of Atrazine by O3/H2O2. Water, 14(9), 1412. https://doi.org/10.3390/w14091412





